Abstract
Background: Alcohol consumption has been in existence in the world for many centuries and it is the major cause of death and injury worldwide. Alcoholic liver disease (ALD) is caused due to excess and chronic alcohol intake. Studies across the globe have identified several pathways leading to ALD. Adipose tissue which has been considered as an energy storage organ is also found to play a major role in ALD progression by secreting hormones and cytokines known as adipokines or adipocytokines. Ethanol affects the metabolic and innate immune activities of adipose tissue contributing to alcohol-induced injury of the tissues.
Objective: We aimed at 1) summarizing the metabolism and progression of ALD 2) summarizing about the structure and effect of ethanol induced oxidative stress on adipose tissue 3) reviewing the available data on the effect of ethanol on adipose tissue mass and adipokine secretion in both rodent models and alcoholic patients.
Methods: The article is summarized based on the original literature and reviews in studying the effect of ethanol on adipose tissue.
Results: Studies on alcoholic patients and rodent models has shown that chronic ethanol consumption reduces adipose tissue mass and causes CYP2E1 mediated oxidative stress and inflammation of adipose tissue. Further hyperlipolysis is observed in adipose tissue that leads to excess fatty acid release that gets transported and deposited in the liver resulting in hepatic steatosis.
Conclusion: Studies show that adipose tissue plays a major role in the progression of ALD. So understanding of the mechanisms linking ethanol induced adipose tissue injury with ALD progression would help us in identifying potential therapeutic targets.
Abbreviations
| ALD | = | Alcoholic liver disease |
| CYP2E1 | = | cytochrome P450 2E1 |
| WAT | = | white adipose tissue |
| BAT | = | brown adipose tissue |
| ROS | = | reactive oxygen species |
Introduction
Alcohol is a psychoactive substance and its consumption may lead to alcoholism or alcohol abuse. Alcohol uptake at harmful levels may cause morbidity and mortality together with socio-economic burden. The pattern and the amount of alcohol consumed determine the extent of alcohol related harm. It is among the world's top 5 risk factors causing injury, disease, disability, or death.Citation1,2 It is the cause for more than 200 disease and injury conditions and is associated with a danger of developing alcohol dependence, liver damage, and cancers.Citation3 According to 2014 WHO global status report on alcohol and health, alcohol consumption is responsible for 5.9% of all global deaths and 5.1% of DALYs (disability-adjusted life years).
Alcohol Metabolism
Alcohol is primarily metabolized in the body via diverse pathways by the catalytic activity of 3 different enzymes–alcohol dehydrogenase, cytochrome P450 2E1 (CYP2E1), and catalase. Chronic ethanol exposure induces CYP2E1 expression. Although the expression of CYP2E1 is greatest in the liver, it is also expressed in adipose tissue of rats after chronic ethanol consumption.Citation4
Asians are more susceptible to negative effects of alcohol and alcohol related cancers due to genetic differences in alcohol dehydrogenase and aldehyde dehydrogenase enzymes.Citation57
Alcohol disease progression
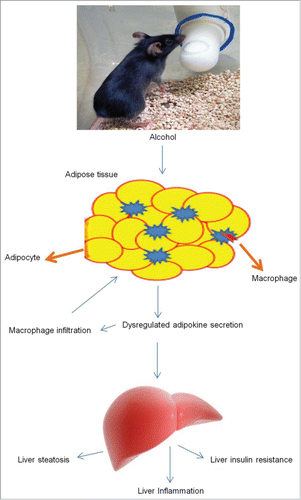
Alcohol consumption affects multiple signaling pathways and it is the cause of a multifactorial disease known as alcoholic liver disease. The disease progression is characterized by pathologic conditions like steatosis (fatty liver), steatohepatitis, fibrosis, end stage liver disease known as cirrhosis, and finally hepatocellular carcinoma.Citation8 Alcohol consumption affects multiple hepatic lipid metabolic pathways like de novo lipogenesis, oxidation of fatty acids, uptake and export of lipids.Citation9,10 However, studies show that alcohol mediated effect on hepatic lipid metabolism is also due to certain extra hepatic factors known as adipokinesCitation11,12 secreted by the adipose tissue.
Adipose tissue histology, locationand function
Based on function and histology, adipose tissue is subdivided into white adipose tissue (WAT) and brown adipose tissue (BAT). BAT plays a role in thermogenesis and body temperature regulation while WAT acts as an energy storage and endocrine organ. In mammals WAT is the major energy storage organ and it provides both thermal and mechanical insulation to the body. The location of WAT is not confined but it is distributed as physically unconnected individual pads throughout the organism. Based on the location WAT can be classified as epididymal, mesenteric, subcutaneous, retroperitoneal, and pericardial fat pads.Citation13,14
Adipose tissue is diverse in its cellular composition comprising of mature adipocytes, pre-adipocytes, macrophages, fibroblasts, and endothelial cells. Mature adipocytes can amass excess energy sources with the help of glucose transporters and lipoprotein lipase enzymes in the form of triglycerides inside the cell without compromising their cellular function.Citation15
WAT has 3 main functions: 1) energy storage 2) triglyceride hydrolysis to free fatty acids for supporting tissue energy needs and 3) adipokine release.Citation16
WAT under positive energy balance conditions stores excess energy in the form of triglycerides whereas under negative energy balance conditions it provides energy for other organs by releasing free fatty acids. Fat storage function disorder of WAT leads to excess influx of fatty acid into the liver leading to steatosis. So, healthy adipose tissue is required to maintain lipid homeostasis throughout the body at the adipose tissue-liver axis.Citation17-19
Alcohol, adipose tissue, CYP2E1 and innate immunity
Ethanol consumption leads to the development of oxidative stress in a number of tissuesCitation20 together with adipose tissue. Activation of CYP2E1 mediated ethanol breakdown results in oxidative stress and endoplasmic reticulum (ER) stress leading to adipokine dysregulation and subsequent progression of ALD. Oxidative stress indicators like 4-hydroxynonenal are detected in adipose tissue after chronic ethanol feeding.Citation21,22 Increased ethanol-induced CYP2E1 expression is found to be critical to the development of adipose tissue inflammation via activating redox-sensitive transcription factors that cause increased ROS production. Alternatively, increased CYP2E1 expression and activity also leads to C1q dependent complement system activation and apoptosis mediated by Bid resulting in an indirect mechanism of CYP2E1 mediated inflammatory responses.Citation23 Also levels of inflammatory cytokine, macrophage migration inhibitory factor (MIF) are found to be increased in alcoholic hepatitis and cirrhosis patients. Although the exact mechanism of MIF increase is not known it is known that CYP2E1 activation is partially responsible for the increased MIF expression.Citation24-26 A summary of the effects of alcohol consumption on adipose tissue mass and inflammatory mediators is presented in and .
Table 1. The effect of alcohol consumption in rodents and humans with respect to adipokine secretion and adipose tissue mass
Endocrine Function of Adipose Tissue
Knowledge about the endocrine function of adipose tissue has emerged after the discovery of leptin expression and production by adipocytes.Citation27 Studies following leptin discovery have shown that adipose tissue secretes a wide variety of peptide molecules (both hormones and cytokines) known as adipokines or adipocytokines. Adipokines exert their activity on metabolism, immune function, as well as neuroendocrine pathways regulating feeding behaviors. All the cell types contribute to the secretory function of WAT. Both physiological environment and adipose tissue expansion status regulate adipokine production Citation27,28
The extent of metabolic activity of adipose tissue fat pads varies with the location and certain fat depots secrete specific adipokines more actively than others. Non-adipose tissues also secrete certain adipokines. For example, resistin is found in mouse brain and pituitary,Citation29 and in humans resistin mRNA is also observed in placentaCitation30 and monocytes.Citation31,32
Effect of alcohol on WAT
Alcohol consumption causes alcoholic fatty liver disease. In human and animal models, alcohol intake causes susceptibility toward non-alcoholic fatty liver disease.Citation33 Studies in both humans and animals have shown that alcohol consumption affects both adipose tissue mass and adipokine secretion. Insulin resistance, increased macrophage infiltration, inflammatory cytokine,Citation34 reduced ability of insulin to stimulate glucose uptake and inhibit lipolysis leading to hepatic steatosisCitation21,4 are observed after chronic ethanol feeding.
Studies on rodents
Reduction in adipose tissue massCitation21,35 and increase in fatty acid uptake by hepatocytes is observed in alcohol exposed rodents.Citation36,37 Ethanol consumption in rats apart from decreasing glucose uptake in rat adipocytes,Citation38 it also increases the rate of degradation of triglycerides in adipose tissue thus resulting in increased free fatty acid circulation.Citation21 Studies on C57BL/6N mice showed that lipid homeostasis at the adipose tissue-liver axis is disturbed on chronic ethanol consumption and this demonstrates that WAT lipolysis is stimulated on alcohol consumption resulting in an excess fatty acid release that get transported to the liver and become deposited as triglycerides.Citation39 Anatomically, VAT is connected to hepatic system via the mesenteric and portal veins. Thus chronic alcohol consumption may cause simultaneous accumulation of free fatty acid in liver cells and mesenteric fat tissues. Adipocyte size is also found to be reduced in chronic alcohol exposed mice.Citation40
Adipose triglyeride lipase and hormone sensitive lipase is found to be activated in association with a significant increase in the release of fatty acid from adipose tissue explants in mice fed with alcohol. Lipolysis in WAT is primarily due to hormone sensitive lipase (HSL) enzyme.Citation41 Up on lipolytic hormones stimulation, HSL is activated via phosphorylation by cAMP/PKA pathway.Citation42 The enzyme is inactivated by protein phosphatase 2A (PP2A) mediated dephosphorylation.Citation43 Methyaltion of PP2A by leucine carboxyl methyltransferase 1 (LCMT1) enhances its activity.Citation44
Studies on mice chronically fed with alcohol containing diet showed hypomethyaltion of PP2A enzyme as evident by reduced SAM/SAH ratio resulting in uninhibited HSL activation leading to increased lipolysis and release of free fatty acids from adipose tissue.Citation45
Alcohol exposure up regulates fatty acid transport proteins, causes accumulation of lipids in the liver, induces insulin intolerance, inactivates adipose protein phosphatase 1, and also upregulates phosphatase and tensin homolog (PTEN) and suppressor of cytokine signaling 3(SOCS3) proteins in mice. WAT dysfunction can directly impact lipid homeostasis of liver by reverse triglyceride transport. Hyperlipolysis is found to be the major functional defect of WAT after chronic ethanol exposure. Studies suggest that alcohol induced adipose tissue hyperlipolysis and insulin resistance are due to inactivation of adipose protein phosphatase 1 and upregulation of negative regulators of insulin signaling like PTEN and SOCS3 there by dysregulating or inhibiting insulin signal transduction pathway. Study by Zhong et al showed a significant loss in WAT in alcohol fed mouse.Citation40
Studies on alcoholics
Clinical studies in alcoholics have shown an association of lower fat mass with higher liver fat.Citation46,47 Lower body mass index (BMI) and fat mass (FM) and higher hepatic fat levels are observed in alcoholic patients.Citation46-48 Alcohol intake enhances cortisol secretion that results in change of fat distribution pattern, together with an increase in abdominal and hepatic fat depositionCitation49 and SAT lipolysis.Citation50 Studies show that alcohol consumption is associated with decreased accumulation of SAT and increased accumulation of VAT. Impaired function of adipocytes or surpass in the storage capacity of SAT results in accumulation of fat outside SAT thus resulting in buildup of VAT.Citation51 VAT accumulation results in glucose intolerance, onset of diabetes, and an increase in apolipoproteins.Citation52-54 The Framingham Offspring Study reported that intake of large amounts of alcohol is associated with decrease of SAT in women and increase of VAT in men.Citation55
Consumption of alcohol more than 14 standard drinks is shown to be related to an increased risk of metabolic syndrome. Further no protective effect on adipose tissue accumulation is shown with low to moderate alcohol intake. Alcohol energy content of 7.1g/kcal is relatively highCitation56 and it increases appetite and promotes energy intake.Citation57 A study on the dietary intake of energy from food and alcohol in Koreans showed that with higher consumption of alcohol there was an increase in total energy intake and further it was observed that there was an increase in VAT accumulation with either decrease or no change in SAT accumulation.Citation58
Alcohol and adipokines
Chronic consumption of alcohol disrupts gut permeability leading to the diffusion of bacterial endotoxin (LPS) into the portal blood circulation.Citation59-61 This result in TLR4 signaling activation mediated via MyD88 dependent and independent pathways resulting in an increased production of inflammatory cytokines.Citation62,63
Alcohol consumption is known to disrupt adipokine release from adipose tissue,Citation11,64 and promote infiltration of macrophages and this leads to adipose tissue phenotype alteration accredited to alcohol metabolism induced oxidative stress.Citation65 Qin and colleagues study results provided evidence for an increased adipose tissue inflammation due to alcohol binging prior to burn injury.Citation66 These observations have important inferences as studies show that increases in pro-inflammatory cytokines not only alter the functional integrity of adipose tissue but also dysregulate metabolism of adipose tissue.Citation67
Studies have shown that consumption of alcohol at moderate levels improves human health, especially cardiovascular related morbidities.Citation68 In vitro and in vivo experimental data demonstrate that alcohol acts as anti-inflammatory agent in presence of stimuli like bacterial lipopolysaccharides that up regulate inflammatory cytokines expression.Citation69 However, in the absence of inflammatory stimuli alcohol acts as a pro-inflammatory agent and upregulates the production of inflammatory cytokines and enzymes like IL-6, TNF-α, iNOS, and COX-2.Citation70,71
Studies on rodents
Adiponectin and leptin are the key adipokines that modulate hepatic lipid homeostasis toward lowering liver lipid content. Reduced adipose tissue weight, serum leptin and adiponectin concentrations together with the development of fatty liver are observed in chronic alcohol exposed rodents.Citation21,35,36,40,72-78 Adiponectin signaling via adiponectin receptor activates adenosine monophosphate-activated protein kinase (AMPK) pathway that stimulates fatty acid oxidation and decreases hepatic lipid influx and de novo lipogenesis thereby regulating hepatic lipid content.Citation11,12,16,79 Chronic consumption of alcohol results in impaired metabolism of methionine leading to hyperhomocysteinemia and decreased ratio of SAM/SAH in adipose tissue. This contributes to alcohol induced reduction in adiponectin secretion from adipocytes.Citation75 Exogenous adiponectin administration or endogenous adiponectin production stimulation or Rosiglitazone, a PPAR-γ agonist that mainly targets adipocytes has been shown to attenuate alcohol induced fatty liver in miceCitation72,73,76,80,81 as adiponectin has anti-inflammatory effects that are mediated via a heme-oxygenase-1 dependent pathway that attenuates TLR4 signaling mediated by MyD88 dependent and independent pathways.Citation62,63 Leptin signaling via leptin receptor b activates AMPK and signal transducer Stat3 pathways.Citation16,79 Decreased serum leptin levels are observed on either chronic alcohol or acute alcohol consumption. Studies show that leptin deficiency is associated with WAT mass reduction and exogenous leptin administration in leptin deficient mice restored alcohol induced hypoleptinemia and hepatic steatosis.Citation82-85 Studies show that during hyperinsulinemic-euglycemic clamp subcutaneous, epididymal, and omental adipose tissue glucose uptake is decreased after chronic ethanol feeding. Studies on male Wistar rats showed macrophage infiltration in epididymal adipose tissue, alteration in mRNA expression levels of adipocytokines like TNFα, IL-6, MCP-1, adiponectin, and RBP4 on chronic ethanol feeding.Citation34
Intragastric feeding of male Wistar rats for 22 weeks showed a decrease in the production of cartonectin and adiponectin in VAT and an increase in the production of leptin, visfatin, chemerin,Citation86 and resistin in both sera and VAT in a dose dependent manner.Citation87
Concentration dependent up regulation of inflammatory genes IL-6 and TNF-α genes and inflammatory enzymes COX-2 and iNOS is observed in 3T3L1 pre-adipocytesCitation88 treated with ethanol.
Studies on alcoholic patients
Higher levels of adiponectin (Acrp30) and resistin are found in patients with ALD when compared to control subjects. The increase in levels of resistin is due to inflammation in alcoholic patients while the increase in adiponectin levels suggests a protective and anti-inflammatory role of adiponectin.Citation89 This may be explained by Behre's hypothesis which states that increased adiponectin blood levels have a protective role and also help the body to adapt during fasting. In ALD progression malnutrition is frequently observed and this might be the reason for increased plasma levels of Acrp30 in alcoholic patients when compared to controls.Citation90
Studies show that moderate alcohol consumption increased adiponectin and grehlin levels while acylation-stimulating protein concentrations are decreased and these concentrations improve insulin sensitivity.Citation91 Recent data shows that high levels of TNFα in the SAT are observed in patients with alcoholic hepatitis and a positive correlation is observed between the liver IL6 and liver histological lesions and SAT TNFα levels.Citation92 Osteopontin expression levels from adipose tissue also correlate with fibrosis of liver in alcohol patients.Citation93 These data suggest the existence of similar pro-inflammatory mechanisms in adipose tissue and liver. Alcohol consumption leads to pro inflammatory cytokine expression from SAT. One week after alcohol withdrawal adipose tissue macrophages polarize into a M2 phenotype and alleviation in SAT inflammation is observed in patients with ALD.Citation94
Serum concentrations of leptin, an adipokine with pro-inflammatory and pro-fibrotic properties, are high in alcoholic patients irrespective of the presence or absence of cirrhosis.Citation64
Studies on patients with ALD established a correlation between TNF-α and IL-10 produced by adipose tissue with histological lesions in the liver and this suggests a relationship between adipose tissue inflammatory process and ALD progression in the liver.Citation92
Studies on alcoholic patients also established a correlation between the osteopontin expression in adipose tissue and hepatic fibrosis emphasizing the concept of adipose tissue inflammation in ALD.Citation93
In vitro studies on human adipose tissue fragments showed a significant anti-inflammatory effect of ethanol in a dose and time dependent manner.Citation95
Conclusion
Alcohol induced effects on tissue are multifactorial and involves crosstalk among various organs and tissues. Studies show that adipose tissue plays a major role in alcohol induced liver disease progression. These effects of ethanol on the adipose tissue innate immunity and metabolic activities lead to ethanol induced tissue injuryCitation96 and they also influence alcohol consumption regulating behaviors.Citation97,98 Research till date suggests that progression of ALD can be reduced by improving the function of adipose tissue. Though much progress has been made in understanding the role of adipose tissue in alcohol related tissue injury, the exact underlying molecular mechanisms linking adipose tissue damage and liver disease progression due to alcohol consumption still needs to be elucidated. As studies show that adipokines play a key role in alcoholic liver disease progression, effective drug leads targeting adipokine signaling pathways need to be developed and tested for ethanol induced liver disease amelioration.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Funding
This work was supported by grants from DST (Department of Science and Technology), CSIR (Council of Scientific and Industrial Research) and by UGC (University Grant Commission) for providing fellowship to Venkata Harini Kema.
References
- Lim SS, Vos T, Flaxman AD, Danaei G, Shibuya K, Adair-Rohani H. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012; 380:2224-60.
- Global status report on alcohol and health 2014; WHO Report.
- Shield KD, Parry C, Rehm J. Chronic diseases and conditions related to alcohol use. Alcohol Res Curr Rev 2013; 35:155-71; PMID:24881324
- Chen X, Sebastian BM, Tang H, McMullen MM, Axhemi A, Jacobsen DW, Nagy LE Taurine supplementation prevents ethanol-induced decrease in serum adiponectin and reduces hepatic steatosis in rats. Hepatology 2009; 49(5):1554-62; PMID:19296466; http://dx.doi.org/10.1002/hep.22811
- Mizoue T, Inoue M, Wakai K, Nagata C, Shimazu T, Tsuji I, Otani T, Tanaka K, Matsuo K, Tamakoshi A, et al. Alcohol drinking and colorectal cancer in Japanese: a pooled analysis of results from five cohort studies. Am J Epidemiol 2008; 167:1397-406; PMID:18420544; http://dx.doi.org/10.1093/aje/kwn073
- Yokoyama T, Yokoyama A, Kato H, Tsujinaka T, Muto M, Omori T, Haneda T, Kumagai Y, Igaki H, Yokoyama M, et al. Alcohol flushing, alcohol and aldehyde dehydrogenase genotypes, and risk for esophageal squamous cell carcinoma in Japanese men. Cancer Epidemiol Biomarkers Prev 2003; 12:1227-33; PMID:14652286
- Brooks PJ, Enoch MA, Goldman D, Li TK, Yokoyama A. The alcohol flushing response: an unrecognized risk factor for esophageal cancer from alcohol consumption. PLoS Med 2009; 6: e50; PMID:19320537
- Hall P. Pathological spectrum of alcoholic liver disease. Alcoholic Liver Disease 1995; 2:41-88.
- Lakshman MR. Some novel insights into the pathogenesis of alcoholic steatosis. Alcohol 2004; 34:45-8; PMID:15670665; http://dx.doi.org/10.1016/j.alcohol.2004.08.004
- Gao B, Bataller R. Alcoholic liver disease: pathogenesis and new therapeutic targets. Gastroenterology 2011; 141:1572-85; PMID:21920463; http://dx.doi.org/10.1053/j.gastro.2011.09.002
- Rogers CQ, Ajmo JM, You M. Adiponectin and alcoholic fatty liver disease. IUBMB Life 2008; 60:790-7; PMID:18709650; http://dx.doi.org/10.1002/iub.124
- You M, Rogers CQ. Adiponectin: a key adipokine in alcoholic fatty liver. Exp Biol Med 2009; 234:850-9; PMID:19491377; http://dx.doi.org/10.3181/0902-MR-61
- Saely CH, Geiger K, Drexel H. Brown versus white adipose tissue: a mini-review. Gerontology 2012; 58:15-23; PMID:21135534; http://dx.doi.org/10.1159/000321319
- Adamczak M, Wiecek A. The Adipose Tissue as an Endocrine Organ. Semin Nephrol 2013; 33(1):2-13; PMID:23374889; http://dx.doi.org/10.1016/j.semnephrol.2012.12.008
- Abel ED, Peroni O, Kim JK, Kim YB, Boss O, Hadro E, Minnemann T, Shulman GI, Kahn BB. Adipose-selective targeting of the GLUT4 gene impairs insulin action in muscle and liver. Nature 2001; 409(6821):729-33; PMID:11217863; http://dx.doi.org/10.1038/35055575
- Marra F, Bertolani C. Adipokines in liver disease. Hepatology 2009; 50:957-69; PMID:19585655; http://dx.doi.org/10.1002/hep.23046
- Lafontan M, Girard J. Impact of visceral adipose tissue on liver metabolism. part I: heterogeneity of adipose tissue and functional properties of visceral adipose tissue. Diabetes Metab 2008; 34:317-27; PMID:18550411; http://dx.doi.org/10.1016/j.diabet.2008.04.001
- Cusi K. The role of adipose tissue and lipotoxicity in the pathogenesis of type 2 diabetes. Curr Diab Rep 2010; 10:306-15; PMID:20556549; http://dx.doi.org/10.1007/s11892-010-0122-6
- Wree A, Kahraman A, Gerken G, Canbay A. Obesity affects the liver: the link between adipocytes and hepatocytes. Digestion 2011; 83:124-33; PMID:21042023; http://dx.doi.org/10.1159/000318741
- Lu Y, Cederbaum AI. CYP2E1 and Oxidative Liver Injury by Alcohol. Free Radic. Biol. Med. 2008; 44(5):723-38; PMID:18078827; http://dx.doi.org/10.1016/j.freeradbiomed.2007.11.004
- Kang L, Chen X, Sebastian BM, Pratt BT, Bederman IR, Alexander JC, Previs SF, Nagy LE. Chronic ethanol and triglyceride turnover in white adipose tissue in rats: inhibition of the anti-lipolytic action of insulin after chronic ethanol contributes to increased triglyceride degradation. J Biol Chem 2007; 282:28465-73; PMID:17686776; http://dx.doi.org/10.1074/jbc.M705503200
- Chen X, Sebastian BM, Nagy LE. Chronic ethanol feeding to rats decreases adiponectin secretion by subcutaneous adipocytes. Am J Physiol Endocrinol Metab 2007; 292: E621-E628; PMID:17047161; http://dx.doi.org/10.1152/ajpendo.00387.2006
- Sebastian BM, Roychowdhury S, Tang H, Hillian AD, Feldstein AE, Stahl GL, Takahashi K, Nagy LE. Identification of a cytochrome P4502E1/Bid/C1q-dependent axis mediating inflammation in adipose tissue after chronic ethanol feeding to mice. J Biol Chem. 2011; 286(41):35989-97; PMID:21856753; http://dx.doi.org/10.1074/jbc.M111.254201
- Barnes MA, Roychowdhury S, Nagy LE. Innate immunity and cell death in alcoholic liver disease: Role of cytochrome P4502E1. Redox Biology 2014; 2:929-35; PMID:25180169; http://dx.doi.org/10.1016/j.redox.2014.07.007
- Kumagi T, Akbar F, Horiike N, Onji M. Increases serum levels of macrophage migration inhibitory factor in alcoholic liver diseases and their expression in liver tissues. Clinical Biochemistry 2001; 34:189-93; PMID:11408016; http://dx.doi.org/10.1016/S0009-9120(01)00214-4
- Barnes MA, McMullen MR, Roychowdhury S, Pisano SG, Liu X, Stavitsky AB, Bucala R, Nagy LE. Macrophage migration inhibitory factor contributes to ethanol-induced liver injury by mediating cell injury, steatohepatitis, and steatosis. Hepatology 2013; 57:1980-91; PMID:23174952; http://dx.doi.org/10.1002/hep.26169
- Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM. Positional cloning of the mouse obese gene and its human homologue. Nature 1994; 372(6505):425-32; PMID:7984236; http://dx.doi.org/10.1038/372425a0
- Kang K. White adipose tissue as an endocrine organ. MA Shiffman (Ed.), Autologous Fat Transfer, Springer-Verlag Berlin Heidelberg; 2010:37-40.
- Morash B, Li A, Murphy PR, Wilkinson M, Ur E (1999). Leptin gene expression in the brain and pituitary glands. Endocrinology, 140, 5995-8; PMID:10579368; http://dx.doi.org/10.1210/endo.140.12.7288
- Yura S, Sagawa N, Itoh H, Kakui K, Nuamah MA, Korita D, Takemura M, Fujii S. Resistin is expressed in the human placenta. J Clin Endocrinol Metab 2003; 88:1394-7; PMID:12629135; http://dx.doi.org/10.1210/jc.2002-011926
- Nagaev I, Smith U. Insulin resistance and type 2 diabetes are not related to resistin expression in human fat cells or skeletal muscle. Biochem Biophys Res Commun 2001; 285:561-4; PMID:11444881; http://dx.doi.org/10.1006/bbrc.2001.5173
- Savage DB, Sewter CP, Klenk ES, Segal DG, Vidal-Puig A, Considine RV, O'Rahilly S. Resistin/FIZZ3 expression in relation to obesity and peroxisome-proliferator activated receptor-gamma action in humans. Diabetes 2001; 50:2199-02; PMID:11574398; http://dx.doi.org/10.2337/diabetes.50.10.2199
- Baker SS, Baker RD, Liu W, Nowak NJ, Zhu L. Role of alcohol metabolism in non-alcoholic steatohepatitis. PLoS One 2010; 5: e9570; PMID:20221393; http://dx.doi.org/10.1371/journal.pone.0009570
- Kang L Sebastian BM Pritchard MT Pratt BT Previs SF Nagy LE. Chronic ethanol-induced insulin resistance is associated with macrophage infiltration into adipose tissue and altered expression of adipocytokines. Alcohol Clin Exp Res 2007; 31(9):1581-8; PMID:17624994; http://dx.doi.org/10.1111/j.1530-0277.2007.00452.x
- Kang X, Zhong W, Liu J, Song Z, McClain CJ, Kang YJ, Zhou Z. Zinc supplementation reverses alcohol-induced steatosis in mice through reactivating hepatocyte nuclear factor-4alpha and peroxisome proliferator-activated receptor-alpha. Hepatology 2009; 50:1241-50; PMID:19637192; http://dx.doi.org/10.1002/hep.23090
- Zhou SL, Gordon RE, Bradbury M, Stump D, Kiang CL, Berk PD. Ethanol up-regulates fatty acid uptake and plasma membrane expression and export of mitochondrial aspartate aminotransferase in HepG2 cells. Hepatology 1998; 27:1064-74; PMID:9537447; http://dx.doi.org/10.1002/hep.510270423
- Berk PD, Zhou S, Bradbury MW (2005). Increased hepatocellular uptake of long chain fatty acids occurs by different mechanisms in fatty livers due to obesity or excess ethanol use, contributing to development of steatohepatitis in both settings. Trans Am Clin Climatol Assoc 2005; 116:335-44; PMID:16555625
- Rachdaoui N, Sebastian BM, Nagy LE. Chronic ethanol feeding impairs endothelin-1-stimulated glucose uptake via decreased G alpha 11 expression in rat adipocytes. Am J Physiol-Endocr M. 2003; 285: E303-10
- Wei X, Shi X, Zhong W, Zhao Y, Tang Y, Sun W, Yin X, Bogdanov B, Kim S, McClain C, et al. Chronic alcohol exposure disturbs lipid homeostasis at the adipose tissue-liver axis in mice: analysis of triacylglycerols using high-resolution mass spectrometry in combination with In vivo metabolite deuterium labeling. PLoS ONE 2013; 8(2):1-10
- Zhong W, Zhao Y, Tang Y, Wei X, Shi X, Sun W, Sun X, Yin X, Sun X, Kim S, et al. Chronic alcohol exposure stimulates adipose tissue lipolysis in mice: role of reverse triglyceride transport in the pathogenesis of alcoholic steatosis. Am J Pathol 2012; 180:998-1007; PMID:22234172; http://dx.doi.org/10.1016/j.ajpath.2011.11.017
- Schweiger M, Schreiber R, Haemmerle G, Lass A, Fledelius C, Jacobsen P, Tornqvist H, Zechner R, Zimmermann R. Adipose triglyceride lipase and hormone-sensitive lipase are the major enzymes in adipose tissue triacylglycerol catabolism. J Biol Chem 2006; 281:40236-41; PMID:17074755; http://dx.doi.org/10.1074/jbc.M608048200
- Anthonsen MW, Rönnstrand L, Wernstedt C, Degerman E, Holm C. Identification of novel phosphorylation sites in hormone-sensitive lipase that are phosphorylated in response to isoproterenol and govern activation properties in vitro. J Biol Chem 1998; 273:215-21; PMID:9417067; http://dx.doi.org/10.1074/jbc.273.1.215
- Wood SL, Emmison N, Borthwick AC, Yeaman SJ. The protein phosphatases responsible for dephosphorylation of hormone-sensitive lipase in isolated rat adipocytes. Biochem J 1993; 295:531-5; PMID:8240253
- Janssens V, Goris J. Protein phosphatase 2A: a highly regulated family of serine/threonine phosphatases implicated in cell growth and signalling. Biochem J 2001; 353:417-39; PMID:11171037; http://dx.doi.org/10.1042/0264-6021:3530417
- Dou X, Xia Y, Chen J, Qian Y, Li S, Zhang X, Song Z. Rectification of impaired adipose tissue methylation status and lipolytic response contributes to hepatoprotective effect of betaine in a mouse model of alcoholic liver disease. Br J Pharmacol 2014; 171(17):4073-86; PMID:24819676; http://dx.doi.org/10.1111/bph.12765
- Addolorato G, Capristo E, Greco AV, Stefanini GF, Gasbarrini G. Energy expenditure, substrate oxidation, and body composition in subjects with chronic alcoholism: new findings from metabolic assessment. Alcohol Clin Exp Res 1997; 21:962-7; PMID:9309302
- Addolorato G, Capristo E, Greco AV, Stefanini GF, Gasbarrini G. Influence of chronic alcohol abuse on body weight and energy metabolism: is excess ethanol consumption a risk factor for obesity or malnutrition? J Intern Med 1998; 244:387-95; PMID:9845854; http://dx.doi.org/10.1046/j.1365-2796.1998.00381.x
- Addolorato G, Capristo E, Marini M, Santini P, Scognamiglio U, Attilia ML, Messineo D, Sasso GF, Gasbarrini G, Ceccanti M. Body composition changes induced by chronic ethanol abuse: evaluation by dual energy X-ray absorptiometry. Am J Gastroenterol 2000; 95:2323-7; PMID:11007236; http://dx.doi.org/10.1111/j.1572-0241.2000.02320.x
- Leggio L, Malandrino N, Ferrulli A, Cardone S, Miceli A, Gasbarrini G, Capristo E, Addolorato G. Is cortisol involved in the alcohol-related fat mass impairment? A longitudinal clinical study. Alcohol Alcohol 2009; 44:211-5; PMID:19144979; http://dx.doi.org/10.1093/alcalc/agn116
- Krsek M, Ruziicka M, Nedvidkova J, Kvasnickova H, Hana V, Marek J, Haluzik M, Lai EW, Pacak K. Increased lipolysis of subcutaneous abdominal adipose tissue and altered noradrenergic activity in patients with Cushing's syndrome: an in-vivo microdialysis study. Physiol Res 2006; 55:421-8; PMID:16238457
- Unger RH. The physiology of cellular liporegulation. Annu Rev Physiol 2003; 65:333-47; PMID:12471167; http://dx.doi.org/10.1146/annurev.physiol.65.092101.142622
- Fox CS, Massaro JM, Hoffmann U, Pou KM, Maurovich-Horvat P, Liu CY, Vasan RS, Murabito JM, Meigs JB, Cupples LA, et al. Abdominal visceral and subcutaneous adipose tissue compartments: association with metabolic risk factors in the Framingham Heart Study. Circulation 2007; 116:39-48; PMID:17576866; http://dx.doi.org/10.1161/CIRCULATIONAHA.106.675355
- Wajchenberg BL. Subcutaneous and visceral adipose tissue: their relation to the metabolic syndrome. Endocr Rev 2000; 21:697-738; PMID:11133069; http://dx.doi.org/10.1210/edrv.21.6.0415
- Matsushita Y, Nakagawa T, Yamamoto S, Takahashi Y, Yokoyama T, Noda M, Mizoue T. Associations of visceral and subcutaneous fat areas with the prevalence of metabolic risk factor clustering in 6,292 Japanese individuals: the Hitachi Health Study. Diabetes Care 2010; 33:2117-9; PMID:20460443; http://dx.doi.org/10.2337/dc10-0120
- Molenaar EA, Massaro JM, Jacques PF, Pou KM, Ellison RC, Hoffmann U, Pencina K, Shadwick SD, Vasan RS, et al. Association of lifestyle factors with abdominal subcutaneous and visceral adiposity: The Framingham Heart Study. Diabetes Care 2009; 32:505-10; PMID:19074991; http://dx.doi.org/10.2337/dc08-1382
- Suter PM, Hasler E, Vetter W. Effects of alcohol on energy metabolism and body weight regulation: is alcohol a risk factor for obesity? Nutr Rev 1997; 55:157-71; PMID:9212692; http://dx.doi.org/10.1111/j.1753-4887.1997.tb06470.x
- Westerterp-Plantenga MS, Verwegen CR. The appetizing effect of an aperitif in over-weight and normal-weight humans. Am J Clin Nutr 1999; 69:205-12; PMID:9989681
- Kim KH, Oh SW, Kwon H, Park JH, Choi H, Cho B. Alcohol Consumption and Its Relation to Visceral and Subcutaneous Adipose Tissues in Healthy Male Koreans. Ann Nutr Metab 2012; 60:52-61; PMID:22327000
- Mathurin P, Deng QG, Keshavarzian A, Choudhary S, Holmes E.W, Tsukamoto. Exacerbation of alcoholic liver by enteral endotoxin in rat. Hepatology 2000; 32:1008-17; PMID:11050051; http://dx.doi.org/10.1053/jhep.2000.19621
- Tamai H, Kato S, Horie Y, Ohki E, Yokoyama H, Ishii H. Effect of ethanol administration on the intestinal absorption of endotoxin in rat. Alcohol.Clin. Exp.Res 2000; 24:390-4; PMID:10776683; http://dx.doi.org/10.1111/j.1530-0277.2000.tb04629.x
- Jarvelainen HA, Fang C, Ingelmann-Sundberg M, Lindros KO. Effect of chronic coadministration of endotoxin and ethanol onrat liver pathology and pro-inflammatory and anti-inflammatory cytokines. Hepatology 1999; 29:1503-10; PMID:10216135; http://dx.doi.org/10.1002/hep.510290508
- Mandal P, Park PH, McMullen MR, Pratt BT, Nagy LE. The anti-inflammatory effects of adiponectin are mediated via a heme oxygenase-1-dependent pathway in rat Kupffer cells. Hepatology 2010; 51(4):1420-9; PMID:20052772; http://dx.doi.org/10.1002/hep.23427
- Mandal P, Pritchard MT, Nagy LE. Anti-inflammatory pathways and alcoholic liver disease: Role of an adiponectin/interleukin-10/heme oxygenase-1 pathway. World J Gastroenterol 2010; 16(11):1330-6; PMID:20238399; http://dx.doi.org/10.3748/wjg.v16.i11.1330
- Nicolas JM, Fernandez-Sola J, Fatjo F, Casamitjana R, Bataller R, Sacanella E, Tobıas E, Badıa E, Estruch R. Increased circulating leptin levels in chronic alcoholism. Alcohol Clin Exp Res 2001; 25:83-88; PMID:11198718; http://dx.doi.org/10.1111/j.1530-0277.2001.tb02130.x
- Tang H, Sebastian BM, Axhemi A, Chen X, Hillian AD, Jacobsen DW, Nagy LE. Ethanol-induced oxidative stress via the CYP2E1 pathway disrupts adiponectin secretion from adipocytes. Alcohol Clin Exp Res 2012; 36:214-22; PMID:21895711; http://dx.doi.org/10.1111/j.1530-0277.2011.01607.x
- Qin Y, Hamilton JL, Bird MD, Chen MM, Ramirez L, Zahs A, Kovacs EJ, Makowski L. Adipose inflammation and macrophage infiltration after binge ethanol and burn injury. Alcohol Clin Exp Res 2013; 38:204-13; PMID:23909743; http://dx.doi.org/10.1111/acer.12210
- Hajer GR, van Haeften TW, Visseren FL. Adipose tissue dysfunction in obesity, diabetes, and vascular diseases. Eur Heart J 2008; 29:2959-71; PMID:18775919; http://dx.doi.org/10.1093/eurheartj/ehn387
- Klatsky A.L. Udaltsova N. Alcohol drinking and total mortality risk. Ann. Epidemiol 2007; 17(5): S63-S67; http://dx.doi.org/10.1016/j.annepidem.2007.01.014
- Syapin PJ, Militante JD, Garrett DK, Ren L. Cytokine-induced iNOS expression in C6 glial cells: transcriptional inhibition by ethanol. J Pharmacol Exp Ther 2001; 298(2):744-52; PMID:11454939
- Nanji A, Miao A.L, Thomas P, Rahemtulla A, Khwaja S, Zhao S, Peters D, Tahan SR, Dannenberg AJ. Enhanced cyclooxygenase-2 g ene expression in alcoholic liver disease in the rat. Gastroenterology 1997; 112(3):943-51; PMID:9041257; http://dx.doi.org/10.1053/gast.1997.v112.pm9041257
- Yuan G J, Zhou X R, Gong Z J, Zhang P, Sun X M, Zheng S H. Expression and activity of inducible nitric oxide synthase and endothelial nitric oxide synthase correlate with ethanol-induced liver injury. World J. Gastroenterol 2006; 12(15):2375-81; PMID:16688828D
- Xu A, Wang Y, Keshaw H, Xu LY, Lam KS, Cooper GJ. The fat-derived hormone adiponectin alleviates alcoholic and nonalcoholic fatty liver diseases in mice. J Clin Invest 2003; 112:91-100; PMID:12840063; http://dx.doi.org/10.1172/JCI200317797
- You M, Considine RV, Leone TC, Kelly DP, Crabb DW. Role of adiponectin in the protective action of dietary saturated fat against alcoholic fatty liver in mice. Hepatology 2005; 42:568-77; PMID:16108051; http://dx.doi.org/10.1002/hep.20821
- Esfandiari F, You M, Villanueva JA, Wong DH, French SW, Halsted CH. S-adenosylmethionine attenuates hepatic lipid synthesis in micropigs fed ethanol with a folate-deficient diet. Alcohol Clin Exp Res 2007; 31:1231-39; PMID:17577393; http://dx.doi.org/10.1111/j.1530-0277.2007.00407.x
- Song Z, Zhou Z, Deaciuc I, Chen T, McClain CJ. Inhibition of adiponectin production by homocysteine: potential mechanism for alcoholic liver disease. Hepatology 2008; 47:867-79; PMID:18167065; http://dx.doi.org/10.1002/hep.22074
- Shen Z, Liang X, Rogers CQ, Rideout D, You M. Involvement of adiponectin-SIRT1-AMPK signaling in the protective action of rosiglitazone against alcoholic fatty liver in mice. Am J Physiol Gastrointest Liver Physiol 2010; 298: G364-G374; PMID:20007851; http://dx.doi.org/10.1152/ajpgi.00456.2009
- Otaka M, Konishi N, Odashima M, Jin M, Wada I, Matsuhashi T, Ohba R, Watanabe S. Effect of alcohol consumption on leptin level in serum, adipose tissue, and gastric mucosa. Dig Dis Sci 2007; 52:3066-9; PMID:17406835; http://dx.doi.org/10.1007/s10620-006-9635-x
- Maddalozzo GF, Turner RT, Edwards CH, Howe KS, Widrick JJ, Rosen CJ, Iwaniec UT. Alcohol alters whole body composition, inhibits bone formation, and increases bone marrow adiposity in rats. Osteoporos Int 2009; 20:1529-38; PMID:19238309; http://dx.doi.org/10.1007/s00198-009-0836-y
- Galic S, Oakhill JS, Steinberg GR. Adipose tissue as an endocrine organ. Mol Cell Endocrinol 2010; 316:129-39; PMID:19723556; http://dx.doi.org/10.1016/j.mce.2009.08.018
- Ajmo JM, Liang X, Rogers CQ, Pennock B, You M. Resveratrol alleviates alcoholic fatty liver in mice. Am J Physiol Gastrointest Liver Physiol 2008; 295: G833-G842; PMID:18755807; http://dx.doi.org/10.1152/ajpgi.90358.2008
- Sun X, Tang Y, Tan X, Li Q, Zhong W, Sun X, Jia W, McClain CJ, Zhou Z. Activation of peroxisome proliferator-activated receptor-γ by rosiglitazone improves lipid homeostasis at the adipose tissue-liver axis in ethanol-fed mice. Am J Physiol Gastrointest Liver Physiol 2012; 302: G548-G557; PMID:22173916; http://dx.doi.org/10.1152/ajpgi.00342.2011
- Greco AV, Mingrone G, Favuzzi A, Capristo E, Gniuli D, Addolorato G, Brunani A, Cavagnin F, Gasbarrini G. Serum leptin levels in post-hepatitis liver cirrhosis. J Hepatol 2000; 33:38-42; PMID:10905584; http://dx.doi.org/10.1016/S0168-8278(00)80157-9
- Santolaria F, Pérez-Cejas A, Alemán MR, González-Reimers E, Milena A, de la Vega MJ, Martínez-Riera A, Gómez-Rodríguez MA. Low serum leptin levels and malnutrition in chronic alcohol misusers hospitalized by somatic complications. Alcohol Alcohol 2003; 38:60-6; PMID:12554610; http://dx.doi.org/10.1093/alcalc/agg015
- Calissendorff J, Brismar K, Röjdmark S. Is decreased leptin secretion after alcohol ingestion catecholamine-mediated? Alcohol Alcohol 2004; 39:281-6; PMID:15208157; http://dx.doi.org/10.1093/alcalc/agh054
- Kalousová M, Zima T, Popov P, Spacek P, Braun M, Soukupová J, Pelinkova K, Kientsch-Engel R. Advanced glycation end-products in patients with chronic alcohol misuse. Alcohol Alcohol 2004; 39:316-20; PMID:15208163; http://dx.doi.org/10.1093/alcalc/agh058
- Ren RZ, Zhang X, Xu J, Zhang HG, Yu CX, Cao MF, Gao L, Guan QB, Zhao JJ. Chronic ethanol consumption increases the levels of chemerin in the serum and adipose tissue of humans and rats. Acta Pharmacologica Sinica 2012; 33:652-9; PMID:22447224; http://dx.doi.org/10.1038/aps.2012.11
- Yu HC, Li SY, Cao MF, Jiang XY, Feng F, Zhao JJ, Gao L. Effects of chronic ethanol consumption on levels of adipokines in visceral adipose tissues and sera of rats. Acta Pharmacologica Sinica 2010; 31:461-9; PMID:20208553; http://dx.doi.org/10.1038/aps.2010.12
- Kiselova-Kaneva YO Tasinov D Vankova DI. Ethanol induces il-6 and tnf-α cytokine and inos and cox-2 enzyme gene expression in 3t3-l1 preadipocytes, Scripta Scientifica Medica 2012; 44 (2):31-5; http://dx.doi.org/10.14748/ssm.v44i2.354
- Kasztelan-Szczerbinska B, Surdacka A, Slomka M, Rolinski J, Celinski K, Smolen A, Szczerbinski M. Association of serum adiponectin, leptin, and resistin concentrations with the severity of liver dysfunction and the disease complications in alcoholic liver disease. Mediators Inflammat 2013; 2013; Article ID 148526:12; 24259947
- Behre CJ. Adiponectin: saving the starved and the overfed. Medical Hypotheses 2007; 69(6):1290-2; PMID:17509773; http://dx.doi.org/10.1016/j.mehy.2007.02.044]
- Beulens JWJ, de Zoete EC, Kok FJ, Schaafsma G, Hendriks HFJ. Effect of moderate alcohol consumption on adipokines and insulin sensitivity in lean and overweight men: a diet intervention study. Eur J Clin Nutrit 2008; 62:1098-05; PMID:17554246; http://dx.doi.org/10.1038/sj.ejcn.1602821
- Naveau S, Cassard-Doulcier AM, Njike-Nakseu M, 20399524 Harmful effect of adipose tissue on liver lesions in patients with alcoholic liver disease. J Hepatol 2010; 52:895-902; PMID:20399524; http://dx.doi.org/10.1016/j.jhep.2010.01.029
- Patouraux S, Bonnafous S, Voican CS, Anty R, Saint-Paul MC, Rosenthal-Allieri MA, Agostini H, Njike M, Barri-Ova N, Naveau S, et al. The osteopontin level in liver, adipose tissue and serum is correlated with fibrosis in patients with alcoholic liver disease. PLoS One 2012; 7: e35612; PMID:22530059; http://dx.doi.org/10.1371/journal.pone.0035612
- Voican CS, Njiké-Nakseu M, Boujedidi H, Barri-Ova N, Bouchet-Delbos L, Agostini H, Maitre S, Prévot A, Cassard-Doulcier AM, Naveau S, et al. Alcohol withdrawal alleviates adipose tissue inflammation in patients with alcoholic liver disease. Liver Int 2014; PMID:24766056; http://dx.doi.org/10.1111/liv.12575
- Wandler A, Bruun JM, Nielsen MP, Richelsen B. Ethanol exerts anti-inflammatory effects in human adipose tissue in vitro. Mol Cell Endocrinol 2008; 296:26-31; PMID:18840498; http://dx.doi.org/10.1016/j.mce.2008.09.006
- Pravdova E, Fickova M Alcohol intake modulates hormonal activity of adipose tissue. Endocr Regul 2006; 40(3):91-104; PMID:17100551
- Wurst FM, Rasmussen DD, Hillemacher T, Kraus T, Ramskogler K, Lesch O, Bayerlein K, Schanze A, Wilhelm J, Junghanns K, et al Alcoholism, craving, and hormones: the role of leptin, ghrelin, prolactin, and the pro-opiomelanocortin system in modulating ethanol intake. Alcohol Clin Exp Res 2007; 31(12):1963-7; PMID:18034691; http://dx.doi.org/10.1111/j.1530-0277.2007.00531.x
- Hillemacher T., Weinland C., Heberlein A., Gröschl M., Schanze A., Frieling H., Wilhelm J., Kornhuber J., Bleich S. Increased levels of adiponectin and resistin in alcohol dependence–possible link to craving. Drug Alcohol Depend 2009; 99(1-3):s 333-33; PMID:18818026; http://dx.doi.org/10.1016/j.drugalcdep.2008.07.019