Abstract
Obesity and diabetes are major health concerns worldwide. Western diets, often calorically rich, paired with sedentary habits are driving the current worldwide epidemic of pediatric and adult obesity. In addition, age related energy imbalances lead to increased adiposity and metabolic disorders later in life, making the middle aged population particularly susceptible. Here we discuss how Forkhead box A3 (Foxa3), a family member of the forkhead box binding proteins, can potentially contribute to pathology by playing a double role in metabolism. Recent data revealed that Foxa3 favors the selective expansion of visceral depots under high caloric conditions (e.g., high fat diet) and suppresses subcutaneous fat tissue energy expenditure during aging. This evidence suggests that Foxa3 acts to both preserve and conserve calories, by accumulating fat and by reducing metabolic burn. In other words, Foxa3 appears to function to enable energy “hoarding,” which may be critical for survival of organisms with intermittent exposure to external caloric sources, but pathologic in circumstances where calories are abundant. Understanding how this “calorie hoarder gene” functions may suggest approaches to combat obesity and associated metabolic disorders.
Obesity, Adipose Tissues and Aging
Obesity and type 2 diabetes represent a health problem globally. In the developed world, the raise in obesity-related illnesses, such as diabetes and cardiovascular disease, has been associated with the increased abundance and readily availability of food and sedentary life. Fat is the primary tissue involved in storing lipids and its excessive expansion leads to obesity. There are 3 types of fat cells -white, brown and beige-, which have been categorized on the basis of their distinct functions and anatomical locations.Citation1-3 White adipose tissues (WATs) have been classically thought to be relevant for energy storage and have now been widely recognized as active secretory and endocrine organs; furthermore, it has been recently shown that adipose tissues located in different areas of the body have distinct gene signatures and confer different propensity to diabetes.Citation4 Indeed, the expansion of the fat around internal organs in the visceral cavity, but not of that present subcutaneously, represents a major risk factor for the development of metabolic disorders.Citation5 Conversely, brown adipose tissue (BAT), located in the interscapular area in rodents, converts energy to heat through the activity of the uncoupling protein 1 (UCP1), a mitochondrial protein involved in uncoupling oxidative phosphorylation from ATP synthesis.Citation4 Brown adipocytes contain numerous lipid droplets of small diameter and a high number of mitochondria. Recent lineage tracing studies have described the existence of cells of smooth muscle-like origin, the beige adipocytes, as novel inducible thermogenic fat cells residing within the white fat tissue.Citation6,7 Beige cells normally express lower levels of UCP1, compared to brown adipocyes, however they are able to induce UCP1 in response to cyclic AMP stimulation, reaching respiration rates comparable to those achieved in stimulated classic brown fat.Citation7-9 Furthermore, beige cells possess a unique gene expression profile that distinguishes them from white and brown fat.Citation7 Similarly to rodents, both brown and beige fat cells are present in adult humans, in the supraclavicular areas,Citation10,11 and can efficiently contribute to energy expenditure.Citation10–13
Aging results from progressive changes in physical, psychological, and neurocognitive functions. A significant number of countries worldwide with improved standards of care are now facing an increasing population of individuals over 65 years of age.Citation14 In parallel with an extension in lifespan, it has been also observed an increase in the risk of developing the metabolic syndrome late in life, possibly due to decreased energy expenditure in part caused by age-dependent loss of brown and beige cells and increased food consumption.Citation15,16 Given the important role of brown and beige adipocytes in energy metabolism, more emphasis has been placed in the identification of molecular pathways that could be targeted to modify the activity of these tissues to ultimately control metabolic disorders.
Forkhead Factor Foxa3 Positively Regulates Adipocyte Differentiation
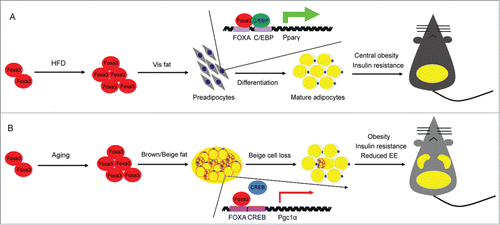
The forkhead box (Fox) proteins are a large family of transcriptional regulators that bind to DNA through a conserved ‘forkhead’ or ‘winged-helix’ DNA-binding domain. To date, 39 distinct fox factors have been identified and divided into 19 subgroups, defined as Fox -A to -S, based on their sequence homology in and around the forkhead domain.Citation17 Many of the forkhead factors have been previously shown to play a wide range of roles in development, organogenesis, cell growth, proliferation and differentiationCitation18,19; however, till 2013, only 3 Fox factors, Foxo1, Foxc2 and FoxA2, had been studied in the context of adipocyte differentiation and shown to suppress it, either directly or indirectly.Citation20-23 To more systematically assess the contribution of each and all individual Fox factors to this process, a few years ago our laboratory carried out a genetic screen in vitro using a siRNA library. Via this approach, Foxa3 was identified as a positive modulator of adipocyte differentiation. Through detailed mechanistic studies we also revealed that Foxa3 cooperates with C/EBPβ and δ to induce PPARγ at the mRNA level during the early phases of adipocyte differentiation (Fig. 1A).Citation24 Overall, these data have provided the first demonstration that members of the Forkhead family can also play an important positive role in fat differentiation.
Foxa3 Regulates Fuel Metabolism in vivo
Prior to our demonstration of the involvement of Foxa3 in adipocyte function in vitro, Foxa3 had been studied predominantly in the context of hepatic physiology.Citation25-27 Foxa3-null mice generated in the late nineties by Kaestner and Schuetz showed no metabolic abnormalities in normal feeding conditions,Citation26 however they developed hypoglycemia when fasted for 72 hours,Citation27 indicating a possible critical role of Foxa3 in glycemic control during metabolic stress. More recently Foxa3 has been shown to contribute to the differentiation of goblet cells and to be involved in the regulation of pulmonary innate immunity.Citation28 Given our in vitro demonstration of Foxa3 role in fat differentiation,Citation24 we assessed Foxa3's function in the physiology of adipose tissue. Our studies revealed that, during high fat diet regimens, Foxa3 is highly induced at the RNA level, selectively in visceral fat tissue and that mice with Foxa3 ablation are specifically protected from the development of visceral obesity. Furthermore, functional analysis of primary cells derived from adipose depots of WT and Foxa3 knock-out mice demonstrated that adipocytes obtained from Foxa3-null visceral fat express lower levels of PPARγ and of differentiation markers, compared to WT cells.
Given the known correlation between aging, increased adiposity and diminished energy expenditure, we assessed whether Foxa3's ablation in mice would also contribute to obesity late in life and demonstrated that mid-age mice lacking Foxa3 have increased browning of inguinal fat tissues, improved thermogenic function, enhanced energy expenditure and decreased adiposity, compared to aged matched WT mice.Citation29 Through molecular analysis involving chromatin immune-precipitation in cells and tissues and luciferase assays, we revealed that these effects are, at least in part, mediated by a direct interference of Foxa3 with the transcriptional regulation of PGC1α by the cAMP responsive element binding protein 1 (CREB; Fig. 1B). This mechanism of action through promoter binding competition appears to be common among Foxa family members, as it resembles that employed by Foxa1 and 2 to regulate IL6 levels.Citation30 A comprehensive identification of Foxa3 responsive elements in distinct fat depots and their relative proximity to cis regulatory sequences recognized by other transcription factors will reveal which target genes execute Foxa3's distinct functions in white, beige and brown fat tissue biology.
In addition to the mechanism of interference between Foxa3 and CREB described, it could also be envisioned that the elevated levels of PGC1α observed in Foxa3 knockout cells could result from the increased accessibility of other Fox factors to the forkhead responsive element in the PGC1α promoter leading to stabilization of CREB binding to the neighboring CRE element. This possibility is consistent with the evidence that diverse Fox factors could act promiscuously on the same promoter by virtue of their highly conserved forkhead box motifsCitation31 and drive opposing transcriptional events via differential recruitment of cofactors through unique protein-protein interaction domains outside of the DNA binding region.
Our analysis of aging Foxa3 knock-out mice has provided novel evidence of the beneficial effects of Foxa3's ablation on lifespan in normal dietary conditions. Given the previous report on the role of the C. elegans gene pha-4, homologous to the 3 mammalian Foxa family members, in diet-restriction-mediated longevity,Citation32 it will be of interest to assess the specific contribution of Foxa3, among the Foxa family members, to life span extension in calorie-restricted conditions and, ultimately, determine whether Foxa3 may exert differential effects on longevity depending on specific dietary regimens.
Our in vitro and in vivo studies have revealed for the first time that Foxa3 mRNA levels are highly induced by high fat diet, selectively only in visceral tissue, and during aging, specifically in brown and inguinal fat tissues. These results illustrate how Foxa3 mRNA may be switched on by nutritional and developmental factors in a fat depot-selective manner. The possibility that Foxa3 may be highly regulated at the expression level by select nutrients is consistent with previous reports demonstrating that Foxa3 mRNAs in liver tissues are sensitive to the amount of protein present in the diet.Citation33 In addition to the tightly controlled transcriptional regulation observed, it is plausible that Foxa3's function may also be regulated post-translationally, perhaps in response to insulin stimulation, as previously shown for other Foxa factors,Citation34,35 and epigenetically, through the modulation of differential cofactors recruitment and by affecting Foxa3's promoter occupancy choices.
Foxa3 is Involved in Calorie Preservation
Almost half a century ago the thrifty gene theory was proposed to explain the existence of genes that enabled efficient energy storage and conferred an advantage during the caloric uncertainty that characterized alternating periods of food availability and scarcity.Citation36,37 According to this hypothesis, the genes ensuring survival at times of “feast and famine” would become the main drivers of obese states in nutrient-rich environments. Although this theory has been criticized by some claiming the lack of evidence that extreme feast and famine characterized the lifestyle of Stone Age men,Citation38,39 the search for central regulatory nodes involved in efficient nutrient storage during metabolic stresses has continued. Based on our data demonstrating a role for Foxa3 in controlling energy accumulation selectively in visceral adipose tissue, we propose that, in addition to its thrifty activity in limiting energy expenditure, Foxa3 could also play a role as a “hoarder” gene. Such calorie hoarding function would enable Foxa3 to increase lipid accumulation only during times of food copiousness via increasing the activation of the PPARγ lipid storing machinery in selective fat depots.
Role of Foxa3 in Human Metabolic Disease
Given our demonstration of Foxa3 critical function in adipose tissue physiology in models of obesity, we recently assessed possible associations between metabolic traits and variants or mutations at the human Foxa3 locus in a cohort of children, adolescent and young adults between the age of 6 and 21.Citation40 This analysis has led to the identification of 14 novel Foxa3 DNA variants and statistical analysis has revealed that the common Foxa3 variant rs28666870 is associated with increased BMI and appendicular lean mass. Additionally, our in vitro functional studies have shown that 2 novel missense mutations, c.185C > T and c.731C > T, we identified in an overweight and an obese subject, respectively, conferred increased differentiation capacity to Foxa3. Overall these genetic data suggest a potential correlation between Foxa3 genotypes and metabolic phenotype in humans.Citation40
From data generated in our laboratory in the last 3 years, Foxa3 has emerged as a new critical player in adipose tissue biology and physiology in mouse and the genetic data we recently obtained in a cohort of children has suggested a potential role of this factor also in human metabolism.Citation24,28,40 Further analysis involving the detailed characterization of genes regulated by Foxa3 in fat tissues and the identification of novel modifiers of Foxa3 levels and function will shed further light into the molecular pathways utilized by Foxa3 to regulate efficient energy storage and preservation in different fat depots. Overall, the combination of the physiological and genetic evidence provided to date suggests that Foxa3 may represent a suitable target to combat obesity and associated metabolic disorders.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Figure 1. Model proposing the pleiotropic function of Foxa3 on energy conservation, during high fat diet and aging. (A) Foxa3 mRNA levels are selectively increased in visceral fat (Vis fat) in response to high fat diet (HFD) and associated with increased lipid accumulation in the visceral depot and the induction of PPARγ mRNA. (B) Foxa3 levels are primarily elevated in subcutaneous fat depots during the aging process. Schematic representation depicting the competition between Foxa3 and CREB for their binding to regulatory elements at the PGC1α promoter, resulting in the modulation of PGC1α mRNA levels. EE, energy expenditure.
Acknowledgments
We thank Pasha Sarraf for thoughtful discussions.
Funding
This research was supported by funds of the NIDDK Intramural Research Program of the National Institutes of Health.
References
- Farmer SR. Molecular determinants of brown adipocyte formation and function. Genes Dev 2008; 22:1269-75; PMID:18483216; http://dx.doi.org/10.1101/gad.1681308
- Mueller E. Understanding the variegation of fat: Novel regulators of adipocyte differentiation and fat tissue biology. Biochim Biophys Acta 2014; 1842:352-7; PMID:23735215; http://dx.doi.org/10.1016/j.bbadis.2013.05.031
- Boss O, Farmer SR. Recruitment of brown adipose tissue as a therapy for obesity-associated diseases. Front Endocrinol (Lausanne) 2012; 3:14; PMID:22654854
- Berry DC, Stenesen D, Zeve D, Graff JM. The developmental origins of adipose tissue. Development 2013; 140:3939-49; PMID:24046315; http://dx.doi.org/10.1242/dev.080549
- Matsuzawa Y, Funahashi T, Nakamura T. The concept of metabolic syndrome: contribution of visceral fat accumulation and its molecular mechanism. J Atheroscler Thromb. 2011; 18:629-39; PMID:21737960; http://dx.doi.org/10.5551/jat.7922
- Long JZ, Svensson KJ, Tsai L, Zeng X, Roh HC, Kong X, Rao RR, Lou J, Lokurkar I, Baur W, et al. A smooth muscle-like origin for beige adipocytes. Cell Metab 2014; 19:810-20; PMID:24709624; http://dx.doi.org/10.1016/j.cmet.2014.03.025
- Wu J, Boström P, Sparks LM, Ye L, Choi JH, Giang AH, Khandekar M, Virtanen KA, Nuutila P, Schaart G, et al. Beige adipocytes are a distinct type of thermogenic fat cell in mouse and human. Cell 2012; 150:366-76; PMID:22796012; http://dx.doi.org/10.1016/j.cell.2012.05.016
- Harms M, Seale P. Brown and beige fat: development, function and therapeutic potential. Nat Med 2013; 19:1252-63; PMID:24100998; http://dx.doi.org/10.1038/nm.3361
- Wang QA, Tao C, Gupta RK, Scherer P.E. Tracking adipogenesis during white adipose tissue development, expansion and regeneration. Nat Med 2013; 19:1338-44; PMID:23995282; http://dx.doi.org/10.1038/nm.3324
- Sharp LZ, Shinoda K, Ohno H, Scheel DW, Tomoda E, Ruiz L, Hu H, Wang L, Pavlova Z, Gilsanz V, et al. Human BAT possesses molecular signatures that resemble beige/brite cells. PLoS One 2012; 7:e49452; PMID:23166672; http://dx.doi.org/10.1371/journal.pone.0049452
- Jespersen NZ, Larsen TJ, Peijs L, Daugaard S, Homøe P, Loft A, de Jong J, Mathur N, Cannon B, Nedergaard J, et al. A classical brown adipose tissue mRNA signature partly overlaps with brite in the supraclavicular region of adult humans. Cell Metab 2013; 17:798-805; PMID:23663743; http://dx.doi.org/10.1016/j.cmet.2013.04.011
- Ouellet V, Labbé SM, Blondin DP, Phoenix S, Guérin B, Haman F, Turcotte EE, Richard D, Carpentier AC. Brown adipose tissue oxidative metabolism contributes to energy expenditure during acute cold exposure in humans. J Clin Invest 2012; 122:545-52; PMID:22269323; http://dx.doi.org/10.1172/JCI60433
- Saito M. Brown adipose tissue as a regulator of energy expenditure and body fat in humans. Diabetes Metab J 2013; 37:22-9; PMID:23441053; http://dx.doi.org/10.4093/dmj.2013.37.1.22
- Mathus-Vliegen EM. Obesity and the elderly. J Clin Gastroenterol 2012; 46:533-44; PMID:22772735; http://dx.doi.org/10.1097/MCG.0b013e31825692ce
- Rogers NH, Landa A, Park S, Smith RG. Aging leads to a programmed loss of brown adipocytes in murine subcutaneous white adipose tissue. Aging Cell 2012; 11:1074-83; PMID:23020201; http://dx.doi.org/10.1111/acel.12010
- Mookerjee SA, Divakaruni AS, Jastroch M, Brand MD. Mitochondrial uncoupling and lifespan. Mech Ageing Dev 2010; 131: 463-72; PMID:20363244; http://dx.doi.org/10.1016/j.mad.2010.03.010
- Hannenhalli S, Kaestner KH. The evolution of Fox genes and their role in development and disease. Nat Rev Genet 2009; 10:233-40; PMID:19274050; http://dx.doi.org/10.1038/nrg2523
- Friedman JR, Kaestner KH. The Foxa family of transcription factors in development and metabolism. Cell Mol Life Sci 2006; 63:2317-28; PMID:16909212; http://dx.doi.org/10.1007/s00018-006-6095-6
- Kaestner KH. The FoxA factors in organogenesis and differentiation. Curr Opin Genet Dev 2010; 20:527-32; PMID:20591647; http://dx.doi.org/10.1016/j.gde.2010.06.005
- Nakae J, Kitamura T, Kitamura Y, Biggs WH 3rd, Arden KC, Accili D. The forkhead transcription factor Foxo1 regulates adipocyte differentiation. Dev Cell. 2003; 4:119-29; PMID:12530968; http://dx.doi.org/10.1016/S1534-5807(02)00401-X
- Davis KE, Moldes M, Farmer SR. The forkhead transcription factor FoxC2 inhibits white adipocyte differentiation. J Biol Chem 2004; 279:42453-61; PMID:15277530; http://dx.doi.org/10.1074/jbc.M402197200
- Wolfrum C, Shih DQ, Kuwajima S, Norris AW, Kahn CR, Stoffel M. Role of Foxa-2 in adipocyte metabolism and differentiation. J Clin Invest 2003; 112:345-56; PMID:12865419; http://dx.doi.org/10.1172/JCI18698
- Gerin I, Bommer GT, Lidell ME, Cederberg A, Enerback S, Macdougald OA. On the role of FOX transcription factors in adipocyte differentiation and insulin-stimulated glucose uptake. J Biol Chem 2009; 284:10755-63; PMID:19244248; http://dx.doi.org/10.1074/jbc.M809115200
- Xu L, Panel V, Ma X, Du C, Hugendubler L, Gavrilova O, Liu A, McLaughlin T, Kaestner KH, Mueller E. The winged helix transcription factor foxa3 regulates adipocyte differentiation and depot-selective fat tissue expansion. Mol Cell Biol 2013; 33:3392-9; PMID:23798556; http://dx.doi.org/10.1128/MCB.00244-13
- Lee CS, Friedman JR, Fulmer JT, Kaestner KH. The initiation of liver development is dependent on Foxa transcription factors. Nature 2005; 435:944-7; PMID:15959514; http://dx.doi.org/10.1038/nature03649
- Kaestner KH, Hiemisch H, Schütz G. Targeted disruption of the gene encoding hepatocyte nuclear factor 3gamma results in reduced transcription of hepatocyte-specific genes. Mol Cell Biol 1998; 18:4245-51; PMID:9632808
- Shen W, Scearce LM, Brestelli JE, Sund NJ, Kaestner KH. Foxa3 (hepatocyte nuclear factor 3gamma) is required for the regulation of hepatic GLUT2 expression and the maintenance of glucose homeostasis during a prolonged fast. J Biol Chem 2001; 276:42812-7; PMID:11546810; http://dx.doi.org/10.1074/jbc.M106344200
- Su N, Thiaville MM, Awad K, Gjymishka A, Brant JO, Yang TP, Kilberg MS. Protein or amino acid deprivation differentially regulates the hepatic forkhead box protein A (FOXA) genes through an activating transcription factor-4–independent pathway. Hepatology 2009; 50: 282-90; PMID:19415718; http://dx.doi.org/10.1002/hep.22971
- Ma X, Xu L, Gavrilova O, Mueller E. Role of forkhead box protein A3 in age-associated metabolic decline. Proc Natl Acad Sci U S A 2014; 111:14289-94; PMID:25225406; http://dx.doi.org/10.1073/pnas.1407640111
- Li Z, White P, Tuteja G, Rubins N, Sackett S, Kaestner KH. Foxa1 and Foxa2 regulate bile duct development in mice. J Clin Invest 2009; 119:1537-45; PMID:19436110; http://dx.doi.org/10.1172/JCI38201
- Duncan SA, Navas MA, Dufort D, Rossant J, Stoffel M. Regulation of a transcription factor network required for differentiation and metabolism. Science 1998; 281:692-5; PMID:9685261; http://dx.doi.org/10.1126/science.281.5377.692
- Panowski SH, Wolff S, Aguilaniu H, Durieux J, Dillin A. PHA-4/Foxa mediates diet-restriction-induced longevity of C. elegans. Nature 2007; 447:550-5; PMID:17476212; http://dx.doi.org/10.1038/nature05837
- Chen G, Korfhagen TR, Karp CL, Impey S, Xu Y, Randell SH, Kitzmiller J, Maeda Y, Haitchi HM, Sridharan A, Senft AP, et al. Foxa3 induces goblet cell metaplasia and inhibits innate antiviral immunity. Am J Respir Crit Care Med 2014; 189:301-13; PMID:24392884; http://dx.doi.org/10.1164/rccm.201306-1181OC
- Potter AS, Casa AJ, Lee AV. Forkhead box A1 (FOXA1) is a key mediator of insulin-like growth factor I (IGF-I) activity. J Cell Biochem 2012; 113:110-21; PMID:21882221; http://dx.doi.org/10.1002/jcb.23333
- Wolfrum C, Besser D, Luca E, Stoffel M. Insulin regulates the activity of forkhead transcription factor Hnf-3beta/Foxa-2 by Akt-mediated phosphorylation and nuclear/cytosolic localization. Proc Natl Acad Sci U S A 2003; 100:11624-9; PMID:14500912; http://dx.doi.org/10.1073/pnas.1931483100
- Neel JV. Diabetes mellitus: a “thrifty” genotype rendered detrimental by “progress”? Am J Hum Genet 1962; 14: 353-62; PMID:13937884
- Neel JV. The “thrifty genotype” in 1998. Nutr Rev 1999; 57:S2-9; PMID:10391020; http://dx.doi.org/10.1111/j.1753-4887.1999.tb01782.x
- Prentice AM, Hennig BJ, Fulford AJ. Evolutionary origins of the obesity epidemic: natural selection of thrifty genes or genetic drift following predation release? Int J Obes (Lond) 2008; 32: 1607-10; PMID:18852700; http://dx.doi.org/10.1038/ijo.2008.147
- Speakman JR. Thrifty genes for obesity, an attractive but flawed idea, and an alternative perspective: the 'drifty gene' hypothesis. Int J Obes (Lond) 2008; 32: 1611-7; PMID:18852699; http://dx.doi.org/10.1038/ijo.2008.161
- Adler-Wailes DC, Alberobello AT, Ma X, Hugendubler L, Stern EA, Mou Z, Han JC, Kim PW, Sumner AE, Yanovski JA, et al. Analysis of variants and mutations in the human winged helix FOXA3 gene and associations with metabolic traits. Int J Obes (Lond) 2015; 1-5; PMID:25135377; http://dx.doi.org/10.1036/1JE.2015.17
