ABSTRACT
Molecular scaffolds are often viewed as passive signaling molecules that facilitate protein-protein interactions. However, new evidence gained from the use of loss-of-function or gain-of-function models is dispelling this notion. Our own recent discovery of 14-3-3ζ as an essential regulator of adipogenesis highlights the complex roles of this member of the 14-3-3 protein family. Depletion of the 14-3-3ζ isoform affected parallel pathways that drive adipocyte development, including pathways controlling the stability of key adipogenic transcription factors and cell cycle progression. Going beyond adipocyte differentiation, this study opens new avenues of research in the context of metabolism, as 14-3-3ζ binds to a variety of well-established metabolic proteins that harbor its canonical phosphorylation binding motifs. This suggests that 14-3-3ζ may contribute to key metabolic signaling pathways, such as those that facilitate glucose uptake and fatty acid metabolism. Herein, we discuss these novel areas of research, which will undoubtedly shed light onto novel roles of 14-3-3ζ, and perhaps its related family members, on glucose homeostasis.
Introduction
Adipocytes, residing in white fat or brown fat depots, have essential roles in regulating glucose and lipid homeostasis. The primary role of white adipocytes is to function as lipid stores for energy; however, the identification of secreted adipokines, such as leptin and adiponectin, has led to the classification of adipocytes as endocrine cells.Citation1,2 Brown adipocytes primarily oxidize fatty acids through non-shivering thermogenesis, but secreted factors specific to brown adipocytes have been recently discovered which expands on its physiological role.Citation3,4 Multiple signal transduction pathways and effector molecules are required in either adipocyte type to facilitate their physiological functions; impairments in the function or expression of signaling effectors may promote the development of abnormal conditions, such as insulin resistance, hyperlipidemia, and obesity.Citation5 Despite the importance of these signaling effectors, it is not fully understood how their activation or localization are spatially or temporally coordinated within a cell, nor is it known what are the endogenous molecules present within the cell to ensure the accurate transduction of downstream signals.
Physiological roles of molecular scaffolds
Molecular scaffolds are well suited to coordinate complex signaling networks due to their ability to promote interactions between signaling effectors and to control the subcellular localization of transcription factors and kinases.Citation6 Despite these critical functions, the requirement of scaffolds in physiology, and more specifically in the regulation of glucose homeostasis and lipid metabolism, has not been investigated in great detail. This is partly due to the paucity of adequate genetic knockout or transgenic models or the assumption that such proteins have only minor roles in the physiological pathways governing glucose homeostasis and metabolism. Scaffolds that have been examined for roles in glucose homeostasis include Akap150, β-arrestin-1, and NLRP,Citation7-9 but there are still numerous unexplored families of scaffolds, such as 14-3-3 proteins, that may contribute to key events that control metabolism.
14-3-3ζ is a critical regulator of adipocyte differentiation
Since their discovery from brain extracts, all seven 14-3-3 proteins, which are encoded by distinct genes, have been examined as potential biomarkers for various neurological diseases and some forms of cancer.Citation10-12 The scaffolding function of 14-3-3 proteins stems from their unique ability to bind to phosphorylated proteins harboring canonical phosphorylated serine or threonine motifs.Citation13 This permits their interaction with receptors, kinases, transcription factors, and ion channels to regulate their activity, stability, and localization within a cell.Citation10,14 When taken together, these pleiotropic functions emphasize that 14-3-3 proteins go beyond being ‘simple’ scaffolds within cells. Loss-of-function and gain-of-function studies have also identified critical roles of 14-3-3 proteins in cell survival and the regulation of autophagy in numerous cell types.Citation15-17 Prior to our study, it was not known if 14-3-3 proteins were involved in metabolic signaling pathways in vivo.
Our efforts to understand the role of 14-3-3 proteins in metabolism started with our report that 14-3-3ζ was essential for pancreatic β-cell survival in vitroCitation16 and we hypothesized that deletion of this isoform in vivo would lead to profound β-cell death and frank diabetes. Instead, the primary defect in vivo was decreased visceral adiposity and the presence of poorly differentiated adipocytes.Citation18 This was due in part to the aberrant expression of the hedgehog signaling effector, Gli3, and the cyclin-dependent kinase inhibitor, p27Kip1, in mesenchymal adipose precursor cells and also from the increase in autophagy-mediated degradation of C/ebpδ, a key transcription factor that promotes Pparγ expression.Citation18,19 The ability of 14-3-3ζ to control differentiation was not surprising given the ability of 14-3-3 proteins to control differentiation in other cell types,Citation20-22 but prior to our study, the role of 14-3-3ζ in the adipocyte had not been examined.
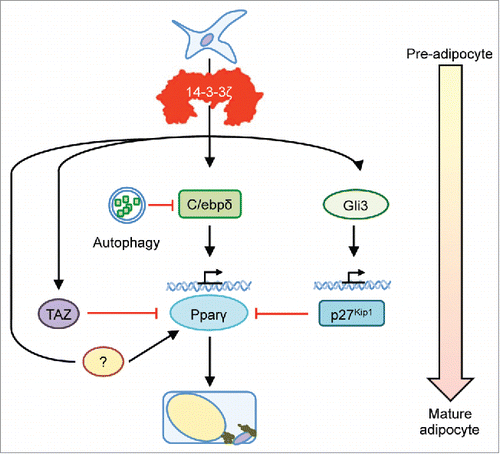
Given its ability to interact with multiple phosphorylated proteins harboring its canonical target motifs, a 14-3-3ζ may also coordinate other parallel pathways that drive the development of a mature adipocyte. For example, TAZ (transcriptional coactivator with PDZ-binding motif) is a 14-3-3 binding protein that is known to modulate mesenchymal stem cell differentiation by competing with Pparγ, the canonical adipogenic transcription factor ().Citation19,23 Binding of TAZ to 14-3-3 proteins induces its sequestration and permits Pparγ to initiate the adipogenic program. Deletion of 14-3-3ζ may promote the anti-adipogenic actions of TAZ in adipose precursor cells, but how the activity of TAZ relates to those of Gli3 and p27Kip1 is not known. Thus, further work is required to identify the interactome of 14-3-3ζ during adipogenesis, and this will define which pathways are regulated by 14-3-3ζ and potentially lead to the identification of new targets to affect adipocyte differentiation.
Figure 1. Potential involvement of 14-3-3ζ in alternative pathways controlling adipogenesis. Although we reported the upstream actions of 14-3-3ζ on C/ebpδ stability and Gli3-regulated p27Kip1 activity during adipogenesis, the ability of 14-3-3ζ to primarily bind to phosphorylated proteins harboring its canonical motifs, and to a lesser extent non-phoshorylated proteins, suggest that it may regulate the activity of known and unknown adipogenic factors. For example, TAZ, which competes with Pparγ occupancy during adipocyte differentiation, is one such 14-3-3 protein binding partner. Discovery of the 14-3-3ζ interactome during adipogenesis may aid in the discovery of novel effects of adipocyte differentiation.
The idea that distinct pools of adipose precursor cells exist in various adipose depots has been confirmed by elegant studies using in vivo lineage tracing or fluorescence-activated cell sorting approaches.Citation24,25 Remarkably, we found that the requirement for 14-3-3ζ was specific to visceral adipose tissue depots, while having no apparent effect on the sub-cutaneous fat we examined. Our study demonstrated that 14-3-3ζ regulates one distinct group of precursor cells (or all precursor cells in a partial manner) because systemic deletion of 14-3-3ζ did not fully ablate all visceral adipose tissue in vivo.Citation18 Progenitor cell heterogeneity in visceral adipose tissue depots has been recently reported by Chau and colleagues, whereby they identified a subset of progenitors that express Wt1.Citation26 In our study, knockout of 14-3-3ζ promoted the aberrant expression of Gli3 and p27Kip1 in adipose precursor cells, but the fact that adipogenesis still occurred suggests that not all adipocytes rely on 143–3ζ for differentiation. This apparent heterogeneity is further reinforced by a recent finding from the Scherer lab, where they identified distinct transcriptional programs that control embryonic versus adult adipocyte maturation.Citation27 Although both Pparγ and C/ebpα have long been postulated to be critical for adipogenesis during embryogenesis or adulthood, mice deficient in C/ebpα displayed essential functions for this transcription factor only in compensatory adipocyte hypertrophy or hyperplasia in adulthood, and not in terminal adipocyte differentiation during embryogenesis.Citation27 The observation that 14-3-3 proteins found to complex with C/ebpβ prior to binding to the Pparγ promoterCitation18,28 suggests a bias of 14-3-3 proteins in the embryonic development of adipocytes. Our study reinforces this notion because the expansion of fat mass following high-fat diet feeding was normal in 14-3-3ζ knockout mice, which indicates normal function of residual adult adipocytes.Citation18 Nonetheless, further work is required to determine the identity of precursors that are dependent on 14-3-3ζ activity.
Indirect role in browning of white adipose tissue?
Factors and mechanisms controlling the conversion of subcutaneous white adipocytes to brown-like, or beige, adipocytes have been an area of great research in adipocyte biology,Citation3 but it is still not fully understood what are the endogenous processes that regulate this event. Reduced circulating insulin, intermittent cold exposure and circulating FGF21 are known to promote browning of white adipocytes,Citation3,4,29 and it has recently been shown that FTO may also have critical roles in mediating this process in human adipocytes.Citation30 Elegant work from Qiu and colleagues described the contributions of the innate immune system on the development of beige fat.Citation31 They identified the importance to catecholamine production from macrophages on browning of subcutaneous fat through the use of mice with macrophages that lack tyrosine-hydroxylase, a key enzyme in catecholamine production.Citation31 Interestingly, one of the earliest established functions of 14-3-3 proteins is their ability to modulate the activity of tyrosine hydroxylase.Citation32 14-3-3ζ knockout mice did not display differences in energy expenditure; however, this observation was obtained at 23°C.Citation18 Further studies performed with intermittent or prolonged cold exposure are required to examine the requirement of 14-3-3ζ on tyrosine-hydroxylase-dependent browning of white adipocytes.
Regulation of insulin-stimulated glucose uptake by 14-3-3ζ
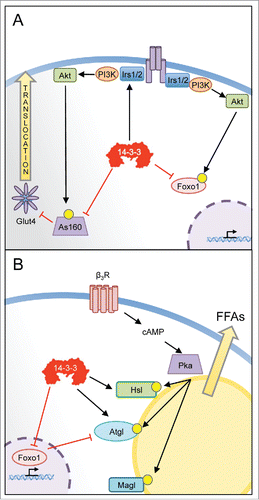
Given the ability of 14-3-3 proteins to bind to various components of the insulin signaling network (),Citation33-38 14-3-3 proteins could have important roles in various insulin-mediated metabolic effects in adipocytes. This was apparent in 14-3-3ζKO mice, as they displayed decreased insulin sensitivity following administration of peripheral exogenous insulin,Citation18 and likely indicates defects in GLUT4-mediated glucose uptake in skeletal muscle or fat. It is uncertain as to whether of deletion of 14-3-3ζ in vivo affects proximal or distal events in the insulin signaling pathway that control glucose uptake, but 14-3-3 proteins are known to bind to and promote the stability of insulin receptor substrate (IRS) molecules, which are key proximal effectors in this pathway.Citation33,34 Deletion of 14-3-3ζ in adipocytes may also impact distal events controlling in the translocation of GLUT4 to the plasma membrane for glucose uptake. The Rab GAP AS160/TBC1D4 controls the activity of Rab molecules involved in transport of GLUT4 containing vesicles to the plasma membrane, following its phosphorylation by Akt, its inhibitory activity is attenuated by interacting with 14-3-3 proteins. Mutations of key residues on AS160/TBC1D4 that mediate 14-3-3 binding promotes reduced insulin sensitivity and glucose uptake.Citation35,36 Thus, deletion of 14-3-3ζ in adipocytes and muscle may promote the inhibitory actions of AS160/TBC1D4 on insulin-stimulated glucose uptake.
Figure 2. Potential actions of 14-3-3ζ in the mature adipocyte. (A) 14-3-3ζ is known to interact with various insulin signaling effectors that facilitate glucose uptake. Biochemical studies have shown that 14-3-3ζ and other isoforms can regulate the stability of insulin receptor substrate molecules (IRS)-1 or -2, which are key proximal effectors. Following their phosphorylation by Akt, 14-3-3 proteins have been shown to control the transcriptional activities of Foxo1, by promoting its retention in the cytosol. 14-3-3 proteins have also been shown to control the inhibitory actions of the Rab-GAP As160/TBD1C4, which regulates the translocation of Glut4-containing vesicles to the plasma membrane for glucose uptake. (B) By recognizing phosphorylated forms hormone sensitive lipase (Hsl) or adipose triglyceride lipase (Atgl), 14-3-3ζ may directly regulate lipolysis of stored triglycerides. The expression of Atgl is regulated in part by Foxo1, whose transcriptional activity is controlled by 14-3-3ζ. Thus, 14-3-3ζ may regulate lipolysis through direct actions on key lipolytic enzymes and by influencing their protein abundance. Further studies are required to directly assess whether 14-3-3ζ has such roles in a mature adipocyte.
Does 14-3-3ζ have a role in lipolysis?
One of the key functions of adipose tissue is to store lipids. Gonadal adipocytes in 14-3-3ζKO mice were still able to expand in size following high-fat diet feeding,Citation18 which demonstrates that processes underlying lipid storage were not affected by deletion of 14-3-3ζ. In contrast, 14-3-3β has been implicated in lipid droplet formation in 3T3-L1 adipocytes by regulating actin remodeling.Citation39 Mobilization of fatty acids from adipocytes following exposure to adrenergic stimuli requires the activities of lipases such as lipoprotein lipase (Lpl), adipose triglyceride lipase (Atgl), and hormone sensitive lipase (Hsl), and although we did not test this directly in our study, the presence of 14-3-3 binding sites on both Atgl and Hsl suggest the ability of 14-3-3ζ, and other isoforms, to regulate lipolysis ().Citation40,41 The phosphorylation of ATGL by AMPK generates phosphorylation sites specific to 14-3-3 proteins,Citation40 and in C. Elegans, this interaction has been shown to control the localization of Atgl from lipid droplets.Citation42 Following its phosphorylation by Akt, Foxo1 has been shown to interact with various 14-3-3 protein isoforms where it gets retained in the cytoplasm.Citation37 While it has been shown that Foxo1 is required for adipocyte differentiation,Citation38 our study demonstrates that the pro-adipogenic roles of 14-3-3ζ lie upstream of Foxo1 and 14-3-3ζ controls Foxo1 expression.Citation18 This does not exclude the possibility that 14-3-3ζ may regulate the function of Foxo1 in mature adipocytes. Gonadal adipose tissue from mice lacking 14-3-3ζ had significantly decreased Atgl mRNA levels.Citation18 Although we speculated that this could be due to residual gonadal white adipocytes being in a poorly differentiated state, it is possible that altered Foxo1 activity stemming from deficiency of 14-3-3ζ could be responsible for this observed decrease in Atgl mRNA. Foxo1 has been found to directly regulate Atgl transcription.Citation43
Conclusion
The discovery of novel physiological roles of 14-3-3ζ has opened exciting, new avenues of metabolic research. Our studies have dispelled the notion that 14-3-3ζ is a casual signaling bystander and instead revealed its critical function in adipogenesis. More work is clearly warranted to better understand which processes are regulated by 14-3-3ζ in the adipocyte and whether other related isoforms may have over-lapping or unique roles. In the end, a better understanding of how these integral signaling proteins coordinate signal transduction networks may aid in the development of highly specific therapeutics to treat various aspects of the metabolic syndrome.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Funding
This work was funded by JDRF (1-2011-652) and Canadian Institutes for Health Research (CIHR, MOP-133692) grants to J.D.J. G.E.L. was supported with postdoctoral fellowships from the JDRF and the Canadian Diabetes Association (CDA).
References
- Kershaw EE, Flier JS. Adipose tissue as an endocrine organ. J Clin Endocrinol Metab 2004; 89:2548-56; http://dx.doi.org/10.1210/jc.2004-0395
- Rosen ED, MacDougald OA. Adipocyte differentiation from the inside out. Nat Rev Mol Cell Biol 2006; 7:885-96; PMID:17139329; http://dx.doi.org/10.1038/nrm2066
- Harms M, Seale P. Brown and beige fat: development, function and therapeutic potential. Nat Med 2013; 19:1252-63; PMID:24100998; http://dx.doi.org/10.1038/nm.3361
- Lee P, Linderman JD, Smith S, Brychta RJ, Wang J, Idelson C, Perron RM, Werner CD, Phan GQ, Kammula US, et al. Irisin and FGF21 are cold-induced endocrine activators of brown fat function in humans. Cell Metab 2014; 19:302-9; PMID:24506871; http://dx.doi.org/10.1016/j.cmet.2013.12.017
- Guilherme A, Virbasius JV, Puri V, Czech MP. Adipocyte dysfunctions linking obesity to insulin resistance and type 2 diabetes. Nat Rev Mol Cell Biol 2008; 9:367-77; PMID:18401346; http://dx.doi.org/10.1038/nrm2391
- Scott JD, Pawson T. Cell signaling in space and time: where proteins come together and when they're apart. Science 2009; 326:1220-4; PMID:19965465; http://dx.doi.org/10.1126/science.1175668
- Hinke SA, Navedo MF, Ulman A, Whiting JL, Nygren PJ, Tian G, Jimenez-Caliani AJ, Langeberg LK, Cirulli V, Tengholm A, et al. Anchored phosphatases modulate glucose homeostasis. EMBO J 2012; 31:3991-4004; PMID:22940692; http://dx.doi.org/10.1038/emboj.2012.244
- Zhuang LN, Hu WX, Xin SM, Zhao J, Pei G. Beta-arrestin-1 protein represses adipogenesis and inflammatory responses through its interaction with peroxisome proliferator-activated receptor-gamma (PPARgamma). J Biol Chem 2011; 286:28403-13; PMID:21700709; http://dx.doi.org/10.1074/jbc.M111.256099
- Vandanmagsar B, Youm YH, Ravussin A, Galgani JE, Stadler K, Mynatt RL, Ravussin E, Stephens JM, Dixit VD. The NLRP3 inflammasome instigates obesity-induced inflammation and insulin resistance. Nat Med 2011; 17:179-88; PMID:21217695; http://dx.doi.org/10.1038/nm.2279
- Morrison DK. The 14-3-3 proteins: integrators of diverse signaling cues that impact cell fate and cancer development. Trends Cell Biol 2009; 19:16-23; PMID:19027299; http://dx.doi.org/10.1016/j.tcb.2008.10.003
- Harris VK, Sadiq SA. Disease biomarkers in multiple sclerosis: potential for use in therapeutic decision making. Mol Diagn Ther 2009; 13:225-44; PMID:19712003; http://dx.doi.org/10.1007/BF03256329
- Neal CL, Yu D. 14-3-3zeta as a prognostic marker and therapeutic target for cancer. Expert Opin Ther Targets 2010; 14:1343-54; PMID:21058923; http://dx.doi.org/10.1517/14728222.2010.531011
- Yaffe MB, Rittinger K, Volinia S, Caron PR, Aitken A, Leffers H, Gamblin SJ, Smerdon SJ, Cantley LC. The structural basis for 14-3-3:phosphopeptide binding specificity. Cell 1997; 91:961-71; PMID:9428519; http://dx.doi.org/10.1016/S0092-8674(00)80487-0
- Fu H, Subramanian RR, Masters SC. 14-3-3 proteins: structure, function, and regulation. Annu Rev Pharmacol Toxicol 2000; 40:617-47; PMID:10836149; http://dx.doi.org/10.1146/annurev.pharmtox.40.1.617
- Brennan GP, Jimenez-Mateos EM, McKiernan RC, Engel T, Tzivion G, Henshall DC. Transgenic overexpression of 14-3-3 zeta protects hippocampus against endoplasmic reticulum stress and status epilepticus in vivo. PloS one 2013; 8:e54491; PMID:23359526; http://dx.doi.org/10.1371/journal.pone.0054491
- Lim GE, Piske M, Johnson JD. 14-3-3 proteins are essential signalling hubs for beta cell survival. Diabetologia 2013; 56:825-37; PMID:23354124; http://dx.doi.org/10.1007/s00125-012-2820-x
- Pozuelo-Rubio M. 14-3-3zeta binds class III phosphatidylinositol-3-kinase and inhibits autophagy. Autophagy 2011; 7:240-2; PMID:21135576; http://dx.doi.org/10.4161/auto.7.2.14286
- Lim GE, Albrecht T, Piske M, Sarai K, Lee JT, Ramshaw HS, Sinha S, Guthridge MA, Acker-Palmer A, Lopez AF, et al. 14-3-3zeta coordinates adipogenesis of visceral fat. Nat Commun 2015; 6:7671; PMID:26220403; http://dx.doi.org/10.1038/ncomms8671
- Cristancho AG, Lazar MA. Forming functional fat: a growing understanding of adipocyte differentiation. Nature Rev Mol Endocrinol 2011; 12:722-34
- Sambandam SA, Kasetti RB, Xue L, Dean DC, Lu Q, Li Q. 14-3-3sigma regulates keratinocyte proliferation and differentiation by modulating Yap1 cellular localization. The Journal of investigative dermatology 2015; 135:1621-8; PMID:25668240
- Toyo-oka K, Wachi T, Hunt RF, Baraban SC, Taya S, Ramshaw H, Kaibuchi K, Schwarz QP, Lopez AF, Wynshaw-Boris A. 14-3-3epsilon and zeta regulate neurogenesis and differentiation of neuronal progenitor cells in the developing brain. J Neurosci 2014; 34:12168-81; PMID:25186760; http://dx.doi.org/10.1523/JNEUROSCI.2513-13.2014
- Patrick DM, Zhang CC, Tao Y, Yao H, Qi X, Schwartz RJ, Jun-Shen Huang L, Olson EN. Defective erythroid differentiation in miR-451 mutant mice mediated by 14-3-3zeta. Genes Dev 2010; 24:1614-9; PMID:20679397; http://dx.doi.org/10.1101/gad.1942810
- Hong JH, Hwang ES, McManus MT, Amsterdam A, Tian Y, Kalmukova R, Mueller E, Benjamin T, Spiegelman BM, Sharp PA, et al. TAZ, a transcriptional modulator of mesenchymal stem cell differentiation. Science 2005; 309:1074-8; PMID:16099986; http://dx.doi.org/10.1126/science.1110955
- Wang QA, Tao C, Gupta RK, Scherer PE. Tracking adipogenesis during white adipose tissue development, expansion and regeneration. Nat Med 2013; 19:1338-44; PMID:23995282; http://dx.doi.org/10.1038/nm.3324
- Rodeheffer MS, Birsoy K, Friedman JM. Identification of white adipocyte progenitor cells in vivo. Cell 2008; 135:240-9; PMID:18835024; http://dx.doi.org/10.1016/j.cell.2008.09.036
- Chau YY, Bandiera R, Serrels A, Martinez-Estrada OM, Qing W, Lee M, Slight J, Thornburn A, Berry R, McHaffie S, et al. Visceral and subcutaneous fat have different origins and evidence supports a mesothelial source. Nat Cell Biol 2014; 16:367-75; PMID:24609269; http://dx.doi.org/10.1038/ncb2922
- Wang QA, Tao C, Jiang L, Shao M, Ye R, Zhu Y, Gordillo R, Ali A, Lian Y, Holland WL, et al. Distinct regulatory mechanisms governing embryonic versus adult adipocyte maturation. Nat Cell Biol 2015; 17:1099-111; PMID:26280538; http://dx.doi.org/10.1038/ncb3217
- Siersbaek R, Rabiee A, Nielsen R, Sidoli S, Traynor S, Loft A, Poulsen LL, Rogowska-Wrzesinska A, Jensen ON, Mandrup S. Transcription factor cooperativity in early adipogenic hotspots and super-enhancers. Cell Rep 2014; 7:1443-55; PMID:24857652; http://dx.doi.org/10.1016/j.celrep.2014.04.042
- Mehran AE, Templeman NM, Brigidi GS, Lim GE, Chu KY, Hu X, Botezelli JD, Asadi A, Hoffman BG, Kieffer TJ, et al. Hyperinsulinemia drives diet-induced obesity independently of brain insulin production. Cell Metab 2012; 16:723-37; PMID:23217255; http://dx.doi.org/10.1016/j.cmet.2012.10.019
- Claussnitzer M, Dankel SN, Kim KH, Quon G, Meuleman W, Haugen C, Glunk V, Sousa IS, Beaudry JL, Puviindran V, et al. FTO Obesity Variant Circuitry and Adipocyte Browning in Humans. N Engl J Med 2015; 373:895-907; PMID:26287746; http://dx.doi.org/10.1056/NEJMoa1502214
- Qiu Y, Nguyen KD, Odegaard JI, Cui X, Tian X, Locksley RM, Palmiter RD, Chawla A. Eosinophils and type 2 cytokine signaling in macrophages orchestrate development of functional beige fat. Cell 2014; 157:1292-308; PMID:24906148; http://dx.doi.org/10.1016/j.cell.2014.03.066
- Itagaki C, Isobe T, Taoka M, Natsume T, Nomura N, Horigome T, Omata S, Ichinose H, Nagatsu T, Greene LA, et al. Stimulus-coupled interaction of tyrosine hydroxylase with 14-3-3 proteins. Biochemistry 1999; 38:15673-80; PMID:10569954; http://dx.doi.org/10.1021/bi9914255
- Neukamm SS, Ott J, Dammeier S, Lehmann R, Haring HU, Schleicher E, Weigert C. Phosphorylation of Serine 1137/1138 of Mouse Insulin Receptor Substrate (IRS) 2 Regulates cAMP-dependent Binding to 14-3-3 Proteins and IRS2 Protein Degradation. J Biol Chem 2013; 288:16403-15; PMID:23615913; http://dx.doi.org/10.1074/jbc.M113.474593
- Ogihara T, Isobe T, Ichimura T, Taoka M, Funaki M, Sakoda H, Onishi Y, Inukai K, Anai M, Fukushima Y, et al. 14-3-3 protein binds to insulin receptor substrate-1, one of the binding sites of which is in the phosphotyrosine binding domain. J Biol Chem 1997; 272:25267-74; PMID:9312143; http://dx.doi.org/10.1074/jbc.272.40.25267
- Chen S, Wasserman DH, MacKintosh C, Sakamoto K. Mice with AS160/TBC1D4-Thr649Ala knockin mutation are glucose intolerant with reduced insulin sensitivity and altered GLUT4 trafficking. Cell Metab 2011; 13:68-79; PMID:21195350; http://dx.doi.org/10.1016/j.cmet.2010.12.005
- Ramm G, Larance M, Guilhaus M, James DE. A role for 14-3-3 in insulin-stimulated GLUT4 translocation through its interaction with the RabGAP AS160. J Biol Chem 2006; 281:29174-80; PMID:16880201; http://dx.doi.org/10.1074/jbc.M603274200
- Brunet A, Bonni A, Zigmond MJ, Lin MZ, Juo P, Hu LS, Anderson MJ, Arden KC, Blenis J, Greenberg ME. Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell 1999; 96:857-68; PMID:10102273; http://dx.doi.org/10.1016/S0092-8674(00)80595-4
- Nakae J, Kitamura T, Kitamura Y, Biggs WH, 3rd, Arden KC, Accili D. The forkhead transcription factor Foxo1 regulates adipocyte differentiation. Dev cell 2003; 4:119-29; PMID:12530968; http://dx.doi.org/10.1016/S1534-5807(02)00401-X
- Yang W, Thein S, Wang X, Bi X, Ericksen RE, Xu F, Han W. BSCL2/seipin regulates adipogenesis through actin cytoskeleton remodelling. Hum Mol Genet 2014; 23:502-13; PMID:24026679; http://dx.doi.org/10.1093/hmg/ddt444
- Ahmadian M, Abbott MJ, Tang T, Hudak CS, Kim Y, Bruss M, Hellerstein MK, Lee HY, Samuel VT, Shulman GI, et al. Desnutrin/ATGL is regulated by AMPK and is required for a brown adipose phenotype. Cell Metab 2011; 13:739-48; PMID:21641555; http://dx.doi.org/10.1016/j.cmet.2011.05.002
- Marvyn PM, Bradley RM, Button EB, Mardian EB, Duncan RE. Fasting upregulates adipose triglyceride lipase and hormone-sensitive lipase levels and phosphorylation in mouse kidney. Biochem Cell Biol 2015; 93:262-7; PMID:25879679; http://dx.doi.org/10.1139/bcb-2014-0150
- Xie M, Roy R. AMP-activated kinase regulates lipid droplet localization and stability of adipose triglyceride lipase in C. elegans dauer larvae. PLoS One 2015; 10:e0130480; PMID:26098762; http://dx.doi.org/10.1371/journal.pone.0130480
- Chakrabarti P, Kandror KV. FoxO1 controls insulin-dependent adipose triglyceride lipase (ATGL) expression and lipolysis in adipocytes. J Biol Chem 2009; 284:13296-300; PMID:19297333; http://dx.doi.org/10.1074/jbc.C800241200
