Of Mice and Men
pp. 161–80
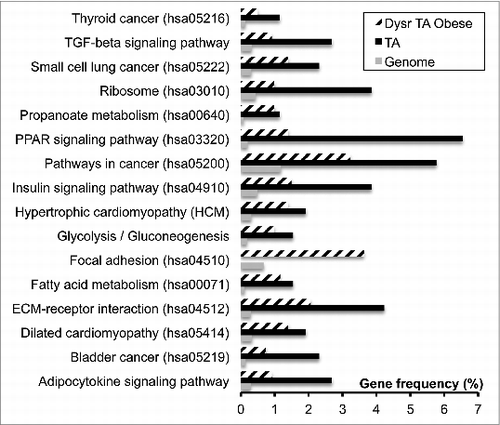
An increase in adipocyte cell size (hypertrophy) and cell number (hyperplasia) has been linked to obesity, and there have been several studies that have looked at the mechanisms that drive adipose tissue differentiation, function, and renewal in rodents and humans. In this research paper, authors Berger et al. sought to identify common threads in both rodents and humans with regards to adipogenesis and/or lipogenesis that maybe dysregulated in obesity. The authors used a combination of real-time assays and bio-informatics and, after identifying 1426 adipose tissue and fatty acid storage related genes that are common between rodents and humans, developed new methods for analyzing lipid uptake and differentiation in both mouse 3T3l1 fibroblasts and human adipose stem cells. These studies revealed that in mice and humans FAT/CD36 was central in regulating both adipogenesis and lipogenesis ().
ECGM may optimize stem cell cultivation
pp. 181–7
Fast, reliable cultivation protocols are now in great demand, especially due to the focus of adipose-derived stem cells in regenerative medicine. Here, authors Storck et al. test endothelial cell growth medium (ECGM) cultivation of human adipose stem cells compared to the common DMEM/F12 medium protocol, finding that the endothelial process both accelerates growth prior to induction and differentiation as well as provides shorter differentiation and induction times. These results relating to mature adipocytes and undifferentiated cells show ECGM to be very effective with regards to the development and cultivation of preadipocytes.
Adipose Stress
pp. 188–202
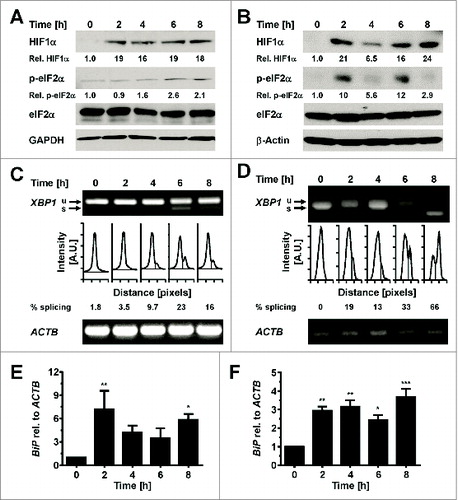
The activation of unfolded protein response (UPR) and endoplasmic reticulum (ER) stress are known to be associated with obesity, and adipose tissue is often exposed to numerous sources of stress via inflammation, hypoxia and the aforementioned ER stress. In this research paper authors Mihai and Schröder seek to identify the physiological triggers of both UPR and ER stress. They find that rather than the lipid overload and hyperinsulinemia often associated with obesity, it is the physiological changes seen in conditions such as glucose starvation and hypoxia that are more likely to be physiological ER stressors of adipocytes ().
Immortalized Primary Adipocytes
pp. 203–11
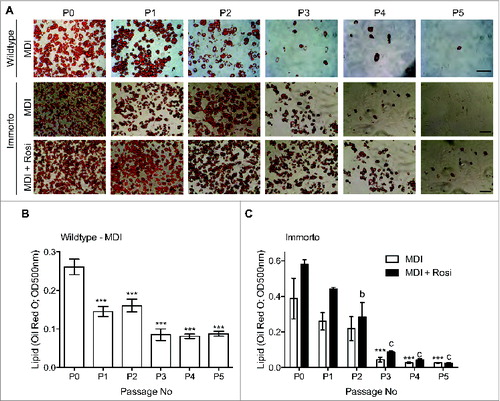
Obesity is a result of an increase in white adipose tissue cell size and mass, and the differentiation of adipocyte precursor (AP) cells is a requirement for the production of new adipocytes. With this in mind, authors Church, Brown, and Rodehaffer attempted to create an immortalized primary adipogenic cell line using isolated APs for studying adipogenesis and adipocyte function. The authors found that SV40T antigen immortalized APs have a limited potential for expansion and adipogenic culture as it loses adipogenic capacity with increasing passage number, due to loss of a committed late adipogenic gene expression profile. These studies suggest that culture conditions that include other factors and/or supportive cells may be needed to maintain adipogenic capacity of APs ().
Amyloid-β effects
pp. 212–6
The peptide Amyloid-β (Aβ) is key in the pathology of Alzheimer disease, and although amyloid precursor protein and Aβ peptide are both expressed in adipose tissue, it is not known whether Aβ has any effect on key adipose tissue functions. This brief report by Wan et al. explores this issue, and attempts to see if Aβ expression affects lipolysis and adipokine secretion. Their findings show an exertion of functional effects on adipose tissue by Aβ peptides that can lead to an increase in the release of pro-inflammatory adipokines as well as free fatty acids.
Wt1, defining visceral WAT identity
pp. 217–21
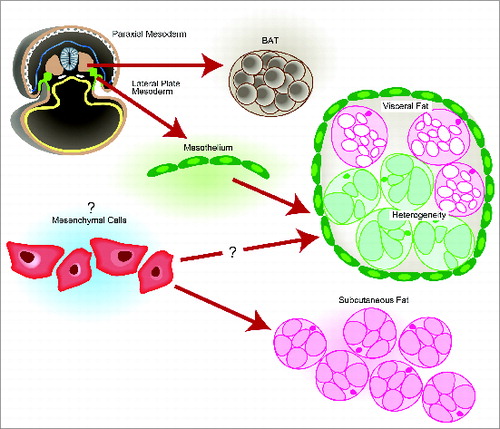
Despite being a focus of study, the amount that we understand with regards to the different developmental origins of visceral white adipose tissue (WAT) depots and subcutaneous WAT is paltry. In this commentary, Chau and Hastie discuss recent studies showing cells with late mouse gestation Wilms' tumor 1 (Wt1) gene expression result in visceral WAT but not subcutaneous WAT. It is also shown that the mesothelium that lines visceral WAT may be the structural source of adipocytes. The implications that Wt1 may actually regulate visceral WAT identity and progenitor population is also discussed ().
Parallel Roles of MicroRNA
pp. 222–4
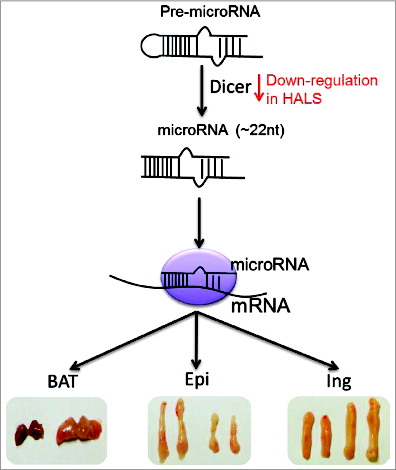
The class of non-coding regulators known as microRNAs play specific functions in adipocyte development, although this development is still not fully understood. In this commentary by Xu and Sun, the authors review their recent study regarding the role of microRNA biogenesis in brown adipocyte feature maintenance as well as a parallel study by Mori et al. which looked at adipose-specific Dicer knockout mice. This study showed that in patients with HIV-associated lipodystrophy (HALS), there was a downregulation of Dicer expression which may provide support that the onset of HALS may have something to do with the dysregulation of microRNA biogenesis ().