ABSTRACT
The adipose organ, which comprises brown, white and beige adipocytes, possesses remarkable plasticity in response to feeding and cold exposure. The development of beige adipocytes in white adipose tissue (WAT), a process called browning, represents a promising route to treat metabolic disorders. While surgical procedures constantly traumatize adipose tissue, its impact on adipocyte phenotype remains to be established. Herein, we studied the effect of trauma on adipocyte phenotype one day after sham, incision control, or surgical injury to the left inguinal adipose compartment. Caloric restriction was used to control for surgery-associated body temperature changes and weight loss. We characterized the trauma-induced cellular and molecular changes in subcutaneous, visceral, interscapular, and perivascular adipose tissue using histology, immunohistochemistry, gene expression, and flow cytometry analysis. After one day, surgical trauma stimulated adipose tissue browning at the site of injury and, importantly, in the contralateral inguinal depot. Browning was not present after incision only, and was largely independent of surgery-associated body temperature and weight loss. Adipose trauma rapidly recruited monocytes to the injured site and promoted alternatively activated macrophages. Conversely, PDGF receptor-positive beige progenitors were reduced. In this study, we identify adipose trauma as an unexpected driver of selected local and remote adipose tissue browning, holding important implications for the biologic response to surgical injury.
Introduction
Understanding beige and brown adipocytes morphology and development has been a much-sought out goal as activating BAT and stimulating browning might reduce metabolic adverse effect of WAT in the context of obesity.Citation1-3 While WAT is the primary site of energy storage and of release of hormones and cytokines,Citation4 brown adipose tissue (BAT) is composed of multilocular brown adipocytes with high mitochondrial content and Uncoupling Protein-(UCP-)1, contributing to heat production in small mammals and humans in response to cold exposure.Citation1,5-7 In prevailing models of browning, the central nervous system triggers pathways such as sympathetic discharge, resulting in the release of norepinephrine (NE) and β-adrenergic receptor activation in WAT and BAT.Citation8 Beige adipocytes arise from both de novo adipogenesis of a subgroup of precursor cells expressing platelet-derived growth factor receptor α (PDGFRα), CD34, and Sca-1 Citation7,9,10,11,12 and pre-existing adipocytes (transdifferentiation),Citation13,14 depending on depot, diet and temperature. During browning, replacement of white adipocytes by de novo generated beige adipocytes is facilitated by alternatively activated macrophages.Citation10,15,16 In addition, alternatively activated macrophages have been shown to produce norepinephrine that is important for the induction of thermogenic geneCitation15,16 such as UCP1, PGC1Citation17, CD137,Citation7 Tbx1 Citation7 or Elovl3.Citation18
Surgical procedures ubiquitously injure adipose tissue. We recently reported that such injury dramatically impacts adipose phenotype (increased inflammation, decreased adipose-derived hormone expression), and that a multitude of clinically relevant factors (age, nutritional status, concomitant innate immunity activation) modulate this response.Citation19-21 Moreover, surgery evokes an acute stress response that activates sympathetic β-agonist adrenergic pathways both locally and systemically. It is possible that mammals have evolved to include adipose browning (induction of UCP-1 in WAT) as a component of their response to trauma, though the actual benefits of such an adaptation are unclear. We therefore used a validated mouse model of surgical injury Citation21 to study the role of adipose tissue repair in beige adipocyte development. We examined inguinal, epididymal, interscapular and perivascular adipose tissue before and one day after sham, incision alone, or surgical injury on the left inguinal adipose compartment to determine if anesthesia and various levels of focal tissue trauma associated with surgery led to any local and/or distant adipose browning.
Methods
Animal studies
Male 26-weeks-old C57BL/6 J mice (Jackson Laboratories, Bar Harbor, ME, USA) were housed in cages at 22°C and maintained on a 12-hour light-dark cycle for ≥1 week pre- experiment and throughout the experiment. They received standard Normal Chow (NC) of 10 kcal% fat (Cat#D12450B, 3.85 kcal/gm, Research Diet Inc.., New Brunswick, NJ), USA with ad libitum access to chow unless otherwise noted. Caloric restricted (CR) animals had free access to a sugar water solution for 72 hours prior to operation. This solution consisted of 12.3% sucrose (wt/vol, contained 0.45 kcal/mL; purity = 99.5%, Cat# S9378, Sigma-Aldrich, St. Louis, MO, USA).
A standardized, mouse model of surgical injury was employed as previously described.Citation21 Briefly, mice were anesthetized by isoflurane inhalation (2% induction, 1.25–1.5% maintenance) and body temperature maintained on a water-circulating heat pad, set at 42°C. Pre and post-operative blood glucose determinations were performed on fresh blood with an Easy Check Diabetes Meter Kit (Home Aide Diagnostics, Deerfield Beach, FL, USA) according to the manufacturer's instructions. The core body temperature was continuously monitored during procedures and before harvest with a rectal probe. In the surgical trauma group, a 2 × 1 cm2 L-shaped skin incision in left flank area was first made. After retracting the skin, ∼2 mm-thick subcutaneous inguinal adipose tissue was harvested (control baseline adipose), one for formalin fixation and one snap frozen in liquid nitrogen. The following standard surgical manipulations (sharp dissection/spreading/cutting/electrocauterization) were then applied to the remaining adipose tissue in the inguinal surgical field. First, blunt dissection into the fat was performed by spreading and closing a hemostat instrument 10 times. This was followed by cauterizing a 4 mm length along the edge of the adipose tissue using a handheld electrocautery instrument. Mice we kept under general anesthesia for 15 min total, then the skin was closed with 6–0 Vicryl absorbable suture, and the mouse allowed to recover.
To determine the effect of the anesthetic agent and potential peri-operative swings in body temperature, sham mice were kept under anesthesia for 15 min (as described above), then allowed to recover. Finally, to determine the effect of incision alone, mice were subjected to the same sized “L”-shaped incision, but no further dissection. Mice we kept under general anesthesia for 15 min total then the skin was closed with 6–0 absorbable suture. The animals were given a single dose of Buprenorphine (0.05–0.1 mg/kg) immediately after the surgery. One to 7 days following after the procedure, the animals were euthanized by cervical dislocation. Adipose tissue from the left (ipsilateral or local) and right (contralateral or distant) inguinal (subcutaneous), epididymal (visceral), interscapular (brown adipose positive control) and perivascular adjacent to the thoracic aorta was harvested and immediately fixed in formalin (one half) or snap frozen in liquid nitrogen (the other half). For the inguinal, epididymal and interscapular fat pad a third fraction was collected in an ice-cold DMEM media with 0.5% FBS for flow cytometry analysis. Morphometry was assessed by histology from 6 μm sections from formalin fixed, paraffin-embedded samples stained with hematoxylin and eosin (H&E).
All animal experiments were performed according to protocols approved by our local Institutional Animal Care and Use Committee and complied with the Guide for the Care and Use of Laboratory Animals (National Institutes of Health Publication No. 85–23, Revised 1996).
Plasma norepinephrine and triglyceride
Retro-orbital blood was collected under general anesthesia. Plasma was prepared from blood centrifugation at 2,000 g for 10 min at 4°C. Plasma levels of NE were measured using the manufacturer protocol (ELISA, BA E-5200, Rocky Mountain Diagnostics, Inc. Colorado Springs, CO, USA). Plasma levels of Triglyceride (TG) were measured using the manufacturer protocol (Triglyceride Quantification Kit, ab65336, Abcam, Cambridge, MA, USA).
Immunohistochemistry
Paraffin sections of adipose tissue were immunostained as previously describedCitation22,23using the following primary antibody: rabbit anti-mouse UCP1 (10983, abcam, Cambridge, MA, USA) at dilution 1:500 and secondary goat-anti rabbit antibody diluted 1:200 (Vector Laboratories, Inc. Burlingame, CA, USA). Negative controls were performed simultaneously.
Quantitative real-time PCR
Quantitative real-time PCR (Q-PCR) analysis was performed on adipose RNA as previously described.Citation24 Differences in total RNA or different efficiency of cDNA synthesis among samples were normalized using mouse Hprt1 expression and results expressed as ratio to baseline.
Adipose tissue isolation and flow cytometry
Harvested adipose tissues were prepared as previously described.Citation25 Adipose stromal-vascular fraction were blocked with CD16/32 monoclonal antibody (mAb) and stained for 30 min at 4°C in the dark with F4/80 APC, CD11b APC/Cy7, CD11c PeCy7, CD206 FITC, CD301 PE, PDGFRα PE, CD34 FITC and Sca1 PE/Cy7 (at 1:100) all from BioLegend (San Diego, CA, USA). Cells were washed and acquired immediately on an LSR II flow cytometer (BD Biosciences, San Jose, CA, USA) and analyzed with FlowJo.
Statistical analysis
Results are expressed as mean ± SEM. All statistical procedure we performed using Sigma Stat for Windows (version 3.5; Systat Software, Chicago, IL, USA) or GraphPad Prism 6.0 (GraphPad Software, San Diego, CA, USA). Student's t-test, one way/2 way/repeat measure ANOVA were performed for 2 and multi- group comparisons. Statistical significance was defined as p < 0.05.
Results
Surgical trauma induces local and distant adipose tissue browning
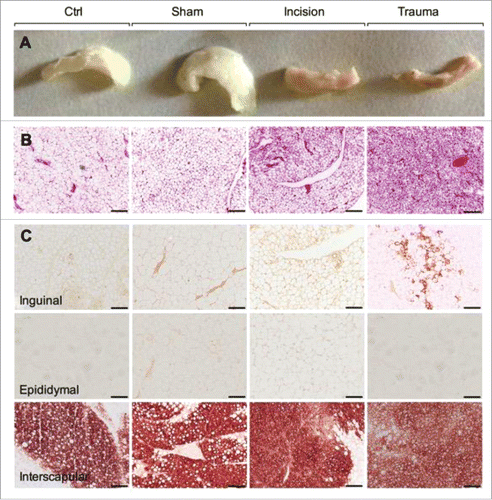
Mice were subjected to focal surgical trauma (sharp dissection/spreading/cutting/electro-cauterization including adipose biopsy), an incision only (to evaluate the effect pain and skin disruption), or sham (to evaluate the effect of anesthesia alone). Macroscopic examination of the inguinal fat pad showed evidence of browning with trauma and, to a lesser extent, one day after incision only (). H&E staining revealed single lipid droplet cells delineated by layers of connective tissue (red) in baseline and sham-treated inguinal depots (). In contrast, inguinal tissue sampled post-trauma, and to a much lesser extent, inguinal depots from the incision group, displayed multilocular adipocytes, consistent with a beige adipose phenotype (). Inguinal, epididymal and interscapular adipose depots were examined for the expression of UCP1, a protein characteristic of brown and beige adipocytes. There were no significant alterations in UCP1 protein in the interscapular adipose tissue (positive brown adipose control) or in the epididymal adipose tissue in sham, incision, and trauma mice (). The subcutaneous inguinal fat, however, had significantly increased levels of UCP1 protein and formation of multilocular adipocytes one day after the surgical injury confirming the inguinal fat pad browning ().
Figure 1. Surgical trauma induces adipose tissue browning. (A) Representative macroscopic morphology from paraffin embedded inguinal adipose tissues. From the left to the right: baseline, sham, incision and trauma group. (B) Representative H&E-stained inguinal adipose tissue sections which differentiated lipid droplets (white) from connective tissue (red). From the left to the right: baseline, sham, incision and trauma group. (C) Representative characterization of brown/beige adipocyte marker UCP1 by immunohistochemistry in inguinal (top), epididymal (middle), and interscapular (bottom) depots. Epididymal or white visceral and interscapular or brown fat represent negative and positive controls respectively. Data are representative of 10 independent animals per group. Bars represent 100 μm.
To investigate whether adipose phenotypic changes were manifest locally or systemically, we investigated distant depots. Surprisingly, H&E stained sections from contralateral inguinal tissue featured browning characteristics, with densely grouped multilocular adipocytes (, upper panel). This finding was confirmed using immunohistochemistry to detect UCP1 protein, which was increased at both the contralateral site as well as the trauma site (). Similarly, in the inguinal depot, brown and beige adipocytes markers UCP1, UCP2 and PGC1α as well as the beige-specific markers CD137 and TBX1 were increased on both sides (). In the interscapular adipose depot (BAT; positive control), a 3- and 15-fold increase in UCP1 and Elovl3 transcript respectively was observed after trauma (). No change was seen in the epididymal fat pad (data not shown). Recent reports suggest a thermogenic function for perivascular fat.Citation26 Thus, UCP1, UCP2 and Elovl3 gene expression in the adipose tissue adjacent to the thoracic aorta was interrogated. One day after left inguinal trauma, UCP1 transcript levels significantly increased in the perivascular fat. No difference was seen in UCP2 and Elovl3 gene expression (). We next investigated the contribution of weight, temperature and glucose. After one day, weight remained similar in the 4 groups (baseline, sham, incision and trauma, p > 0.05 versus day 0) (Fig. S1A). At harvest, compared to day 0, we observed a small decrease in body temperature in the incision group (0.45°C, p = 0.01) and in trauma group (0.37°C, p = 0.02) (Fig. S1B). Interestingly, blood glucose was decreased at day 1 and day 3 following left inguinal trauma, compared to the sham animals (Fig. S1C). Moreover, plasma triglyceride levels were reduced from day 1 and up to 7 days following surgery (Fig. S1D). To examine the relevance of these acute changes, we harvested inguinal adipose tissues at day 1, 3, and 7 following left inguinal trauma. Inguinal depots (left traumatized and right contralateral) displayed multilocular adipocytes that were positive for UCP1, consistent with a beige adipose phenotype () up to day 5. Browning was not detected at day 7 following trauma ()
Figure 2. Trauma induces distant browning. (A, upper panel) Representative H&E stained inguinal fat from baseline and one day post trauma (site and contralateral). Trauma site and contralateral showed reduce lipid droplets to form packed multilocular adipocytes. (A, lower panel) Immunostaining using antibody against UCP1 showed protein expression in the traumatized (trauma Site) and contralateral adipose tissue. (B-D) Q-PCR against UCP1, UCP2, PGC1α, CD137 and TBX1 gene in the inguinal adipose depot (B) and against UCP1, UCP2 and Elovl3 gene in the interscapular (C) and perivascular fat depot (D) at baseline and one day after left inguinal trauma (trauma site and contralateral). Expression values were normalized to their respective baseline. Data are mean±SEM of 5–6 independent animals per group. Bars represent 20 μm. *p < 0.05, **p < 0.01, ***p < 0.001 vs. baseline.
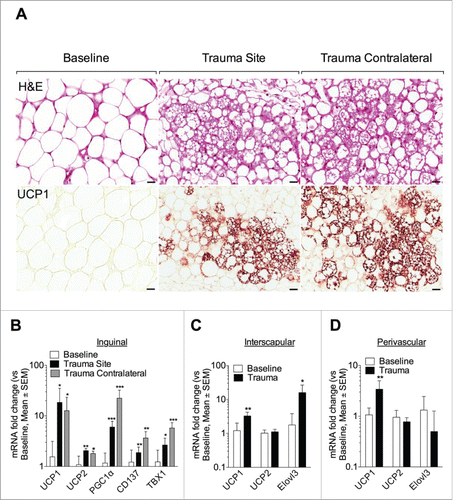
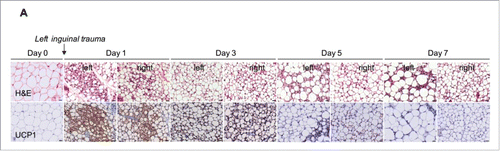
Figure 3. Trauma induces adipose browning up to day 5 following surgery. Representative H&E and UCP1-stained inguinal adipose tissue sections at different time points after surgical trauma as indicated. Data are representative of 4 independent animals per group. Bars represent 20 μm.
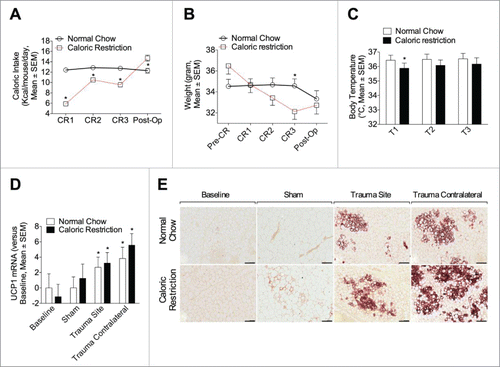
Under physiological conditions, cold exposure is the most powerful stimulus for BAT activation.Citation27 To evaluate the contribution of weight loss and reduced body temperature associated with the observed browning phenomenon, we subjected mice to a 3-day glucose-water-only regimen (caloric restriction, CR)Citation28 before the procedure. Here, Mice on CR consumed less calories (except on the post operative day), lost weight, and had a lower body temperature (). There were modest differences in UCP1 mRNA () or protein () levels after surgical trauma, compared to their respective NC control, likely related to their reduction in body temperature. However, the differential pales in comparison to the overall browning induction due to the surgical trauma.
Figure 4. Short-term caloric restriction minimally modulates adipose browning relative to surgical trauma. (A, B) Caloric intake (A) and weight (B) were measured in animal fed with a NC or CR one day before diet randomization (Pre-CR, weight only), one, 2 and 3 days after (CR1, 2 and 3) and at one day post-op (Post-op). Caloric intake is expressed as Kcal/mouse/day and weight as gram. (C) Body temperature was continuously monitored during procedures (15 min) with a rectal probe. T1 (2 ∼ 3 min after the rectal probe insertion), T2 (15 min after the rectal probe insertion), and T3 (the highest temperature during measurement) and is expressed in degrees Celsius (°C). Data are mean±SEM of 6 independent animals per group. *p < 0.05 versus NC. (D, E) Animals were randomly exposed to a 3 day CR or NC before the indicated procedure (sham or trauma). (D) Q-PCR analysis of UCP1 in inguinal adipose tissue, at baseline or at one day (sham, trauma site and trauma contralateral). (E) Representative UCP1 immunohistochemistry in inguinal adipose tissue. Upper panel: NC group at baseline, sham, trauma site and contralateral (from left to the right). Lower panel: CR group at baseline, sham, trauma site and contralateral (from left to the right). Data are representative of 10 independent animals per group. Bars represent 100 μm. *p < 0.05 vs. baseline.
Trauma-induced browning is independent of pdgfrα+-beige precursor invasion
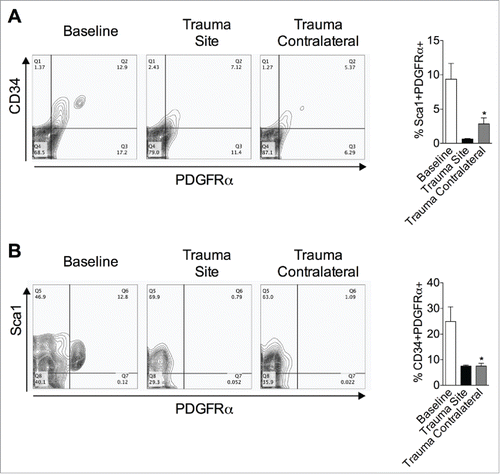
Beige adipocytes arise from both de novo precursors Citation10 and pre-existing adipocytes (transdifferentiation).Citation13 Thus, expression of PDGFRα+, CD34+ and Sca1+ was assessed in inguinal WAT at baseline and one day after surgery () to characterize putative newly recruited beige precursors. One day after the procedure, both PDGFRα+CD34+ () and PDGFRα+Sca1+ () cells were reduced in the left (trauma site) and right (trauma contralateral) inguinal depot, arguing against an involvement of these precursors.
Figure 5. PDGFRα+ cells decrease after surgical injury. (A, B) Cells were isolated from inguinal adipose tissue at baseline and one day post left inguinal injury (trauma site) and analyzed for the expression of PDGFRα, CD34 (A) and Sca1 (B). The percentages of cells are indicated on the flow profile. The graphs show percentage of cells in inguinal depot at baseline and one day after left inguinal trauma (trauma site). Data are mean±SEM of 5–10 independent animals per group. *p < 0.05 versus baseline.
Focal trauma is associated with monocyte infiltration and alternatively activated macrophage at the site of injury only, and not at remote browning locations
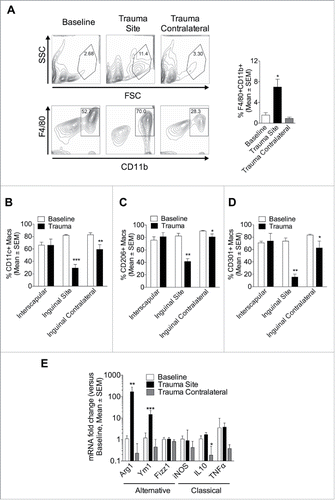
The adipose remodeling toward browning is believed to involve alternatively activated macrophage as well as beige precursor recruitment.Citation10 To investigate macrophage contribution to trauma-induced browning, flow cytometry was conducted at baseline and on inguinal, epididymal, and interscapular fat one day after left inguinal fat pad trauma. At baseline (control), interscapular tissue contained fewer macrophages than epidymal or inguinal (Fig. S2A). One day after trauma a 4-fold increase in F4/80+CD11b+ macrophages at the site of injury was observed (). However, macrophages were not increased in the contralateral inguinal fat (). In baseline inguinal fat (control), most macrophages were CD11c+ (82%), CD206+ (82%), and 73% were CD301+. In contrast, one day after trauma, the majority of infiltrating macrophages did not express these surface markers ( and Fig. S2B–D), which is typical of newly infiltrated monocytes that have not yet upregulated these differentiation markers. In the contralateral inguinal site, there was a slight reduction in macrophage markers (CD11c+, CD206+ and CD301+), suggesting that there was some infiltration of monocytes, although this was minimal (overall macrophage numbers were not increased). One day after the procedure, gene expression profiling revealed increased expression of the alternatively activated macrophage markers Arginase (Arg) 1 and Ym1 mRNA in the traumatized inguinal depot only (). In contrast, expression of classically activated markers, inducible nitric oxide synthase (iNOS), Interleukin-(IL-)10 and Tumor necrosis factor (TNF) genes were unchanged, except for IL-10 reduced in the contralateral depot (). Together, these findings suggest that blood-borne monocytes infiltrated the site of trauma, displaying an alternatively activated phenotype. However, adipose can brown without local macrophage infiltration under the conditions of this focal trauma model.
Figure 6. Surgical trauma promotes local monocyte recruitment and alternatively activated macrophages, but the remote browning can occur without increased macrophages in the browning adipose. (A-D) Cells were isolated from adipose tissue at baseline and one day post left inguinal injury (trauma site) and labeled for flow cytometry. (A) FACS analysis on cell surface marker expression of F4/80 and CD11b at baseline and one day after surgery. The graphs show percentage of F4/80+CD11b+ macrophages in inguinal depot at baseline and one day after left inguinal trauma (trauma site). (B-D) Macrophages were then assessed for the expression of CD11c (B), CD206 (C) and CD301 (D). Data are mean±SEM of 10 independent animals per group. *p < 0.05, **p < 0.01 vs. baseline. (E) Q-PCR against alternatively activated Arg1, Ym1, Fizz1 and against classically activated iNOS, IL-10, TNF macrophages genes in inguinal depot at baseline and one day after left inguinal trauma (trauma site and contralateral). Expression values were normalized to their respective baseline. Data are mean±SEM of 6 independent animals per group. *p < 0.05, **p < 0.01, ***p < 0.001 versus baseline.
Systemic NE levels are increased after trauma but not after incision alone
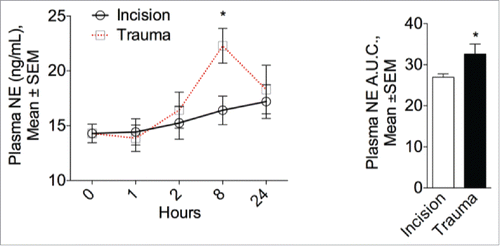
Although adipose depot browning appears to related to β-adrenergic activation by the nervous system, exposure to cold has been reported to promote alternatively activated macrophages that produce NE.Citation16 We reasoned that the local increase in alternatively activated macrophages after trauma may impact NE levels. Consequently, plasma NE plasma was measured at one, 2, 8 hours and one day after the procedure. In the surgical trauma group, circulating NE plasma levels peaked after 8 hours but remained unaffected after incision ().
Figure 7. Surgical trauma increases plasma norepinephrine levels. Left panel: Physiological modulation of the sympathetic system in mice one, 2, 8 hours and one day after surgery or incision. NE circulating plasma levels peaked after 8 hours in the surgical trauma group but remained unaltered after incision compared to baseline (0 hour). Right panel: Quantitative assessment of plasma NE expressed as the area under the curve (A.U.C.). Data are mean±SEM of 5 independent animals per group and time point. *p < 0.05 vs. incision.
Discussion
Using a validated murine model of surgical injury, we show that, after one day, surgical trauma induces WAT browning. Perhaps most striking are our findings that browning also occurs in the distant contralateral inguinal and perivascular adipose depots, suggesting a systemic physiologic adaption in response to focal adipose trauma.
In adult humans, in addition to its role in non-shivering thermogenesis, the amount of BAT is inversely correlated with body-mass index.Citation4,27 In mice, both activation and transplantation of BAT were shown to improve whole body metabolism and insulin sensitivity.Citation1,29 Furthermore, human white adipocytes can be manipulated in vitro to develop “brown” characteristics (UCP1 expression) by forced adenoviral overexpression of PGC-1α.Citation30,31 As a result, promotion of BAT (through an increase in BAT mass or turning WAT into BAT) is increasingly acknowledged as a potential therapeutic strategy for obesity and obesity related diseases.Citation4,6,27,29,32
Importantly, these data evidence remarkably rapid (one day) adipose tissue browning. Surgical injury leads to a 12-fold induction of UCP1 transcript after one day, which can be compared to the 9-fold induction in the same strain mice acclimated to 5°C cold for 4 weeks Citation33 or the 2.5 fold induction after 2 weeks of treadmill exercise in BALB/C mice.Citation18 Of note, this acute browning persisted up to day 5 following the trauma. Moreover, plasma triglyceride were reduced up to day 7 following surgery, which could be linked to the adipose browning, although more definitive studies are required. In the interscapular adipose depot there was a 3-fold increase in UCP1 transcript after trauma. Though this tissue has baseline high UCP1 expression, this upregulation by remote surgical trauma may have substantial impact on whole body UCP1 content. Beige cells that are induced by cold exposure or β3 agonist treatment arise through transdifferentiation Citation13 of existing white adipocytes or by de novo adipogenesis from a subgroup of precursor cells.Citation7,11,12 Here, adipose tissue injury resulted in loss of precursor PDGFRα+CD34+ and PDGFRα+Sca1+ cells, suggesting that those precursors are likely not responsible for surgery-induced browning, as suggested by previous reports.Citation13,14
Surprisingly, surgical trauma not only induces browning locally, but also in the contralateral inguinal and perivascular adipose depots, suggesting that surgical injury triggers systemic pathway(s). Cold exposure is the most powerful and physiological stimulus for BAT activation Citation27 and changes in body temperature may modulate adaptive thermogenesis. At harvest, compared to day 0, a small decrease in the incision group (mean 0.45°C) and in trauma group (mean 0.37°C) core temperatures were measured. However, as browning was not widely induced after incision, it is unlikely that these fluctuation in body temperature are causally involved in the distant browning in inguinal and perivascular adipose depots. Furthermore, CR modestly modulated adipocyte phenotype despite weight loss and mild hypothermia, thus validating the direct influence of adipose trauma on browning.
Macrophages are instrumental in facilitating healing and are invariably recruited to sites of injury and inflammation. In addition to clear dying white adipocytes, and recruit new beige adipocytes from progenitors,Citation7,10-12 alternatively activated macrophages have been implicated in the browning process, acting as an alternative catecholamine source.Citation16 In the current study, after one day, the injured inguinal depot displayed a 4-fold increase in F4/80+CD11b+ monocytes/macrophages. Despite adipose browning, macrophages were not increased in the contralateral inguinal fat. The physiologic stimulation of β-adrenergic receptors on adipocytes activates PGC1α to induce the expression of UCP1.Citation18 We found that trauma specifically led to transiently increased plasma NE levels, peaking 8 hours after surgical injury but remained unchanged after incision only. If systemic catecholamines drive browning, then one would expect all WAT to brown, and this clearly is not the case. Thus, if NE is mechanistically tied to browning then there are likely compartmental effects that are beyond the resolution of the current experiments. Interestingly, in the inguinal adipose depot, this correlated with the apparition of multilocular adipocytes, consistent with a beige adipocyte phenotype and suggesting a β-adrenergic-dependent mechanism. In light of increased alternatively activated macrophages at the site of trauma it is possible that the local production of these cells led to increased systemic NE levels, and thus, distant browning in the contralateral inguinal and perivascular adipose depots. Thus, a limitation of our report is that we measured systemic NE, though differential local compartment signaling levels of NE may be more relevant for the tissue specific browning findings.
A limitation to the current report is that we examine only one type of focal surgical trauma, and other injuries (e.g. laparotomy) and aspects of browning (functional endpoints beyond simple browning markers) may yield additional insights. Only a single age and sex mouse was studied, and largely just the early 24 hour time point (strategy based on our prior report Citation34). Full examination of cellular dynamics regarding cell recruitment, maturation, and death is limited at a single time point, but the single snapshot does give some insights into the underlying cellular dynamics. Additionally, examination of browning in other dietary settings (diet induced obesity, diabetic mouse models, etc.) would inform on clinically relevant potential variations in the browning response.
In summary, we provide evidence that adipose tissue injury leads to local and, importantly, distant adipose tissue browning as early as one day after the procedure. Such browning may influence local metabolism, tissue repair, and/or resistance to infection. Strategies to manipulate adipose remodeling after trauma may enhance the biologic response to interventions such as surgery. Moreover, identifying the molecular relationship between adipose trauma and browning provides opportunities for the understanding and treatment of metabolic diseases.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
1111971_Supplemental_Material.docx
Download MS Word (1.2 MB)Funding
This work was supported by grants from the NIH (NIDDK DK090629; NIA AG036712) to JRM, NIH (National Heart, Lung, and Blood Institute T32HL007734, 1F32HL117521) to CRM, the American Heart Association (12GRNT9510001, 12GRNT1207025) and the Lea Carpenter DuPont Vascular Surgery Fund to CKO, NIH R01-AG25872 to BSK, the Deutsche Forschungsgemeinschaft Research Fellowship (BA 4925/1–1) to AB and the Swiss National Science Foundation (P1LAP3_158895) to AL.
References
- Bartelt A, Bruns OT, Reimer R, Hohenberg H, Ittrich H, Peldschus K, Kaul MG, Tromsdorf UI, Weller H, Waurisch C, et al. Brown adipose tissue activity controls triglyceride clearance. Nat Med 2011; 17(2): 200-5. Epub 2011/01/25; PMID:21258337; http://dx.doi.org/10.1038/nm.2297
- Lidell ME, Betz MJ, Dahlqvist Leinhard O, Heglind M, Elander L, Slawik M, Mussack T, Nilsson D, Romu T, Nuutila P, et al. Evidence for two types of brown adipose tissue in humans. Nat Med 2013; 19(5): 631-4. Epub 2013/04/23; PMID:23603813; http://dx.doi.org/10.1038/nm.3017
- Li J, Guo BC, Sun LR, Wang JW, Fu XH, Zhang SZ, Poston G, Ding KF. TNM staging of colorectal cancer should be reconsidered by T stage weighting. World J Gastroenterol: WJG. 2014; 20(17): 5104-12. Epub 2014/05/08; PMID:24803826
- Cypess AM, Lehman S, Williams G, Tal I, Rodman D, Goldfine AB, Kuo FC, Palmer EL, Tseng YH, Doria A, et al. Identification and importance of brown adipose tissue in adult humans. New Eng J Med 2009; 360(15): 1509-17; PMID:19357406; http://dx.doi.org/10.1056/NEJMoa0810780
- Shabalina IG, Petrovic N, de Jong JM, Kalinovich AV, Cannon B, Nedergaard J. UCP1 in brite/beige adipose tissue mitochondria is functionally thermogenic. Cell Rep 2013; 5(5): 1196-203. Epub 2013/12/03; PMID:24290753; http://dx.doi.org/10.1016/j.celrep.2013.10.044
- Ye L, Wu J, Cohen P, Kazak L, Khandekar MJ, Jedrychowski MP, Zeng X, Gygi SP, Spiegelman BM, et al. Fat cells directly sense temperature to activate thermogenesis. Proc Natl Acad Sci U S A 2013; 110(30): 12480-5. Epub 2013/07/03; PMID:23818608; http://dx.doi.org/10.1073/pnas.1310261110
- Wu J, Bostrom P, Sparks LM, Ye L, Choi JH, Giang AH, Khandekar M, Virtanen KA, Nuutila P, Schaart G, et al. Beige adipocytes are a distinct type of thermogenic fat cell in mouse and human. Cell 2012; 150(2): 366-76. Epub 2012/07/17; PMID:22796012; http://dx.doi.org/10.1016/j.cell.2012.05.016
- Bartelt A, Heeren J. Adipose tissue browning and metabolic health. Nat Rev Endocrinol 2014; 10(1): 24-36. Epub 2013/10/23; PMID:24146030; http://dx.doi.org/10.1038/nrendo.2013.204
- Liu H, Zhou L, Shi S, Wang Y, Ni X, Xiao F, Wang S, Li P, Ding K, et al. Oligosaccharide G19 inhibits U-87 MG human glioma cells growth in vitro and in vivo by targeting epidermal growth factor (EGF) and activating p53/p21 signaling. Glycobiology. 2014; 24(8): 748-65. Epub 2014/05/07; PMID:24799378; http://dx.doi.org/10.1093/glycob/cwu038
- Lee YH, Petkova AP, Granneman JG. Identification of an adipogenic niche for adipose tissue remodeling and restoration. Cell Metabol 2013; 18(3): 355-67. Epub 2013/09/10; PMID:24011071; http://dx.doi.org/10.1016/j.cmet.2013.08.003
- Wang QA, Tao C, Gupta RK, Scherer PE. Tracking adipogenesis during white adipose tissue development, expansion and regeneration. Nat Med 2013; 19(10): 1338-44. Epub 2013/09/03; PMID:23995282; http://dx.doi.org/10.1038/nm.3324
- Xia Y, Wang X, Wen X, Ding K, Zhou J, Yang Y, Zhang Y. Overall functional gene diversity of microbial communities in three full-scale activated sludge bioreactors. Appl Microbiol Biotechnol 2014; 98(16): 7233-42. Epub 2014/05/13; PMID:24816624; http://dx.doi.org/10.1007/s00253-014-5791-7
- Rosenwald M, Perdikari A, Rulicke T, Wolfrum C. Bi-directional interconversion of brite and white adipocytes. Nat Cell Biol 2013; 15(6): 659-67. Epub 2013/04/30; PMID:23624403; http://dx.doi.org/10.1038/ncb2740
- Ding S, Zhao Z, Sun D, Wu F, Bi D, Lu J, Xing N, Sun L, Wu H, Ding K, et al. Eg5 inhibitor, a novel potent targeted therapy, induces cell apoptosis in renal cell carcinoma. Tumour Biol 2014; 35(8): 7659-68. Epub 2014/05/08; PMID:24801905
- Xun H, Ding KZ, Yang M, Chen HB. [Histological observation of the central nervous system in Simulium (Wilhelmia) xingyiense (Diptera:Simuliidae)]. Zhongguo ji sheng chong xue yu ji sheng chong bing za zhi = Chinese journal of parasitology & parasitic diseases. 2013; 31(6): 443-7. Epub 2014/05/14; PMID:24818409
- Nguyen KD, Qiu Y, Cui X, Goh YP, Mwangi J, David T, Mukundan L, Brombacher F, Locksley RM, Chawla A, et al. Alternatively activated macrophages produce catecholamines to sustain adaptive thermogenesis. Nature 2011; 480(7375): 104-8. Epub 2011/11/22; PMID:22101429; http://dx.doi.org/10.1038/nature10653
- Ding K, Wang H, Xu J, Li T, Zhang L, Ding Y, Zhu L, He J, Zhou M. Melatonin stimulates antioxidant enzymes and reduces oxidative stress in experimental traumatic brain injury: the Nrf2-ARE signaling pathway as a potential mechanism. Free Rad Biol Med 2014; 73: 1-11. Epub 2014/05/09
- Bostrom P, Wu J, Jedrychowski MP, Korde A, Ye L, Lo JC, Rasbach KA, Boström EA, Choi JH, Long JZ, et al. A PGC1-alpha-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature 2012; 481(7382): 463-8. Epub 2012/01/13; PMID:22237023; http://dx.doi.org/10.1038/nature10777
- Mauro CR, Nguyen B, Yu P, Tao M, Gao I, Seidman MA, Nguyen LL, Ozaki CK. Inflammatory “adiposopathy” in major amputation patients. Annals Vascul Sur 2013; PMID:23498310
- Mauro CR, Ilonzo G, Nguyen BT, Yu P, Tao M, Gao I, Seidman MA, Nguyen LL, Ozaki CK. Attenuated adiposopathy in perivascular adipose tissue compared with subcutaneous human adipose tissue. Am J Surg. 2013; PMID:23352378
- Nguyen B, Tao M, Yu P, Mauro C, Seidman MA, Wang YE, Mitchell J, Ozaki CK. Preoperative diet impacts the adipose tissue response to surgical trauma. Surgery 2013; PMID:23274098
- Longchamp A, Alonso F, Dubuis C, Allagnat F, Berard X, Meda P, Saucy F, Corpataux JM, Déglise S, Haefliger JA. The use of external mesh reinforcement to reduce intimal hyperplasia and preserve the structure of human saphenous veins. Biomaterials 2014. Epub 2014/01/17; PMID:24429385
- Tao M, Mauro CR, Yu P, Favreau JT, Nguyen B, Gaudette GR, Ozaki CK. A simplified murine intimal hyperplasia model founded on a focal carotid stenosis. Am J Pathol 2013; 182(1): 277-87; PMID:23159527; http://dx.doi.org/10.1016/j.ajpath.2012.10.002
- Tao M, Yu P, Nguyen BT, Mizrahi B, Savion N, Kolodgie FD, Virmani R, Hao S, Ozaki CK, Schneiderman J. Locally Applied Leptin Induces Regional Aortic Wall Degeneration Preceding Aneurysm Formation in Apolipoprotein E-Deficient Mice. Arterioscler Thromb Vasc Biol. 2012. Epub 2012/12/12; PMID:23220275
- Kalwa H, Sartoretto JL, Martinelli R, Romero N, Steinhorn BS, Tao M, Ozaki CK, Carman CV, Michel T. Central role for hydrogen peroxide in P2Y1 ADP receptor-mediated cellular responses in vascular endothelium. Proc Natl Acad Sci U S A. 2014; 111(9): 3383-8. Epub 2014/02/20; PMID:24550450; http://dx.doi.org/10.1073/pnas.1320854111
- Chang L, Villacorta L, Li R, Hamblin M, Xu W, Dou C, Zhang J, Wu J, Zeng R, Chen YE. Loss of perivascular adipose tissue on peroxisome proliferator-activated receptor-gamma deletion in smooth muscle cells impairs intravascular thermoregulation and enhances atherosclerosis. Circulation 2012; 126(9): 1067-78. Epub 2012/08/03; PMID:22855570; http://dx.doi.org/10.1161/CIRCULATIONAHA.112.104489
- Yoneshiro T, Aita S, Matsushita M, Kayahara T, Kameya T, Kawai Y, Iwanaga T, Saito M. Recruited brown adipose tissue as an antiobesity agent in humans. J Clin Invest 2013; 123(8): 3404-8. Epub 2013/07/23; PMID:23867622; http://dx.doi.org/10.1172/JCI67803
- Verweij M, van de Ven M, Mitchell JR, van den Engel S, Hoeijmakers JH, Ijzermans JN, de Bruin RW. Glucose supplementation does not interfere with fasting-induced protection against renal ischemia/reperfusion injury in mice. Transplantation 2011; 92(7): 752-8. Epub 2011/09/20; PMID:21926943; http://dx.doi.org/10.1097/TP.0b013e31822c6ed7
- Stanford KI, Middelbeek RJ, Townsend KL, An D, Nygaard EB, Hitchcox KM, Markan KR, Nakano K, Hirshman MF, Tseng YH, Langin D. Brown adipose tissue regulates glucose homeostasis and insulin sensitivity. J Clin Invest 2013; 123(1): 215-23. Epub 2012/12/12; PMID:23221344; http://dx.doi.org/10.1172/JCI62308
- Tiraby C, Tavernier G, Lefort C, Larrouy D, Bouillaud F, Ricquier D, Langin D. Acquirement of brown fat cell features by human white adipocytes. J Biol Chem 2003; 278(35): 33370-6. Epub 2003/06/17; PMID:12807871; http://dx.doi.org/10.1074/jbc.M305235200
- Cao L, Choi EY, Liu X, Martin A, Wang C, Xu X, During MJ. White to brown fat phenotypic switch induced by genetic and environmental activation of a hypothalamic-adipocyte axis. Cell Metabol 2011; 14(3): 324-38. Epub 2011/09/13; PMID:21907139; http://dx.doi.org/10.1016/j.cmet.2011.06.020
- van der Lans AA, Hoeks J, Brans B, Vijgen GH, Visser MG, Vosselman MJ, Hansen J, Jörgensen JA, Wu J, Mottaghy FM. Cold acclimation recruits human brown fat and increases nonshivering thermogenesis. J Clin Invest 2013; 123(8): 3395-403. Epub 2013/07/23; PMID:23867626; http://dx.doi.org/10.1172/JCI68993
- Vegiopoulos A, Muller-Decker K, Strzoda D, Schmitt I, Chichelnitskiy E, Ostertag A, Berriel Diaz M, Rozman J, Hrabe de Angelis M, Nüsing RM, et al. Cyclooxygenase-2 controls energy homeostasis in mice by de novo recruitment of brown adipocytes. Science 2010; 328(5982): 1158-61. Epub 2010/05/08; PMID:20448152; http://dx.doi.org/10.1126/science.1186034
- Nguyen B, Tao M, Yu P, Mauro C, Seidman MA, Wang YE, Mitchell J, Ozaki CK. Preoperative diet impacts the adipose tissue response to surgical trauma. Surgery 2013; 153(4): 584-93. Epub 2013/01/01; PMID:23274098; http://dx.doi.org/10.1016/j.surg.2012.11.001
