Abstract
By regulating Akt membrane compartmentalization, ClipR-59 modulates adipocyte glucose transport. To elucidate the role of ClipR-59 in the regulation of whole body glucose homeostasis, we have generated adipose tissue specific transgenic mice and examined how forcing expression of ClipR-59 in adipose tissue affects body glucose homeostasis. We found that ClipR-59 adipose transgenic mice showed lower blood glucose level with increased glucose tolerance and enhanced insulin sensitivity. Moreover, ClipR-59 adipose transgenic mice were lean with reduced fat mass and against diet induced obesity. Finally, we examined the potential impact of ClipR-59 on adipose endocrine function and found that ClipR-59 expression enhanced adiponectin secretion in both 3T3-L1 adipocytes and adipose tissue, accompanied with increased circulating adiponectin and enhanced AMPKα phosphorylation at Thr172 in adipose tissue and skeletal muscle. Overall, these studies demonstrate that ClipR-59 is likely an important regulator of body glucose homeostasis and adipocyte function.
Abbreviations
| ACC1 | = | Acetyl-CoA carboxylase |
| adipoQ | = | Adiponectin |
| CAP-Gly | = | Cytoskeleton associated protein-glycine rich |
| Clip | = | Cytoplasm linker protein |
| ClipR-59 | = | Clip Related Protein 59kD |
| FASN | = | Fatty acid synthatase |
| GTT | = | Glucose tolerance assay |
| ITT | = | Insulin tolerance assay |
| TG | = | Transgenic mice |
| Trib3 | = | Tribbles 3 |
Introduction
Adipose tissue is a vital organ in the regulation of body glucose and energy homeostasis. Impaired adipose function results in the development of metabolic syndrome and type II diabetes.Citation1,2 The regulation of body glucose and energy homeostasis by adipose tissue depends on 2 distinct adipocyte functions: glucose uptake through insulin dependent Glut4 membrane translocation to modulates blood glucose levelsCitation3 and producing adipokines which act on other tissues to modulate peripheral insulin sensitivity and energy metabolismCitation4,5 Insulin dependent glucose uptake and adipokine production in adipose tissue appears interlinked in adipose tissue. Glut4 specific knockout mice resulted in several insulin resistance, impaired adipokine production and glucose intolerance.Citation6,7 On the other hand, adipokines, of particular adiponectin has been shown to regulate insulin sensitivity in adipocytes.Citation8 It is therefore believed that the regulator of adipocyte glucose uptake will have profound impact on body glucose homeostasis as well as energy homeostasis.
ClipR-59 is a membrane associated protein characterized with 3 distinct structural domains including a Glu-Pro rich domain, 3 ankryin repeats and 2 putative CAP-Gly domains.Citation9 CAP-Gly domains are signature domain of ClipR-170 protein family that binds microtubule and regulates microtubule dynamics.Citation10,11 In addition, ClipR-59 also undergoes palmitoylation at Cys534 and Cys535, which mediates the association of ClipR-59 with membrane.Citation12,13 Interestingly, ClipR-59 CAP-Gly domains show no microtubule binding activity. Instead, it functions as protein-protein interaction module to interact with protein kinase Akt. As a result, ClipR-59, via its membrane association and Akt interaction, modulates Akt plasma membrane compartmentalization.Citation14,15 In addition, ClipR-59 also interacts with AS160 and facilitates AS160 phosphorylation by Akt in adipocyte.Citation14,15 Akt is the major downstream kinase and AS160 is well-known Akt substrate in insulin regulated Glut4 membrane translocation in insulin dependent Glut4 membrane translocation.Citation16,17 Hence, by modulating Akt membrane compartmentalization and AS160 phosphorylation, ClipR-59 is involved in insulin dependent Glut4 membrane translocation in adipocyte.Citation14,15
Given the critical role of insulin dependent Glut4 membrane translocation in the regulation of body glucose homeostasis, the regulation of Glut4 membrane translocation in adipocytes implies that ClipR-59 likely plays a role in the regulation of body glucose homeostasis. Here we report that we have generated adipose specific ClipR-59 transgenic mice and found that adipose ClipR-59 transgenic mice exhibited higher glucose tolerance and against diet induced obesity.
Results
Generation of ClipR-59 adipocyte transgenic mice
ClipR-59 interacts with Akt and modulates Akt membrane localization and thereby adipocyte insulin dependent Glut4 membrane translocation in adipocyte.Citation14,15 Insulin dependent Glut4 membrane translocation is a basic mechanism to regulate body homeostasis.Citation3 This implies that ClipR-59 play a role in the regulation of whole-body glucose homeostasis. To examine this, we generated adipose tissue-specific ClipR-59 transgenic mice with the aP2-ClipR-59 transgenic gene. Specifically, a Flag-tagged ClipR-59 cDNA was cloned into an adipose-specific aP2 promoter (), and the ClipR-59 transgenic mouse was generated at the Tufts Transgenic Core Facility by pronuclear injection of the aP2-ClipR-59 transgenic gene into embryonic cells within the C6Black genetic background. We obtained a total of 8 pups of which 2 mice (no. 84 and 85) have the transgene judged by PCR analysis of individual mouse-tail DNA ().
Figure 1. Generation of ClipR-59 transgenic mice. (a) Diagram of the Ap2-ClipR-59 transgene, which contains a Flag-tagged and a 150bp CREB 5’ UTR. (b) PCR analysis of tail DNA by ClipR-59-specific primers reveals that mice 84 and 85 harbor the transgenic gene (left bottom panel). The right bottom panel shows the PCR product of mouse 85's tail genomic DNA digested with EcoRI (ClipR-59). Note: The PCR product of ClipR-59 contains an EcoRI restriction site which gives 100bp and 150bp fragments following EcoRI digestion. (c) Genotype of the F1 generation of ClipR-59 transgenic mice. (d) Expression of the ClipR-59 transgenic gene in transgenic and wildtype mice. Total RNA were prepared from Fat (F), liver (L) and Kidney (K) from wildtype and ClipR-59 transgenic mice and its wildtype littermates and subjected to RT-PCR.
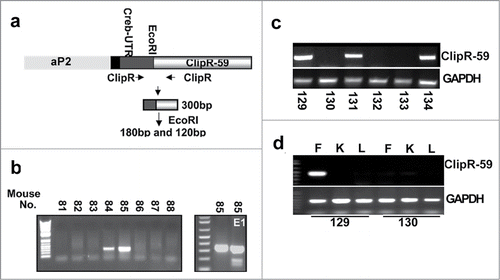
The ClipR-59 transgenic mice were inbred. The F1 mice appear normal and fertile, indicating that the expression of the ClipR-59 transgenic gene has no adverse effect on their development. shows a representative analysis of the genotype of the F1 generation. Of these 6 F1 littermates, 3 have the ClipR-59 transgene, indicating that we have successfully generated ClipR-59 transgenic mice.
Next, we analyzed the expression of the ClipR-59 transgenic gene in several tissues, including fat, liver, and kidney. Shown in , the ClipR-59 transgenic gene is expressed in adipose tissue derived from mouse 129 but not from mouse 130. In addition, there is no detectable amount of ClipR-59 transgene expression in the liver and kidney of either mouse. From these data, we conclude that we have successfully generated ClipR-59 adipose tissue transgenic mice.
Glucose homeostasis of ClipR-59 adipose transgenic mice
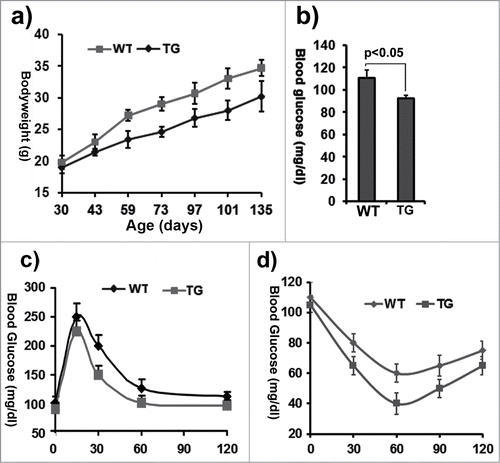
To determine the impact of ClipR-59 adipose transgenic gene on mice, aP2-ClipR-59 transgenic mice were propagated from lines 131 and 134, respectivly. First, we monitored growth and weight gain of these mice. We observed that they were about 10-20% lighter than wildtype littermates through the first 4 months (). Next, we measured the level of fasting blood glucose at age 3 months. The ClipR-59 transgenic mice (TG) had a slightly lower level of fasting blood glucose than the wildtype (WT) littermates (TG: 93 ± 3 mg/dl vs WT: 110 ± 5.8 mg/dl, n = 4) (). The key parameter by which to determine how glucose homeostasis is affected is to measure how the mouse copes with blood glucose changes. Therefore, glucose tolerance tests were done to determine how aP2-ClipR-59 transgenic mice deal with changes of blood glucose (). After glucose injection, aP2-ClipR-59 transgenic mice showed a higher rate of blood glucose disposal compared to wildtype mice, an indication that expression of ClipR-59 in adipose tissue increased glucose tolerance. Next, we performed an insulin tolerance assay to measure insulin activity. As shown in , ClipR-59 transgenic mice clearly showed higher insulin sensitivity.
Figure 2. Characterization of ClipR-59 transgenic mice. (a) Growth rates of ClipR-59 transgenic mice and their wildtype littermates (n = 6). (b) Fast glucose blood levels of ClipR-59 transgenic mice (TG) and their wildtype littermates. The bar graph shows means ± STDV, n =6. P < 0.05. (c) Glucose tolerance of wildtype and ClipR-59 transgenic mice (n = 6). (d) Insulin tolerance assay of wildtype and ClipR-59 transgenic mice (n = 6).The error bar shows ± STDV (n = 6).
The impact of ClipR-59 expression on adipose tissue
At 4 months of age, we scarified the mice and examined the impact of ClipR-59 expression on adipose tissue. A simple view of the dissected mice shows that transgenic mice had less fat mass (). For a more accurate measure, we expressed the fat mass as a ratio of epididymal fat pad weight to total body weight. We found that the epididymal fat pad of induced ClipR-59 adipose mice (0.015 + 0.005 fat mass/bodyweight) was almost 60% less than that of non-induced ClipR-59 mice (0.045 + 0.007 fat mass/ bodyweight) (). We also assessed the size of white adipocytes from wildtype and ClipR-59 transgenic mice through hematoxylin and eosin staining. In agreement with the finding that ClipR-59 transgenic mice were leaner, the adipocytes from ClipR-59 transgenic mice were smaller in size than those from wildtype littermates (). These data indicate that exogenous ClipR-59 expression in adipocytes has an impact on total fat mass.
Figure 3. The impact of ClipR-59 expression on adipose growth. (a) The appearance of male control (WT) and ClipR-59 transgenic (TG) mice. (b) The ratio of epididymal pad mass and bodyweight. The bar graph shows means ± STDV, n = 6. P < 0.04. (c) H&E staining of white adipose tissues of wildtype and ClipR-59 transgenic mice shows reduced adipocyte size in ClipR-59 transgenic mice. Only representative tissues were presented. (d) ClipR-59 transgenic mice on high calorie diet. ClipR-59 adipose transgenic (TG) and wildtype male mice (6 per group) at age of 4 weeks were feed with regular chow or high calorie diet (HD) were feed for 12 weeks. The bodyweights were measured for every 7-10 days. The bar graph shows means ± STDV, n = 6.
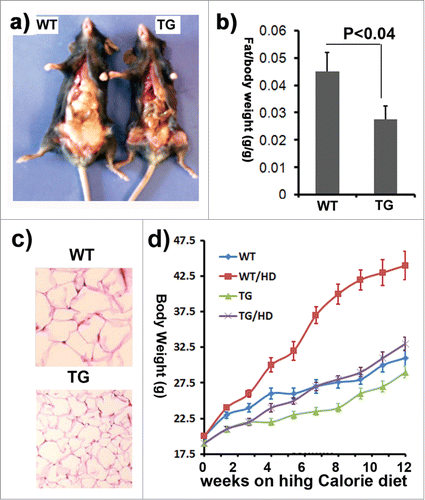
The impact of adipose mass implies that ClipR-59 might affect the development of obesity. To determine how ClipR-59 transgenic mice copy with diet induced obesity, ClipR-59 transgenic mice and their wildtype littermates were fed with regular chow or high calorie diet (60%) up to 12 weeks. Wildtype mice become obese within 8 weeks on high calorie diet judged by more than 20% bodyweight gain. On the other hand, at this time, ClipR-59 transgenic mice showed no significant differences in bodyweight from wildtype mice on regular chow, suggesting that ClipR-59 transgenic mice were resistant against diet induced obesity ().
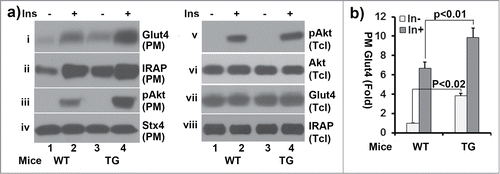
In our early studies, we showed that ClipR-59, via recruiting Akt to membrane, regulates Glut4 membrane translocation.Citation14,15 To determine whether there is a differ in adipose glucose membrane translocation between wildtype and ClipR-59 transgenic mice, the subcellular fractionation assay was performed in adipocytes from ClipR-59 transgenic mice and their wildtype littermates. As shown in , insulin promotes Glut4 membrane translocation and Akt activation as expected in wildtype adipocytes. In ClipR-59 transgenic adipocytes, the levels of Glut4 and phospho-Akt on PM were significantly higher than that of wildtype (panel i, and iii). In addition, we also examined membrane translocation of IRAP, the major cargo of Glut4 vesicle. Similarly, the levels of IRAP in PM were also significantly higher in ClipR-59 transgenic adipocytes than that in wildtype one. The difference in Glut4 in PM observed here is not because of sample variation as a comparable levels of syntaxin 4, a PM protein whose levels is not regulated by insulin were observed in each sample (panel iv). Since, there is no different between wildtype and ClipR-59 transgenic adipose tissue in the expression of Glut4, IRAP and overall Akt activation (panel v, iiv and viii), these results further demonstrate that ClipR-59 in adipose tissue promotes Akt membrane association and Glut4 membrane translocation as we have previously reported.
Figure 4. Subcellular fractionation assays of adipocytes from wildtype and ClipR-59 transgenic mice. (a) Adipocytes were prepared from epididymal pads from either wildtype or ClipR-59 transgenic mice. Then, the adipocytes were treated with or without 10nM insulin and plasma membrane were prepared as described in Methods and Materials. The plasma membrane fractions were analyzed in Western Blot with Anti-Glut4, anti-IRAP, anti-phospho-Akt, anti-Akt and anti-syntaxin 4 (stx4) antibodies, respective as indicated. PM: plasma membrane. Tcl: total cell lystates. (b) Quantification of Glut4 membrane translocation in (a). The Glut4 levels in PM in wildtype adipocytes without insulin simulation were set as 1 after normalized to the levels of syntaxin 4 on PM. Bar graphs show means ± STDV, n = 3.
ClipR-59 regulates adiponectin production
Glut4 transgenic mice show increase adipose mass and adiposity.Citation18,19 The view thatClipR-59 transgenic mice exhibited increase Glut4 membrane translocation, yet with decreased fat mass implies that ClipR-59 could regulate other adipose function, i.e. adipokine production. Adipose tissue produces a number of adipokines include adiponectin, the most abundant adipokine to regulate energy metabolism.Citation20 To examine this, adipose pad were isolated from 3 month wildtype and ClipR-59 transgenic mice and cultured in serum free medium for 6 hours. Then, the levels of adiponectin, in medium were determined. As shown , the amount of adiponectin in medium of ClipR-59 transgenic adipose tissue was about twice more than that of control adipose tissue (panel i and for quantified result). In agreement with notion that ClipR-59 adipose transgenic mice expressed higher ClipR-59, the levels of ClipR-59 in ClipR-59 transgenic adipose tissue were higher than control adipose tissue (, panel iii).
Figure 5. The impact of ClipR-59 expression on adiponectin production. (a) ClipR-59 transgenic adipose show increased adiponectin production. Epididymal pads were isolated from ClipR-59 transgenic mice and their wildtype littermates and incubated in serum-free medium for 6 hrs. Then medium and total tissue extracts were harvested for Western Blot with indicated antibody. (b) Quantitative presentation of Data in a). The medium levels of adiponectin in wildtype adipose tissue were set as 100%. Bar graphs show means ± STDV, n = 6. (c) Overexpression of ClipR-59 in 3T3-L1 adipocytes increases adiponectin production. 3T3-L1 adipocytes were transduced with adenoviral vectors that express either GFP or ClipR-59 in triplicates. 48h posttransduction, the medium was changed to serum free medium. 6 hrs later, the medium and total cell lysates were harvested for Western blot with anti-adiponectin antibody (i) and (ii). The levels of Flag-ClipR-59 expressed from adenoviral vectors is shown in panel iii. (d) Quantitative presentation of Data in a). The medium levels of adiponectin in control medium were set as 100%. Bar graphs show means ± STDV, n = 3. (e and f) Knockdown of ClipR-59 in adipocytes reduces adiponectin production. The experiments were carried out similar to (c) and (d) except adenoviral vectors that express either luciferase shRNA or ClipR-59 shRNA were used. Med: Medium. Cell: cell lysates.
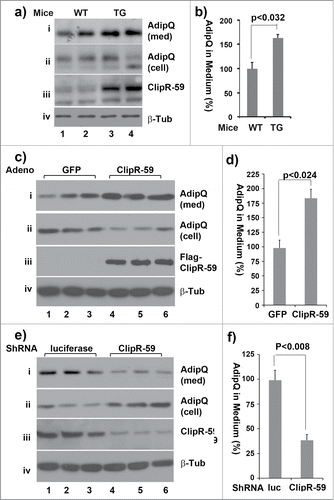
To further examine the regulation of adiponectin production by ClipR-59, we assessed the adiponectin production in 3T3-L1 adipocytes that were transduced with adenoviral vectors that expressed either ClipR-59 cDNA or GFP (served as a control). As shown in , the amount of adiponectin in medium from ClipR-59 overexpressing adipocytes was twice about that from control cells (panel i and the quantified result ). Correspondingly, the cellular levels of adiponectin were decreased about 50% in ClipR-59 overexpressing cells (panel ii). Next, we examined the impact of ClipR-59 knockdown on adiponectin production. As shown in , the levels of adiponectin in cell culture medium decreased by more than 50% (panel i and , the quantified result) with corresponding decreases the cellular levels of adiponectin (panel ii). As expected, expression of ClipR-59 shRNA decreased the levels of ClipR-59 by more than 70% (panel iii).
The notion that ClipR-59 expression regulates adiponectin production promotes us examining the circulating adiponectin in ClipR-59 transgenic mice. Toward this, we collected blood samples from both wildtype and aP-ClipR-59 transgenic mice and assessed the levels of adiponectin in these samples. As shown in , the circulating levels of adiponectin in ClipR-59 transgenic mice were twice more than that in wildtype mice. Next, we examined the circulating adiponectin under regular chow and high fat diet. As shown in , in agreement with the view that obesity reduces circulating adiponectin, the plasma levels of adiponectin were reduced by more than 60% under high calorie diet. Similarly, the plasma levels of adiponectin were high in ClipR-59 transgenic mice, which are only modestly reduced under high calorie diet.
Figure 6. ClipR-59 transgenic mice show increased circulating adiponectin. (a) Western Blot analysis of plasma sera from 3-month old wildtype (WT) and ClipR-59 adipose transgenic mice (TG) fasted for 6 hours. 50ug of total plasma sera were separated on SDS page and probed with anti-adiponectin antibody. (b). Quantification of Western Blot in a). The plasma levels of adiponectin from wildtype mice were set as 1. Bar graphs show means ± STDV, n = 3. *P < 0.005. (c) Western Blot analysis of plasma sera from wildtype (WT) and ClipR-59 adipose transgenic mice (TG) under regular chow (RC) and high calorie diet (HD) for 12 weeks with anti-adiponectin antibody. (d) Quantification of Western Blot in (a). The plasma levels of adiponectin from wildtype mice under normal chow were set as 1. Bar graphs show means ± STDV, n = 3. *P < 0.042. **P < 0.002. (e) Western blot of analysis of tissue (adipose: adipo and skeletal muscle) extracts from wildtype (WT) and ClipR-59 adipose transgenic (TG) with anti-phospho-AMPKα at Thr172 (i and iii), anti-AMPKα (ii and iv) and anti-ClipR-59 (v) antibodies, respectively. (f) Quantification of adipose tissue pAMPKα in (e). The levels of pAMPKα in wildtype mice were set as 1 after normalized to total level of AMPKα. Bar graphs show means ± STDV, n = 3. *P < 0.043. (g) Quantification of muscle pAMPKα in (e). The levels of pAMPKα in wildtype mice were set as 1 after normalized to total level of AMPKα. *P < 0.0082. (h) RT-PCR analysis of the expression of PGC1a, ACC1, FASN, SREBP1c and ClipR-59 in ClipR-59 transgenic adipose tissue. Bar graphs show means ± STDV, n = 3. *P < 0.002, ** P < 0.03.
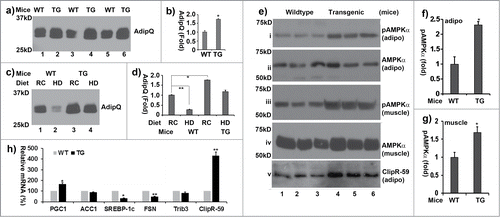
In cells, adiponectin binds to its receptor and activates AMPK through induction of AMPKα subunit phosphorylation at Thr172 to regulate energy metabolism.Citation21-23 With this mind, we examined phosphorylation levels of AMPKα at Thr172 in both adipose tissue and skeletal muscle from wildtype and ClipR-59 adipose transgenic mice. As shown in , the levels of phospho-AMPKα at Thr 172 in ClipR-59 transgenic were about 2 fold higher than that in wildtype mice in both adipose tissue (panel I, ) and muscle (panel iii, ),. Taken together, these data demonstrate that ClipR-59 is involved in adipocyte adiponectin production which altered the activation of AMPKα.
One of adiponectin activities is to regulate lipid metabolism through modification of lipid metabolic genes such as PGC1α,Citation24 Fatty acid synthatase (FASN)Citation25 and SREBP-1c.Citation26 To determine whether there are alterations in the expression of these genes in ClipR-59 adipose transgenic mice, we examined the expression of these in ClipR-59 transgenic adipose tissue by RT-PCR. We observed that the expression of PGC1a was increased whereas that of SREBP1c, FASN was decreased (). We also examined the expression of ACC1, Trib3 and ClipR-59 in ClipR-59 transgenic mice. No appreciable changes in the expression of ACC1 and Trib3 were observed. As expected, ClipR-59 expression was markedly higher in ClipR-59 transgenic mice. Taken together, we believe that ClipR-59 expression in adipose tissue altered the expression of lipid metabolic genes.
Discussion
To explore the role in ClipR-59 in metabolic regulation, we have generated ClipR-59 adipose transgenic mice. Based on our in vitro studies that ClipR-59 regulates insulin dependent membrane translocation, we anticipate that ClipR-59 adipose transgenic mice will exhibit a lower blood glucose level and an higher glucose tolerance, all which were found to be true (), underscoring the role of ClipR-59 in the regulation of whole body glucose homeostasis.
Glut4 adipose transgenic mice showed increased glucose tolerance, but also increased adiposity.Citation18,19 The view that ClipR-59 adipose tissue transgenic mice consist of less fat mass implies that ClipR-59 might potentially affect the other function of adipocytes. With this mind, we examined the possible effect of ClipR-59 on the secretion of adiponectin. Adiponectin is the most abundant adipokine produced from adipocyte and has been demonstrated to play a critical role in the regulation of glucose and energy metabolism. Our results showed that ClipR-59 modulates adiponectin production as forcing expression of ClipR-59 enhanced whereas knockdown of ClipR-59 decreased adiponectin production (). Moreover, we have observed that the circulating levels of adiponectin in ClipR-59 transgenic mice are higher than that in wildtype mice and () under both regular chow and high fat diet. Adiponectin is the most abundant adipokine and plays a critical role in the regulation of energy metabolism and peripheral insulin sensitivity.Citation21 In cells, binding adiponectin to its receptor (AdipR) activates AMPK, which in turn regulates lipid and energy metabolism.Citation27,28 Impaired Adiponectin signaling results insulin resistance, obesity and type II diabetes.Citation20 It is therefore plausible that regulation of adiponectin production by ClipR-59 in adipocytes would contribute to the phenotype observed in ClipR-59 adipose tissue transgenic mice, namely lean mice and enhances insulin sensitivity.
At present, how ClipR-59 regulates adiponectin production is not known. ClipR-59 is a palmitoylated protein which is associated with plasma membrane and trafficking vesicles cells and regulate vesicle trafficking and membrane dynamics.Citation12,13 Thus, it is possible that the regulation of adiponectin production is because the function of ClipR-59 in plasma membrane. Further studies are required to elucidate the mechanism under which ClipR-59 to regulate adiponectin secretion.
As endocrine organ, adipose tissue produces a number of adipokines such as leptin, etc, which play critical role in energy metabolism.Citation29 In current studies, we have not examined whether ClipR-59 is involved in the production of other adipokines. However, it will be great interesting to examine this in future.
In summary, we have examined the potential role of ClipR-59 in the regulation of glucose homeostasis in vivo and found that ClipR-59 potentially regulates tow important functions of adipocyte, Glut54 membrane translocation and adiponectin production, thereby, glucose homeostasis. To further establish the role of ClipR-59 in adipocyte function, ClipR-59 adipose tissue conditional knockout mice, which is currently in the production, is required.
Methods and Materials
Reagents
Anti-Flag antibody (F3165) was from Sigma. All common used chemicals from Thermos Scientific. Anti-adiponectin (2789), anti-phospho-AMPKα at Thr172 (Cat# 2535S), anti-AMPKα (Cat# 5831S), anti-IRAP (Cat# 6918), anti-Akt (4685) and anti-phospho-Akt (Cat# 8200S) antibodies were from Cell Signaling. anti-Glut4 (Cat# SC-53566), Anti-β-tubulin (SC-23950), anti-syntaxin 4 (Cat# SC-101301) were from Santa Cruz. aP2 promoter construct (Cat# 11424) was from Addgene. High calorie diet (Cat# F3282) was from Bio-serv laboratory. Anti-ClipR-59 antibody has been described.Citation14,15
Generation of ClipR-59 transgenic mice
Flag-tagged ClipR-59 cDNA, which consists of CREB 5’UTR was cloned into pBS-aP2 between SmaI and Not I site. Then, ClipR-59 transgenic gene were released by XhoI site. The resulted transgenic gene was injected to C6Black mouse embryo in Tufts Medical Center transgenic core facility.
Genotyping and PCR
Mouse Genotyping were carried out by genomic PCR. Specifically, mouse genomic DNA were prepared with DNeasy kit (Qiagen Cat# 69506). Then, PCR were carried with Forward primer corresponding CREB UTR of transgene and Reverse primer corresponding ClipR-59 transgene: forward 5′-GGAGAAGCCGAGTGTTGGTG-3 (with in CREB UTR) and reverse 5′-GGTGCACAACAGGCTTCTG-3′ (within ClipR-59). The cycles were 94°C, 30." 56°C 30" and 72°C 30" for total of 25 cycles. The PCR products were analyzed on 1.5% agarose gel. Since there primers are specifically to the transgene, transgene is the only one that will be amplified in PCR. In addition, the amplified transgene consists of an EcoRI site which will facilitate to confirm the amplified transgene. All works with mice were performed according to approved mouse protocol by DLAM of Tufts Medical Center.
mRNA and RT-PCR
Tissue RNAs were prepared with RNeasy kit (Qiagen Cat# 74106). The cDNA generation were carried out with RT kit from Ambion (10928-034) with above primers. The primers for endogenous ClipR-59 are the following: forward 5′- ATGACTAAGACGGATCCTG-3 and reverse 5′-GGTGCACAACAGGCTTCTG-3′. The primers for PGC1a, ACC1, SREBP1c, FASN and Trib3 and the procedure for quantitative PCR have been describedCitation30 . The realtime PCR was carried out as the following: 94°C; 45;" 56°C 45" and 72°C 30" for total of 29 cycles.
Isolation of primary adipocytes and subcellular fractionation assay
The isolation of primary adipocytes from fat pad and subcellular fractionation assay have been described.Citation14,15 Briefly, the epididymal fat pads were taken from mice and minced. Then, the minced adipose tissue were digested with collagenase type IIa (Sigma, Cat# C6885) in TESCA buffer (50 mM TES, 0.36 mM Calcium chloride, pH 7.4) at 37°C for 2 hours. After digestion, the digested tissues were filtered and cultured in DMEM. For subcellular fractionation assay, the primary adipocytes were serum-derived from 6 hours and following treated with or without 100nM insulin for 30 mins. For examining adiponectin secretion, the culture medium were taken after 6 hours incubation.
Cell culture
3T3-L1 preadipocytes (ATCC Cat#CL-183,) were cultured in DMEM supplemented with 10% bovine serum and 1x antibotic-antinycotic.HEK293 Cells were cultured in DMEM (Cat# 11995073, Life technologies) supplemented with 10% FBS (Cat# 26140079, Life technologies) and 1x antibiotic-antimycotic (Cat#15240112, Life technologies). The differentiation of 3T3-L1 pre-adipocytes were carried out as previsously described.Citation14,15 Briefly, after grown to confluence, 3T3-L1 preadipocytes were cultured for 2 more days. Then, the medium was switched to differentiation medium (DMED supplemented with 10% FBS, 1uM Dexamethesone. 1mM IBMX and 2ug insulin/ml. Four days later, the medium regular medium supplemented with 1ug/ml insulin. The cells were used for experiments. The adenoviral expression vectors that express either ClipR-59 cDNA or ClipR-59 shRNA have been described.Citation14,15 and puried thorugh double CsCl banding as described.Citation31 To transduce 3T3-L1 adipocytes, the adenoviruses were incubated with 3T3-L1 adipocytes for overnight. The amount of viruses used is to ensure that more than 75% cells were transduced based on the GPF positive cells. The adenoviral vectors consist of a GFP gene which expressed separately from targeted gene.Citation32,33 For adiponectin secretion studies, the 3T3-L1 adipocytes were incubated in serum-free medium for 4-6 hours. Then, the cell culture medium and total cell lysates were prepared for Western blot.
The blood sample and tissue extract preparation
Blood specimens (usually 50 ul) were collected from mice. Then, the specimens were centrifuged for 3000 rpm for 15 min. The supernatants (plasma sera were collected for Western blot. To obtain tissue extract, the individual tissue were taken from mice and minced in a tissue culture plate. Then, the tissues were homogenized in SDS-UREA buffer (8M Urea, 1xPBS, 1 mM NaF, 1mM NaVO, 3.5 mM β-glycerophosphate and 5% Glycerol). The protein concentration was determine with BCA kit (Thomas Fisher Scientific)
Western blot
Cells were washed twice with PBS and lysed with cell lysis buffer (20 mM Tris pH7.6, 150 mM NaCl, 0.5 mM EDTA, 0.5 mM DTT, 10 mM, 1% Triton X-100 or 1% NP-40, 10% glycerol, protease and phosphatase inhibitors). Equal amounts of protein (20–30 ug) were subjected to SDS-PAGE electrophoresis and transferred to polyvinylidene fluoride membrane (PVDF) (Biorad Cat# 162-0177). The membranes were incubated with each primary antibody, followed by incubation with a horseradish peroxidase-conjugated secondary antibody (Biorad). The protein bands were visualized using the ECL detection system (Thomas Fisher Scientific).
H&E Staining
Mouse adipose tissues were fixed in 4% formalin solution. The tissue slides prepared from Tuft Pathology laboratory and stained with Hematoxylin and Eosin as described.Citation34
Glucose measurement, Glucose tolerance assay and insulin tolerance assay
Age matched wildtype and transgenic mice (6 per group) were fasting for 6 hours. Then mice will be weighed, fasting blood glucose was measured, and the mice will be injected intraperitoneally with a 20% D-glucose solution (2 grams D-glucose/kg body weight). After 15, 30, 60, and 120 minutes, blood will be sampled from the tail and glucose levels measured as previsously described.Citation31 For insulin tolerance assay, baseline glucose level will be determined as described above. Subsequently, the mice will be injected intraperitoneally with human insulin (Lily, U-500) (0.75 units per kg body weight). After 30, 60, and 120 minutes, blood will be sampled from the tail, and glucose levels will be measured as described.Citation31
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Funding
This work was supported by NIH grant RO1 DK084319
References
- Govers R. Molecular mechanisms of GLUT4 regulation in adipocytes. Diabetes Betab 2014; 40:400-10; PMID:24656589; http://dx.doi.org/10.1016/j.diabet.2014.01.005
- Frayn KN, Tan GD, Karpe F. Adipose tissue: a key target for diabetes pathophysiology and treatment? Horm Metab Res 2007; 39:739-42; PMID:17952837; http://dx.doi.org/10.1055/s-2007-990270
- Huang S, Czech MP. The GLUT4 glucose transporter. Cell Metab 2007; 5:237-52; PMID:17403369; http://dx.doi.org/10.1016/j.cmet.2007.03.006
- Waki H, Tontonoz P. Endocrine functions of adipose tissue. Annu Rev Pathol 2007; 2:31-56; PMID:18039092; http://dx.doi.org/10.1146/annurev.pathol.2.010506.091859
- Ahima RS, Flier JS. Adipose tissue as an endocrine organ. Trend Endocrinol Metab 2000; 11:327-32; PMID:10996528; http://dx.doi.org/10.1016/S1043-2760(00)00301-5
- Abel ED, Peroni O, Kim JK, Kim YB, Boss O, Hadro E, Minnemann T, Shulman GI, Kahn BB. Adipose-selective targeting of the GLUT4 gene impairs insulin action in muscle and liver. Nature 2001; 409:729-33; PMID:11217863; http://dx.doi.org/10.1038/35055575
- Minokoshi Y, Kahn CR, Kahn BB. Tissue-specific ablation of the GLUT4 glucose transporter or the insulin receptor challenges assumptions about insulin action and glucose homeostasis. J Biol Chem 2003; 278:33609-12; PMID:12788932; http://dx.doi.org/10.1074/jbc.R300019200
- Mora S, Pessin JE. An adipocentric view of signaling and intracellular trafficking. Diabetes/metab Res Rev 2002; 18:345-56; PMID:12397577; http://dx.doi.org/10.1002/dmrr.321
- Perez F, Pernet-Gallay K, Nizak C, Goodson HV, Kreis TE, Goud B. CLIPR-59, a new trans-Golgi/TGN cytoplasmic linker protein belonging to the CLIP-170 family. J Cell Biol 2002; 156:631-42; PMID:11854307; http://dx.doi.org/10.1083/jcb.200111003
- Maekawa H, Schiebel E. CLIP-170 family members: a motor-driven ride to microtubule plus ends. Dev Cell 2004; 6:746-8; PMID:15177023; http://dx.doi.org/10.1016/j.devcel.2004.05.017
- Galjart N. CLIPs and CLASPs and cellular dynamics. Nat Rev Mol Cell Biol 2005; 6:487-98; PMID:15928712; http://dx.doi.org/10.1038/nrm1664
- Lallemand-Breitenbach V, Quesnoit M, Braun V, El Marjou A, Pous C, Goud B, Perez F. CLIPR-59 is a lipid raft-associated protein containing a cytoskeleton-associated protein glycine-rich domain (CAP-Gly) that perturbs microtubule dynamics. J Biol Chem 2004; 279:41168-78; PMID:15262990; http://dx.doi.org/10.1074/jbc.M406482200
- Ren W, Sun Y, Du K. DHHC17 palmitoylates ClipR-59 and modulates ClipR-59 association with the plasma membrane. Mol Cell Biol 2013; 33:4255-65; PMID:24001771; http://dx.doi.org/10.1128/MCB.00527-13
- Ding J, Du K. ClipR-59 interacts with Akt and regulates Akt cellular compartmentalization. Mol Cell Biol 2009; 29:1459-71; PMID:19139280; http://dx.doi.org/10.1128/MCB.00754-08
- Ren W, Cheema S, Du K. The association of ClipR-59 protein with AS160 modulates AS160 protein phosphorylation and adipocyte Glut4 protein membrane translocation. J Biol Chem 2012; 287:26890-900; PMID:22689584; http://dx.doi.org/10.1074/jbc.M112.357699
- Welsh GI, Hers I, Berwick DC, Dell G, Wherlock M, Birkin R, Leney S, Tavaré JM. Role of protein kinase B in insulin-regulated glucose uptake. Biochem Soc Transact 2005; 33:346-9; PMID:15787603; http://dx.doi.org/10.1042/BST0330346
- Klip A, Sun Y, Chiu TT, Foley KP. Signal transduction meets vesicle traffic: the software and hardware of GLUT4 translocation. Am J Physiol Cell Physiol 2014; 306:C879-86; PMID:24598362; http://dx.doi.org/10.1152/ajpcell.00069.2014
- Carvalho E, Kotani K, Peroni OD, Kahn BB. Adipose-specific overexpression of GLUT4 reverses insulin resistance and diabetes in mice lacking GLUT4 selectively in muscle. Am J Physiol Endocrinol Metab 2005; 289:E551-61; PMID:15928024; http://dx.doi.org/10.1152/ajpendo.00116.2005
- Gnudi L, Shepherd PR, Kahn BB. Over-expression of GLUT4 selectively in adipose tissue in transgenic mice: implications for nutrient partitioning. Proc Nutrit Soc 1996; 55:191-9; PMID:8832791; http://dx.doi.org/10.1079/PNS19960020
- Lee B, Shao J. Adiponectin and energy homeostasis. Rev Endocr Metab Disord 2014; 15:149-56; PMID:24170312; http://dx.doi.org/10.1007/s11154-013-9283-3
- Whitehead JP, Richards AA, Hickman IJ, Macdonald GA, Prins JB. Adiponectin–a key adipokine in the metabolic syndrome. Diabetes Obes Metab 2006; 8:264-80; PMID:16634986; http://dx.doi.org/10.1111/j.1463-1326.2005.00510.x
- Passos MC, Goncalves MC. Regulation of insulin sensitivity by adiponectin and its receptors in response to physical exercise. Horm Metab Res 2014; 46:603-8; PMID:25126860; http://dx.doi.org/10.1055/s-0034-1377026
- Yamauchi T, Kamon J, Minokoshi Y, Ito Y, Waki H, Uchida S, Yamashita S, Noda M, Kita S, Ueki K, et al. Adiponectin stimulates glucose utilization and fatty-acid oxidation by activating AMP-activated protein kinase. Nat Med 2002; 8:1288-95; PMID:12368907; http://dx.doi.org/10.1038/nm788
- Iwabu M, Yamauchi T, Okada-Iwabu M, Sato K, Nakagawa T, Funata M, Yamaguchi M, Namiki S, Nakayama R, Tabata M, et al. Adiponectin and AdipoR1 regulate PGC-1alpha and mitochondria by Ca(2+) and AMPK/SIRT1. Nature 2010; 464:1313-9; PMID:20357764; http://dx.doi.org/10.1038/nature08991
- Xu A, Wang Y, Keshaw H, Xu LY, Lam KS, Cooper GJ. The fat-derived hormone adiponectin alleviates alcoholic and nonalcoholic fatty liver diseases in mice. J Clin Invest 2003; 112:91-100; PMID:12840063; http://dx.doi.org/10.1172/JCI200317797
- Awazawa M, Ueki K, Inabe K, Yamauchi T, Kaneko K, Okazaki Y, Bardeesy N, Ohnishi S, Nagai R, Kadowaki T. Adiponectin suppresses hepatic SREBP1c expression in an AdipoR1/LKB1/AMPK dependent pathway. Biochem Biophys Res Commun 2009; 382:51-6; PMID:19254698; http://dx.doi.org/10.1016/j.bbrc.2009.02.131
- Kadowaki T, Yamauchi T, Okada-Iwabu M, Iwabu M. Adiponectin and its receptors: implications for obesity-associated diseases and longevity. Lancet Diabetes Endocrinol 2014; 2:8-9; PMID:24622657; http://dx.doi.org/10.1016/S2213-8587(13)70120-7
- Akingbemi BT. Adiponectin receptors in energy homeostasis and obesity pathogenesis. Prog Mol Biol Transl Sci 2013; 114:317-42; PMID:23317789; http://dx.doi.org/10.1016/B978-0-12-386933-3.00009-1
- Galic S, Oakhill JS, Steinberg GR. Adipose tissue as an endocrine organ. Mol Cell Endocrinol 2010; 316:129-39; PMID:19723556; http://dx.doi.org/10.1016/j.mce.2009.08.018
- Ren W, Sun Y, Cheema S, Du K. Interaction of constitutive photomorphogenesis 1 protein with protein-tyrosine phosphatase 1B suppresses protein-tyrosine phosphatase 1B activity and enhances insulin signaling. J Biol Chem 2013; 288:10902-13; PMID:23439647; http://dx.doi.org/10.1074/jbc.M112.369371
- Du K, Herzig S, Kulkarni RN, Montminy M. TRB3: a tribbles homolog that inhibits Akt/PKB activation by insulin in liver. Science 2003; 300:1574-7; PMID:12791994; http://dx.doi.org/10.1126/science.1079817
- He TC, Zhou S, da Costa LT, Yu J, Kinzler KW, Vogelstein B. A simplified system for generating recombinant adenoviruses. Proc Natl Acad Sci U S A 1998; 95:2509-14; PMID:9482916; http://dx.doi.org/10.1073/pnas.95.5.2509
- Luo J, Deng ZL, Luo X, Tang N, Song WX, Chen J, Sharff KA, Luu HH, Haydon RC, Kinzler KW, et al. A protocol for rapid generation of recombinant adenoviruses using the AdEasy system. Nat Protoc 2007; 2:1236-47; PMID:17546019; http://dx.doi.org/10.1038/nprot.2007.135
- Humphrey RK, Newcomb CJ, Yu SM, Hao E, Yu D, Krajewski S, Du K, Jhala US. Mixed lineage kinase-3 stabilizes and functionally cooperates with TRIBBLES-3 to compromise mitochondrial integrity in cytokine-induced death of pancreatic beta cells. J Biol Chem 2010; 285:22426-36; PMID:20421299; http://dx.doi.org/10.1074/jbc.M110.123786
