ABSTRACT
Human brown adipocytes are able to burn fat and glucose and are now considered as a potential strategy to treat obesity, type 2 diabetes and metabolic disorders. Besides their thermogenic function, brown adipocytes are able to secrete adipokines. One of these is visfatin, a nicotinamide phosphoribosyltransferase involved in nicotinamide dinucleotide synthesis, which is known to participate in the synthesis of insulin by pancreatic β cells. In a therapeutic context, it is of interest to establish whether a potential correlation exists between brown adipocyte activation and/or brite adipocyte recruitment, and adipokine expression. We analyzed visfatin expression, as a pre-requisite to its secretion, in rodent and human biopsies and cell models of brown/brite adipocytes. We found that visfatin was preferentially expressed in mature adipocytes and that this expression was higher in brown adipose tissue of rodents compared to other fat depots. However, using various rodent models we were unable to find any correlation between visfatin expression and brown or brite adipocyte activation or recruitment. Interestingly, the situation is different in humans where visfatin expression was found to be equivalent between white and brown or brite adipocytes in vivo and in vitro. In conclusion, visfatin can be considered only as a rodent brown adipocyte biomarker, independently of tissue activation.
Introduction
Obesity participates for a great part in the pathogenesis of type 2 diabetes (T2D), due to its association with insulin resistance. Obesity has reached worldwide epidemic proportions, with more than 1.9 billion of adults suffering from overweight and 600 million considered as clinically obese.Citation1,2 Unfortunately, except for morbidly obese patients undergoing bariatric surgery, medical obesity treatments are so far not satisfactory.
Overweight and obesity are consequent to energy balance disequilibrium, in favor of inappropriate energy intake increasing white fat mass. In this context, one of the strategies which deserves consideration is the use of the thermogenic capacity of brown adipocytes to counteract the excess of energy intake to equilibrate the energy balance.Citation3 Brown adipocytes are characterized by a high mitochondrial content and expression of the specific uncoupling protein 1 (UCP1). These cells are endowed with a high capacity of glucose and lipid oxidation making them a potential target for decreasing plasma glucose, fatty acids and lowering the risks of T2D and obesity.Citation4,5 Indeed, the recent discovery of brown adipocytes, thermogenically competent, in human adults opens a new field in obesity associated T2D therapy (reviewed inCitation6). In addition to brown adipose tissue (BAT) – consisting in brown adipocytes – white adipose tissue (WAT) contains thermogenic fat cells called “brown-in-white” (“brite”) or “beige” adipocytes.Citation7 These brite adipocytes originate either from the differentiation of specific progenitors resident in the tissue,Citation8,9 or from the conversion of differentiated white adipocytes.Citation10,11 In addition to the preventive function of BAT in the occurrence of T2D, i.e. in fat gain and glucose tolerance regulation, this tissue seems to offer beneficial effects on metabolic disorders. Indeed, transplantation of BAT in mice resulted in a reversal of high-fat diet-induced insulin resistance Citation12 and reversed streptozotocin-induced diabetes.Citation13 In healthy humans, cold exposure leads to a decrease in body fat by recruiting brown/brite adipocytes and increasing nonshivering thermogenesis.Citation14-16 A recent study showed that these adipocytes may function as an anti-diabetic tissue in humans.Citation17 Altogether, these data explore new avenues emphasizing thermogenic adipocytes as novel important candidates to control body weight and carbohydrate metabolism through modulation of energy expenditure.
Visfatin (Nampt/PBEF) is an enzyme (nicotinamide-ribosyl-transferase) synthesising nicotinamide mononucleotide (NMN) from nicotinamide.Citation18 NMN is the substrate for nicotinamide dinucleotide (NAD) synthesis, involved in numerous cell functions.Citation19 Visfatin, secreted from various cells including adipocytes, has been found in the circulation and is considered as an adipokine.Citation18 Several studies demonstrate the crucial role of visfatin in β-cell function, i.e., insulin secretion, insulin receptor phosphorylation and signaling as well as transcriptional regulation of various key genes.Citation18,20 Thus, visfatin could be involved in metabolic disorders involving β-cell failure, such as T2D.
Since dysregulation of adipokine synthesis is associated with obesity, we hypothesize that brown adipocyte activation could participate in an increased production of visfatin and be involved in the regulation of insulin secretion and hence glucose metabolism. To evaluate visfatin as a potential target for future therapies of obesity and associated diseases, we analyzed here the expression of visfatin in several in vivo and in vitro models of brown adipocyte activation and brite adipocyte recruitment in rodents and humans.
Results
Visfatin mRNA is preferentially expressed in adipocytes
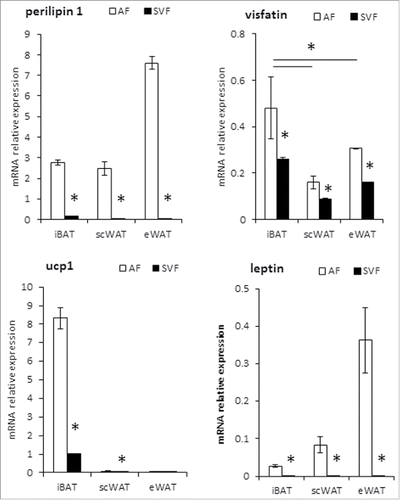
Since adipose tissue is composed of adipocytes and several cell types of the stromal vascular fraction (SVF), we separated by enzymatic digestion these fractions from mouse adipose tissues isolated from interscapular (iBAT), subcutaneous (scWAT) and epididymal depots (eWAT). We measured mRNA expression of key adipocyte markers; i.e. perilipin-1 as control of adipocyte purification, leptin and UCP1 as white and brown adipocyte markers respectively. As expected, perilipin-1 and leptin were only expressed in the adipocyte fraction of the 3 adipose depots, while UCP1 mRNA expression was confined to the adipocyte fraction of iBAT (). In contrast to perilipin, leptin and UCP1, visfatin mRNA was expressed in both adipocyte and stromal vascular fractions, but with a fold2- higher expression in adipocyte fraction for the 3 adipose tissue depots ().
Figure 1. Visfatin expression in mouse brown and white adipocytes and stromal vascular fractions. mRNA expression determined by RT-qPCR in Stroma Vascular Fraction (SVF) and adipocyte fraction (AF) from scWAT, eWAT and iBAT of C57BL/6 mice. Perilipin-1 was used as control of adipocyte purification, and leptin as a white adipocyte marker. Histograms represent mean ± sem of 8 mice. *: p<0 .05.
Visfatin is highly expressed in brown adipocytes
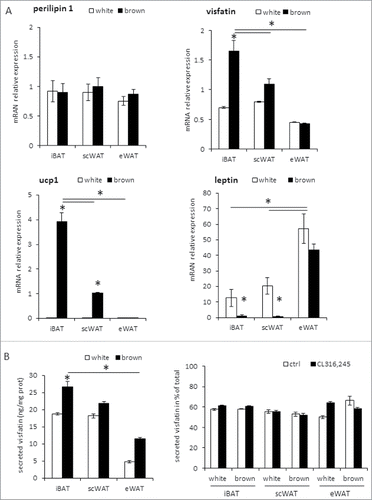
In order to determine whether visfatin expression was correlated to brown adipocyte differentiation, we analyzed its expression in in vitro differentiated mouse adipocytes. For that purpose, SVF cells from the 3 adipose tissue depots were induced to differentiate either into white or brown/brite adipocytes (). As expected, UCP1 mRNA was not detected in white adipocytes and leptin mRNA levels were low in brown/brite adipocytes. In addition, UCP1 was highly expressed in brown adipocytes derived from iBAT progenitors, lower in brown adipocytes derived from scWAT progenitors (considered as brite adipocytes) and not detected in those derived from eWAT (). Conversely, visfatin mRNA was expressed in SVF differentiated either in white or in brown/brite adipocytes with a significantly higher expression in iBAT-derived brown adipocytes. Then, we analyzed visfatin secretion in conditioned media and found that secreted visfatin protein levels were correlated to visfatin mRNA expression, with a higher secretion in brown adipocytes (). Furthermore, visfatin secretion was not affected by β3-adrenergic receptor stimulation ().
Figure 2. Visfatin expression in mouse brown and white adipocytes. (A) Cells from SVF of scWAT, eWAT and iBAT depots were differentiated in white or brown adipocytes and used for mRNA level evaluation by RT-qPCR. Perilipin-1 was used as control of adipogenesis, and leptin as white adipocyte marker. (B) Intracellular and extracellular visfatin protein levels were quantified by EIA in SVF-derived white and brown adipocytes after 16 h of incubation in absence or presence of 16 μM CL316,243. Histograms represent mean ± sem of 3 independent experiments (B). *: p<0 .05.
Visfatin is preferentially found in iBAT, but its expression is not correlated with BAT activation
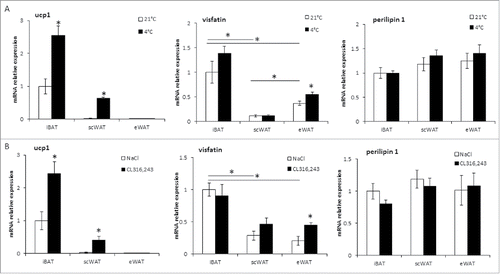
To determine whether a correlation exists between visfatin mRNA expression and BAT activation, mice were exposed to cold (4°C, 4 days) or received a daily injection of CL316,243 for 1 week, known to activate brown adipocytes. These stimulations induced a significant increase in UCP1 mRNA levels in iBAT. However, this was not correlated to an increase in visfatin mRNA expression (). As expected, both cold and β3-receptor agonist treatments led to an increased UCP1 expression in scWAT, but not in eWAT () corresponding to the recruitment and activation of inducible brite adipocytes in the scWAT. Again, visfatin mRNA levels were not significantly modified under these stimulations (). Interestingly, a significant increase of visfatin mRNA was observed in eWAT under both stimulations ().
Figure 3. Visfatin is preferentially expressed in mouse BAT, but is not correlated to brown adipocyte recruitment/activation. mRNA expression was determined by RT-qPCR in iBAT, scWAT and eWAT from C57BL/6 mice exposed (4 days) at a temperature of 4±2°C (A) or treated with CL316,243 for 1 week (B). Control animals were respectively maintained at housing temperature (21±2°C) or injected with the vehicle (NaCl 0.9%, w/v). Perilipin-1 was used as a control marker of adipose tissue. Histograms represent mean ± sem of 8 mice. *: p<0 .05.
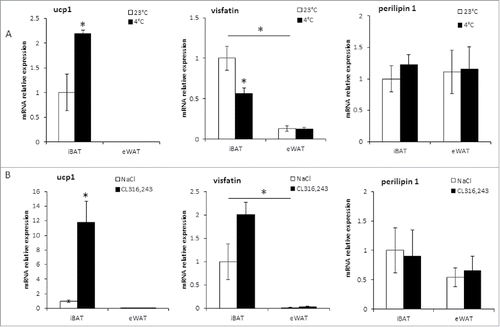
Visfatin mRNA expression was higher in mice iBAT compared to white depots, i.e., eWAT and scWAT (). A similar profile was found in rat tissues with a higher expression in iBAT compared to eWAT (). Concerning the correlation with BAT activation in rat adipose tissue depots, no significant increase in visfatin mRNA levels was observed upon CL316,243 stimulation (). Under acute cold exposure, visfatin expression decreased ().
Figure 4. Visfatin is preferentially expressed in rat iBAT, but is not induced with BAT activation. mRNA expression was determined by RT-qPCR in iBAT and eWAT from Wistar rats exposed overnight at a temperature of 4±2°C (A) or treated with CL316,243 for 1 week (B). Control animals were maintained near to rat thermoneutral temperature (23±2°C) or injected with the vehicle (NaCl 0.9%, w/v). Perilipin-1 was used as a control marker of adipose tissue. Histograms represent mean ± sem of 4 rats. *: p<0 .05.
Visfatin is expressed and secreted by human brown and brite adipocytes
We further analyzed the expression of visfatin in scWAT and BAT biopsies from healthy volunteers (evaluated by FDG-PET/CT analysis). UCP1 mRNA expression was confined to BAT, whereas visfatin mRNA was expressed in both tissues at similar levels ().
Figure 5. Visfatin expression and secretion in human white and brown adipocytes. (A) mRNA levels were evaluated by qRT-PCR in matched biopsies of scWAT and BAT from 6 healthy human adult patients. mRNA expression determined in human subcutaneous adipose tissue SVF-derived white and brown adipocytes (B) or hMADS adipocytes (C). Perilipin-1 was used as control of adipogenesis and leptin as white adipogenic marker. (D) Intracellular and extracellular visfatin EIA quantification in hMADS white and brown adipocytes after 16 h of incubation with or without 1μM CL316,243. Histograms represent mean ± sem of 6 tissue samples (A) or 3 independent experiments (B, C, D). *: p<0 .05.
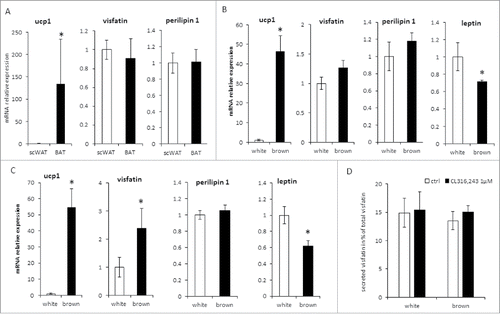
We analyzed the expression of visfatin mRNA in 2 human cellular models. SVF from 2 human subcutaneous adipose tissue samples (hADSC) were induced to differentiate into white or brown adipocytes. As expected, UCP1 mRNA expression was restricted to brown adipocytes and leptin mRNA was highly expressed in white adipocytes. By contrast, visfatin mRNA was expressed at similar levels in both white and brown adipocytes (). Next, we used hMADS cells, which are able to differentiate into white adipocytes and then convert into functional brite adipocytes. Whereas UCP1 mRNA expression was confined to brite adipocytes, visfatin mRNA was expressed in both cell types, brite adipocytes showing an expression 2 times higher than white adipocytes (). To determine a potential functional impact of this up-regulation, we analyzed the secretion of visfatin in the conditioned media. We found that secreted visfatin protein levels, evaluated to be 15% of the total visfatin (cellular + secreted), were equivalent between white and brite hMADS adipocytes, even in the presence of CL316,243 (). The increased visfatin mRNA expression might lead to an increase of the intracellular visfatin level. Indeed, a slightly higher, but non-significant level of visfatin protein was found in cell lysates of brite adipocytes (0.053 ± 0.016vs/ 0.088 ± 0.034 ng visfatin/μg protein respectively for white and brite adipocytes).
Discussion
Visfatin is an essential NMN/NAD biosynthetic enzyme considered as a novel adipokine, with both intra- and extracellular enzymatic functions. It is expressed in white and brown adipose tissues of rodents.Citation18 Secreted visfatin acutely potentiates glucose stimulated insulin secretion, without affecting β-cell survival.Citation21 Moreover, NMN, the product of visfatin activity, has been shown to restore insulin secretion in mouse models of impaired β-cell function.Citation22 Finally, visfatin is found in the blood circulation acting like a cytokine. Thus, targeting visfatin function or expression might represent a novel therapeutic strategy for the control of β-cell function in diabetes.
Here, we have confirmed that while visfatin was expressed in several adipose tissue depots, it was preferentially expressed in brown adipocytes. However, sympathetic activation in rodents did not reveal a positive correlation between visfatin expression and BAT activation. It is now well established that brown-like adipocytes appear in white adipose tissue after chronic sympathetic stimulation. These brite, or beige, adipocytes are found preferentially in subcutaneous white adipose tissue depots, where they form clusters of thermogenic competent cells able to burn lipids and glucose.Citation10 Our results have demonstrated that visfatin was highly expressed and secreted in genuine brown adipocytes compared to white ones, but not in brite adipocytes derived from scWAT progenitors.
Surprisingly, visfatin mRNA expression was found up-regulated in mouse eWAT upon cold exposure or CL316,243 treatment. This was not related to brite adipocytes recruitment, as eWAT is less sensitive to these treatments and does not display high amounts of UCP1 positive cells. However, eWAT is a highly lipolytic tissue under the control of adrenergic stimulation. Therefore, we can assume that visfatin expression could be related rather to the lipolytic function of white adipocytes.
As BAT found in adult humans corresponds more to the inducible brite adipocytes described in rodents,Citation23 it was not surprising to find comparable levels of visfatin mRNA in BAT and WAT biopsies from adult volunteers. Similar observations were obtained in vitro using primary cultures of cells from scWAT and differentiated in white or brite/brown adipocytes.
Brite adipocytes originate from either specific white adipose tissue resident progenitors or from the conversion of mature white adipocytes.Citation11 Using hMADS cell models of white to brite adipocyte conversion, we have shown that visfatin mRNA expression was slightly increased during the conversion of white to brite adipocytes. Furthermore, this increase in mRNA expression was not correlated to an enhanced secretion of visfatin protein. As visfatin has been described to be a PPARγ target gene in macrophages,Citation24 and as white to brite conversion in vitro was triggered by the addition of the PPARγ agonist, rosiglitazone, it is tempting to suggest that this increased visfatin expression was due to PPARγ activation.
Conflicting data have been reported for visfatin plasma levels in various cohorts of diabetic and obese patients as well as in rodents. Unexpectedly, several studies described a positive correlation of plasma visfatin levels with obesity, T2D or metabolic syndrome; situations associated in fine to β-cell dysfunction.Citation25-27 Other studies did not reveal any association between visfatin circulating levels and visceral fat mass or insulin sensitivity parameters in humans and rodents.Citation28,29 Altogether, these data highlight a paradox between pathologic visfatin circulating levels and its positive involvement in β-cell function.
In conclusion, visfatin can be considered as a rodent brown adipocyte biomarker. Nevertheless, its expression and thus secretion does not appear related to brown adipocyte activation or brite adipocyte recruitment in humans and rodents. While the currently available data do not permit to herald visfatin as a beneficial BAT-derived cytokine for the treatment of obesity and T2D, the search for other potential brown/brite adipocyte secreted molecules, acting in concert or not with visfatin, remains a burning issue.
Material and methods
Cell culture
Mouse primary adipocytes
Animals were euthanized by cervical dislocation for preparation of stromal vascular fractions. Interscapular, perigonadal and subcutaneous fat depots were rapidly excised, washed in PBS, and minced. Adipose tissue samples were then digested with mild agitation for 45 min at 37°C in DMEM (Lonza, BE12–707F) containing 2 mg/ml collagenase A (Roche Diagnostics, 11088793011) and 20 mg/ml BSA (Sigma-Aldrich Chemie Gmbh, A7030). Digested tissues were filtrated through 250, 100 and 27 μm nylon sheets, and centrifuged for 5 min at 500 g. Adipocyte fractions were obtained after centrifugation of the first 250 μm filtration at 500 g for 5 min, floating adipocytes were sampled for molecular analysis. The pellet containing stromal-vascular fraction (SVF) cells was submitted to red blood cell lysis procedure. SVF cells were either used directly for molecular analysis or plated and maintained in DMEM containing 10% (v/v) FCS until confluence. Differentiation was induced in the same medium supplemented with 1 μM dexamethasone (Sigma-Aldrich Chemie Gmbh, D4902), 0.5 mM isobutylmethylxanthine (Sigma-Aldrich Chemie Gmbh, I5879) and 860 nM insulin (Invitrogen, 12585014) for 2 d. Cells were then maintained for 7–10 d in presence of 10 nM insulin for white adipogenesis or a mixture containing 10 nM insulin, 1 μM rosiglitazone (BertinPharma, 71740) and 0.2 nM triiodothyronine (Sigma-Aldrich Chemie Gmbh, T6397) for brown or brite adipogenesis.
hMADS and hADSC
The establishment and characterization of the multipotency and self- renewal capacity of hMADS cells have been described.Citation30 White and brite adipocyte differentiations were performed as previously described.Citation31-33 For human adipose-derived stem cells (hADSC), subcutaneous adipose tissue samples were obtained as res nullus from surgeries (non-pathologic abdominoplasty). hADSC cells were isolated by enzymatic digestion as described in the mouse primary adipocytes section. Cells were then plated and maintained in culture using the protocol used for hMADS cells.
Isolation and analysis of RNA
Total RNA was extracted using a TRI-Reagent kit according to the manufacturer's instructions (Euromedex, RT-111), with an additional step for tissues consisting to the homogenization in TRI-Reagent using an ULTRA TURRAX T25 (Ika). Reverse transcription and polymerase chain reaction (RT-PCR) analysis were performed as described previously.Citation33 The oligonucleotide sequences are shown in . Quantitative PCR (qPCR) was performed using SYBR qPCR premix Ex TaqII from Takara (Ozyme, TAKRR820W) and assays were run on a StepOne Plus ABI real-time PCR machine (PerkinElmer Life and Analytical Sciences). The expression of selected genes was normalized to that of 36B4 housekeeping genes and then quantified using the comparative-ΔCt method.
Table 1. Sequence of primers used for gene expression analysis.
Visfatin secretion assay
Differentiated SVF from mouse adipose tissue or differentiated hMADS cells were incubated for 16h in fresh media (serum free, BSA 0.05%) stimulated or not with 1 μM CL316,243. Conditioned media were sampled and centrifuged for 5 min at 400 g to remove cell debris. Attached cells were lysed in a buffer containing 25 mM Tris-Cl (pH 7.4), 100 mM NaCl, 1 mM EDTA, 1% (v/v) Triton X-100, 0.5% (v/v) Nonidet P40, 1x protease inhibitor cocktail (Roche, 11697498001). Visfatin levels were quantified in cell lysates and conditioned media using specific ELISA kit from Enzo Life Sciences for mouse (AG-45A-0007EK-KI01) or RayBiotech for human samples (Clinisciences, EIA-VIS-1). Assays were performed following the manufacturer's instructions.
Animals
The experiments were conducted in accordance with the French and European regulations for the care and use of research animals and were approved by the local experimentation committee CIEPAL-Azur N°28 (protocol NCE-2012–57, NCE-2014–166 and MESR-00500.02). Animals were maintained under constant temperature (mice: 21 ± 2°C; rats: 23 ± 2°C) and 12:12-hour light-dark cycles, with ad libitum access to standard chow diet and water. C57Bl/6J mice (males, 10 to 14 weeks-old) and Wistar rats (males, 12 to 16 weeks-old) were from Janvier Labs (http://www.janvier-labs.com/). Brown adipocyte activation was induced by a daily intra-peritoneal injection of a β3-adrenergic receptor agonist CL316,243 (1 mg/kg in a saline solution, Sigma-Aldrich Chemie Gmbh, C5976) for 7 d Control animals were injected with vehicle only. For cold exposure, mice were exposed for 4 d and rats for one night at 4°C ± 2°C in a climate chamber.
Subjects
The study protocol was reviewed and approved by the ethics committee of the Hospital District of Southwestern Finland, and subjects provided written informed consent following the committee's instructions. The study was conducted according to the principles of the Declaration of Helsinki. All potential subjects were screened for metabolic status, and only those with normal glucose tolerance and normal cardiovascular status (as assessed on the basis of electrocardiograms and measured blood pressure) were included. The age range of the subjects was 23–49 y. We studied a group of 6 healthy volunteers (2 men and 4 women). BAT was sampled in positive FDG-PET scan site, and subcutaneous WAT was derived via the same incision.
Statistical analyses
Data are expressed as mean values ± SEM and were analyzed using InStat software (GraphPad Software). Data were analyzed by one-way ANOVA followed by a Student-Newman-Keuls post-test, or Student's t-test to assess statistical differences between experimental groups. Differences were considered statistically significant when p < 0 .05.
Disclosure of potential conflicts of interestxs
No potential conflicts of interest were disclosed.
Acknowledgments
The authors greatly acknowledge IBV and IRCAN Animal core facilities, as well as Octalia Technologies for manuscript editing.
Funding
This work was supported by CNRS, INSERM, Université de Nice Sophia Antipolis, EU FP7 project DIABAT (HEALTH-F2–2011–278373), French Agence Nationale de la Recherche (ANR-10-BLAN-1105 miRBAT and ANR-RPV12004AAA- DiamiR), Aviesan/AstraZeneca, “Diabetes and the vessel wall injury” program, “Investments for the Future” LABEX SIGNALIFE #ANR-11-LABX-0028–01 and European Foundation for the Study of Diabetes (EFSD/Lilly; European Diabetes Research Program).
References
- Kopelman PG. Obesity as a medical problem. Nature 2000; 404:635-43; PMID:10766250
- WHO. Obesity and overweight. http://wwwwhoint/mediacentre/factsheets/fs311/en/ 2014; Fact sheet N°311
- Langin D. Recruitment of brown fat and conversion of white into brown adipocytes: strategies to fight the metabolic complications of obesity? Biochim Biophys Acta 2010; 1801:372-6; PMID:19782764; http://dx.doi.org/10.1016/j.bbalip.2009.09.008
- Nedergaard J, Golozoubova V, Matthias A, Asadi A, Jacobsson A, Cannon B. UCP1: the only protein able to mediate adaptive non-shivering thermogenesis and metabolic inefficiency. Biochim Biophys Acta 2001; 1504:82-106; PMID:11239487; http://dx.doi.org/10.1016/S0005-2728(00)00247-4
- Nedergaard J, Bengtsson T, Cannon B. New powers of brown fat: fighting the metabolic syndrome. Cell Metab 2011; 13:238-40; PMID:21356513; http://dx.doi.org/10.1016/j.cmet.2011.02.009
- Virtanen KA, van Marken Lichtenbelt WD, Nuutila P. Brown adipose tissue functions in humans. Biochim Biophys Acta 2013; 1831:1004-8; PMID:23274235; http://dx.doi.org/10.1016/j.bbalip.2012.12.011
- Petrovic N, Walden TB, Shabalina IG, Timmons JA, Cannon B, Nedergaard J. Chronic peroxisome proliferator-activated receptor gamma (PPARgamma) activation of epididymally derived white adipocyte cultures reveals a population of thermogenically competent, UCP1-containing adipocytes molecularly distinct from classic brown adipocytes. J Biol Chem 2010; 285:7153-64; PMID:20028987; http://dx.doi.org/10.1074/jbc.M109.053942
- Wang QA, Tao C, Gupta RK, Scherer PE. Tracking adipogenesis during white adipose tissue development, expansion and regeneration. Nat Med 2013; 19:1338-44; PMID:23995282; http://dx.doi.org/10.1038/nm.3324
- Wu J, Bostrom P, Sparks LM, Ye L, Choi JH, Giang AH, Khandekar M, Virtanen KA, Nuutila P, Schaart G, et al. Beige adipocytes are a distinct type of thermogenic fat cell in mouse and human. Cell 2012; 150:366-76; PMID:22796012; http://dx.doi.org/10.1016/j.cell.2012.05.016
- Barbatelli G, Murano I, Madsen L, Hao Q, Jimenez M, Kristiansen K, Giacobino JP, De Matteis R, Cinti S. The emergence of cold-induced brown adipocytes in mouse white fat depots is determined predominantly by white to brown adipocyte transdifferentiation. Am J Physiol Endocrinol Metab 2010; 298:E1244-53; PMID:20354155; http://dx.doi.org/10.1152/ajpendo.00600.2009
- Rosenwald M, Perdikari A, Rulicke T, Wolfrum C. Bi-directional interconversion of brite and white adipocytes. Nat Cell Biol 2013; 15:659-67; PMID:23624403; http://dx.doi.org/10.1038/ncb2740
- Stanford KI, Middelbeek RJ, Townsend KL, An D, Nygaard EB, Hitchcox KM, Markan KR, Nakano K, Hirshman MF, Tseng YH, et al. Brown adipose tissue regulates glucose homeostasis and insulin sensitivity. J Clin Invest 2013; 123:215-23; PMID:23221344; http://dx.doi.org/10.1172/JCI62308
- Kim JH, Bae KH, Choi YK, Go Y, Choe M, Jeon YH, Lee HW, Koo SH, Perfield JW 2nd, Harris RA, et al. cprThe FGF21 analogue, LY2405319, lowers blood glucose in streptozotocin-induced insulin-deficient diabetic mice by restoring brown adipose tissue function. Diabetes Obes Metab 2014; 17(2):161-9
- Blondin DP, Labbe SM, Tingelstad HC, Noll C, Kunach M, Phoenix S, Guérin B, Turcotte EE, Carpentier AC, Richard D, et al. Increased brown adipose tissue oxidative capacity in cold-acclimated humans. J Clin Endocrinol Metab 2014; 99:E438-46; PMID:24423363
- van der Lans AA, Hoeks J, Brans B, Vijgen GH, Visser MG, Vosselman MJ, Hansen J, Jörgensen JA, Wu J, Mottaghy FM, et al. Cold acclimation recruits human brown fat and increases nonshivering thermogenesis. J Clin Invest 2013; 123:3395-403; PMID:23867626; http://dx.doi.org/10.1172/JCI68993
- Yoneshiro T, Aita S, Matsushita M, Kayahara T, Kameya T, Kawai Y, Iwanaga T, Saito M. Recruited brown adipose tissue as an antiobesity agent in humans. J Clin Invest 2013; 123:3404-8; PMID:23867622; http://dx.doi.org/10.1172/JCI67803
- Chondronikola M, Volpi E, Borsheim E, Porter C, Annamalai P, Enerback S, Lidell ME, Saraf MK, Labbe SM, Hurren NM, et al. Brown Adipose Tissue Improves Whole Body Glucose Homeostasis and Insulin Sensitivity in Humans. Diabetes 2014; 63(12):4089-99; PMID:25056438
- Revollo JR, Korner A, Mills KF, Satoh A, Wang T, Garten A, Dasgupta B, Sasaki Y, Wolberger C, Townsend RR, et al. Nampt/PBEF/Visfatin regulates insulin secretion in β cells as a systemic NAD biosynthetic enzyme. Cell Metab 2007; 6:363-75; PMID:17983582; http://dx.doi.org/10.1016/j.cmet.2007.09.003
- Lin SJ, Guarente L. Nicotinamide adenine dinucleotide, a metabolic regulator of transcription, longevity and disease. Curr Opin Cell Biol 2003; 15:241-6; PMID:12648681; http://dx.doi.org/10.1016/S0955-0674(03)00006-1
- Brown JE, Onyango DJ, Ramanjaneya M, Conner AC, Patel ST, Dunmore SJ, Randeva HS. Visfatin regulates insulin secretion, insulin receptor signalling and mRNA expression of diabetes-related genes in mouse pancreatic β-cells. J Mol Endocrinol 2010; 44:171-8; PMID:19906834; http://dx.doi.org/10.1677/JME-09-0071
- Spinnler R, Gorski T, Stolz K, Schuster S, Garten A, Beck-Sickinger AG, Engelse MA, de Koning EJ, Körner A, Kiess W, et al. The adipocytokine Nampt and its product NMN have no effect on β-cell survival but potentiate glucose stimulated insulin secretion. PLoS One 2013; 8:e54106; PMID:23342086; http://dx.doi.org/10.1371/journal.pone.0054106
- Caton PW, Kieswich J, Yaqoob MM, Holness MJ, Sugden MC. Nicotinamide mononucleotide protects against pro-inflammatory cytokine-mediated impairment of mouse islet function. Diabetologia 2011; 54:3083-92; PMID:21901281; http://dx.doi.org/10.1007/s00125-011-2288-0
- Lidell ME, Betz MJ, Dahlqvist Leinhard O, Heglind M, Elander L, Slawik M, Mussack T, Nilsson D, Romu T, Nuutila P, et al. Evidence for two types of brown adipose tissue in humans. Nat Med 2013; 19:631-4; PMID:23603813; http://dx.doi.org/10.1038/nm.3017
- Mayi TH, Duhem C, Copin C, Bouhlel MA, Rigamonti E, Pattou F, Staels B, Chinetti-Gbaguidi G. Visfatin is induced by peroxisome proliferator-activated receptor gamma in human macrophages. FEBS J 2010; 277:3308-20; PMID:20608974; http://dx.doi.org/10.1111/j.1742-4658.2010.07729.x
- El-Mesallamy HO, Kassem DH, El-Demerdash E, Amin AI. Vaspin and visfatin/Nampt are interesting interrelated adipokines playing a role in the pathogenesis of type 2 diabetes mellitus. Metabolism 2011; 60:63-70; PMID:20605615; http://dx.doi.org/10.1016/j.metabol.2010.04.008
- Filippatos TD, Derdemezis CS, Kiortsis DN, Tselepis AD, Elisaf MS. Increased plasma levels of visfatin/pre-B cell colony-enhancing factor in obese and overweight patients with metabolic syndrome. J Endocrinol Invest 2007; 30:323-6; PMID:17556870; http://dx.doi.org/10.1007/BF03346300
- Haider DG, Schindler K, Schaller G, Prager G, Wolzt M, Ludvik B. Increased plasma visfatin concentrations in morbidly obese subjects are reduced after gastric banding. J Clin Endocrinol Metab 2006; 91:1578-81; PMID:16449335; http://dx.doi.org/10.1210/jc.2005-2248
- Berndt J, Kloting N, Kralisch S, Kovacs P, Fasshauer M, Schon MR, Stumvoll M, Blüher M. Plasma visfatin concentrations and fat depot-specific mRNA expression in humans. Diabetes 2005; 54:2911-6; PMID:16186392
- Pagano C, Pilon C, Olivieri M, Mason P, Fabris R, Serra R, Milan G, Rossato M, Federspil G, Vettor R. Reduced plasma visfatin/pre-B cell colony-enhancing factor in obesity is not related to insulin resistance in humans. J Clin Endocrinol Metab 2006; 91:3165-70; PMID:16720654; http://dx.doi.org/10.1210/jc.2006-0361
- Rodriguez AM, Elabd C, Delteil F, Astier J, Vernochet C, Saint-Marc P, Guesnet J, Guezennec A, Amri EZ, Dani C, et al. Adipocyte differentiation of multipotent cells established from human adipose tissue. Biochem Biophys Res Commun 2004; 315:255-63; PMID:14766202; http://dx.doi.org/10.1016/j.bbrc.2004.01.053
- Elabd C, Chiellini C, Carmona M, Galitzky J, Cochet O, Petersen R, Pénicaud L, Kristiansen K, Bouloumié A, Casteilla L, et al. Human multipotent adipose-derived stem cells differentiate into functional brown adipocytes. Stem Cells 2009; 27:2753-60; PMID:19697348; http://dx.doi.org/10.1002/stem.200
- Pisani DF, Ghandour RA, Beranger GE, Le Faouder P, Chambard JC, Giroud M, Vegiopoulos A, Djedaini M, Bertrand-Michel J, Tauc M, et al. The omega6-fatty acid, arachidonic acid, regulates the conversion of white to brite adipocyte through a prostaglandin/calcium mediated pathway. Mol Metab 2014; 3:834-47; PMID:25506549; http://dx.doi.org/10.1016/j.molmet.2014.09.003
- Pisani DF, Djedaini M, Beranger GE, Elabd C, Scheideler M, Ailhaud G, Amri EZ. Differentiation of Human Adipose-Derived Stem Cells into “Brite” (Brown-in-White) Adipocytes. Front Endocrinol (Lausanne) 2011; 2:87; PMID:22654831
