ABSTRACT
Obesity is characterized by an excessive fat accumulation in adipose tissues leading to weight gain and is increasing in prevalence and is strongly associated with metabolic and cardiovascular disorders. The renin-angiotensin system (RAS) has emerged as a key pathogenic mechanism for these disorders; activated RAS and angiotensin (Ang) II production results in worsening of cardiovascular diseases and angiotensin converting enzyme 2 (ACE2) negatively regulates RAS by metabolizing Ang II into Ang 1-7. ACE2 is expressed in the adipocytes and its expression is upregulated in response to high fat diet induced obesity in mice. Loss of ACE2 results in heart failure with preserved ejection fraction which is mediated in part by epicardial adipose tissue inflammation. Angiotensin 1-7 reduces the obesity associated cardiac dysfunction predominantly via its role in adiponectin expression and attenuation of epicardial adipose tissue inflammation. Human heart disease is also linked with inflammed epicardial adipose tissue. Here, we discuss the important interpretation of the novel of ACE2/Ang 1-7 pathway in obesity associated cardiac dysfunction.
Introduction
Obesity is characterized by the excessive fat accumulation in the adipose tissues across the body leading to android type (males; central distribution) and gynoid type (females; gluteal/femoral accumulation) fat distributions. It is the most common nutritional disorder in industrialized countries and is associated with an increased mortality and morbidity of cardiovascular diseases (CVD). In fact, obesity itself is an independent risk factor for the development of heart failure with preserved ejection fraction (HFPEF) independent of other co-morbid conditions.Citation1-4 Human heart is surrounded by 2 different depots of adipose tissue; epicardial adipose tissue (EAT) and pericardial adipose tissue. Anatomically, epicardial and pericardial adipose tissue are very distinct in terms of their locations; while EAT is located between the myocardium and the visceral layer of pericardium, pericardial adipose tissue is located between visceral and parietal pericardium. However, due to its anatomical proximity to the myocardium and the lack of an absolute anatomical boundary between the EAT and the heart, EAT likely has a dominant role in cardiovascular biology.Citation5 Furthermore, the location of EAT in respect to heart eg. peri-atrial, peri-ventricular, determines the transcriptomic signature of the EAT.Citation6 The importance of EAT in the cardiac disease is illustrated by the association between EAT inflammation and cardiac dysfunction.Citation7,8
Renin-angiotensin system and its role in adipose tissue inflammation
The renin-angiotensin system (RAS) is a family of peptides with endocrine and paracrine functions. Angiotensinogen, the precursor of RAS, is converted to Ang I and Ang II by the enzymes renin and angiotensin converting enzyme (ACE) respectively, where Ang II is the active peptide which is involved in the regulations of blood pressure, fluid balance etc. ACE2, a newly discovered member of the RAS family,Citation9,10 is a monocarboxypeptidase which degrades (and inactivates) Ang II into Ang 1-7, a ligand for Mas receptor (MasR)Citation11 showing physiological antagonism to Ang II effects. Thus ACE2/Ang 1–7/MasR axis is a negative regulator of ACE/Ang II/AT1 receptor (AT1R) axis. Angiotensinogen is expressed and constitutively secreted from adipose tissues by mature adipocytes in both humans and experimental animal models.Citation12 Similarly, other components of RAS, including AT1R, ACE2 and MasR are also expressed in the white and brown adipose tissues.Citation13,14 The angiotensin type 1 (AT1R) and type 2 (AT2R) receptors may mediate the effect of Ang II and cause upregulation of adipose tissue lipogenesis (mediated via AT2R) and downregulation of lipolysis (mediated via AT1R).Citation15,16
Activated RAS, and resultant production of Ang II is critically involved in obesity associated inflammation.Citation17,18 Macrophages express the AT1 receptors and their activation by Ang II worsens the extent and complexity of end-organ inflammation by macrophage polarization toward the classically activated or CD11c+ M1 phenotype.Citation17 Macrophages also express the MasR transcripts and their activation by Ang 1–7 treatment decreases LPS-induced expression of pro-inflammatory cytokines.Citation19 Thus Ang II/AT1R activation is involved in the macrophage polarization resulting in increased inflammation, whereas Ang 1–7 decreases expression of the pro-inflammatory cytokines. Importantly, ACE2 expression in the bone marrow cells regulate macrophage polarization and adipose tissue inflammation,Citation20 providing further evidence for a crucial role of ACE2 in adipose tissue inflammation. In addition to the effects on adipose tissue and macrophages, we have also shown a promising cardioprotective role of ACE2/Ang 1–7 axis in murine models of different cardiac diseases.Citation21-26
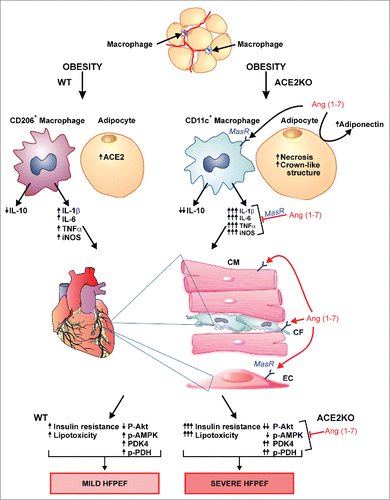
In light of these previous reports, and our observed upregulation of ACE2 in EAT, we hypothesized that the ACE2/Ang 1–7 axis plays a critical role in obesity-associated EAT inflammation leading to impaired cardiac metabolism and heart failure with preserved ejection fraction.Citation27 We showed the critical role of ACE2 and Ang 1–7 in the regulation of obesity associated heart failure.Citation27 In this study, high fat diet feeding-induced obesity (DIO) in ACE2-null (ACE2KO) mice resulted in increased glucose intolerance in spite of reduced obesity. In addition, DIO worsened the EAT inflammation and the macrophage polarization leading to increased resident CD11c+ adipose tissue macrophages (ATM) and increased gene expressions of pro-inflammatory cytokines, TNF-α, IL-1β, IL-6 and iNOS (). In light of the previously illustrated fundamental role of macrophages in adipose tissue inflammation and regulation of insulin sensitivity,Citation28 our data provides an essential role of ACE2 in negative regulation obesity-induced EAT inflammation. Epicardial adipose tissue inflammation in obesity is associated with the progression of cardiac dysfunction.Citation29-32 Increased EAT inflammation in obese-ACE2KO mice also resulted in worsening of myocardial insulin resistance resulting in impaired cardiac metabolism, which was also associated with increased lipotoxicity, oxidative stress and impaired molecular signaling ().Citation27 Angiotensin 1–7 administration to obese-ACE2KO mice for 4 weeks attenuated the EAT inflammation, impaired cardiac metabolism, lipotoxicity, oxidative stress resulting in preserved cardiac function.Citation33 Ang 1–7 effects are predominantly mediated by the activation of its endogenous G-protein coupled receptor, Mas, which is widely expressed including adipocytes.Citation33,34 Importantly, in our study, Ang 1–7 administration for 4 weeks resulted in marked increase in myocardial adiponectin levels as assessed by Western blot and immunofluorescence analyses, in response to Ang 1–7 treatment,Citation27 which might be mediated via increased gene expression in response to Ang 1–7/MasR signaling or may be secondary to the anti-inflammatory effects of Ang 1–7/MasR. However, it is critical to assess the effect of Ang 1–7 on adiponectin gene/protein expression in the EAT. It is noteworthy that Ang 1–7/MasR activation in macrophages is known to mediate anti-inflammatory effects resulting in decreased expression of pro-inflammatory cytokines. Adiponectin is an anti-inflammatory adipokine, synthesized exclusively in the adipocytes. In addition to its classic endocrine effects on heart, adiponectin is also thought to have paracrine effects on the myocardium.Citation35,36 Due to the anatomical proximity between both tissues, increased myocardial adiponectin level in Ang 1–7 treated mice could have resulted from paracrine secretion from the EAT adipocytes. Moreover, the increased myocardial adiponectin levels could also be secreted from other adipose tissues leading to endocrine effects on the heart and assessment of plasma adiponectin levels is essential to understand the endocrine vs paracrine effects of adiponectin. Based on our study, we conclude that ACE2 and Ang 1–7 are negative regulators of obesity associated EAT inflammation and cardiac dysfunction. Our results, however, raise important questions which should be addressed in future studies such as whether the ACE2/Ang 1–7 mediated anti-inflammatory effects are limited to EAT or include other adipose tissue depots. Indeed, the role of ACE2/Ang 1–7 in the peri-renalCitation37 as well as peri-vascular adipose tissue are particularly important.
Figure 1. Role of ACE2 and Ang 1–7 in obesity associated epicardial adipose tissue (EAT) inflammation and cardiac dysfunction. Obesity results in ACE2 upregulation in the adipose tissue of WT mice along with the greater increase in alternatively activated CD206+ macrophages resulting in mild EAT inflammation, cardiac insulin resistance and metabolic impairment leading to mild heart failure with preserved ejection fraction (HFPEF). Obesity in ACE2KO mice results in increased EAT inflammation, worsened cardiac insulin resistance, lipotoxicity and metabolic impairment leading to worsened HFPEF. Angiotensin 1–7 suppresses the EAT inflammation, increases myocardial adiponectin levels and attenuates HFPEF by suppressing the lipotoxicity and correcting the metabolic alterations. ACE2KO: ACE2-null; AMPK: AMP-activated protein kinase; Ang 1–7: Angiotensin 1–7; IL: interleukin; iNOS: inducible nitric oxide synthase; PDH: pyruvate dehydrogenase; PDK4: pyruvate dehydrogenase kinase-4; TNFα: tumor necrosis factor-α; WT: wildtype.
The use of human epicardial adipose tissue
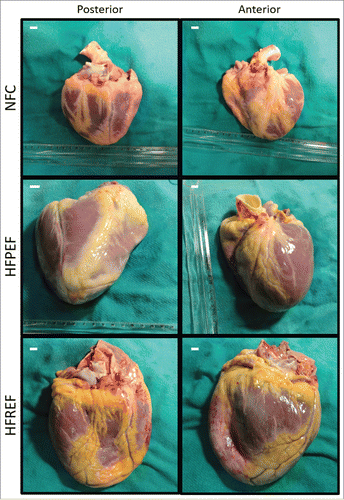
Murine models are not ideal models to study EAT inflammation especially as it relates to human diseases. Human tissues are very different than mice in terms of the amount and localization of EAT. In mice, EAT is localized to atrioventricular groove, whereas the human heart has a much greater abundance of EAT and is nearly completely surrounded by EAT ().Citation38 Importantly, obese patients with heart failure (preserved or reduced ejection fraction) show increased deposition of EAT around the heart when compared with non-obese non-failing controls (). Therefore, we think the human tissues are the ultimate candidate to assess translational nature of the scientific findings, particularly the investigations involving EAT. Therefore, in addition to mice experiments, we also assessed the ACE2 levels and inflammatory status in the EAT obtained from non-failing control and obese patients with HFPEF, which essentially showed similar results validating our experimental findings.Citation27
Figure 2. Epicardial adipose tissue in human hearts. Representative anterior and posterior views of human explanted hearts as a non-failing control (NFC) (weight = 290 g), heart failure with preserved ejection fraction (HFPEF) (ejection fraction = 55%; weight = 590 g) and heart failure with reduced ejection fraction (HFREF) (ejection fraction = 23%; weight = 678 g) depicting the extensive epicardial adipose tissue. Scalebar indicates 1 cm.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Funding
VBP is supported by Alberta Innovates-Health Solutions (AI-HS) Fellowship. We acknowledge funding support from Canadian Institutes of Health Research, Heart and Stroke Foundation and AI-HS.
References
- Kenchaiah S, Sesso HD, Gaziano JM. Body mass index and vigorous physical activity and the risk of heart failure among men. Circulation 2009; 119:44-52; PMID:19103991; http://dx.doi.org/10.1161/CIRCULATIONAHA.108.807289.
- Kenchaiah S, Evans JC, Levy D, Wilson PW, Benjamin EJ, Larson MG, Kannel WB, Vasan RS. Obesity and the risk of heart failure. N Engl J Med 2002; 347:305-13; PMID:12151467; http://dx.doi.org/10.1056/NEJMoa020245.
- Owan TE, Hodge DO, Herges RM, Jacobsen SJ, Roger VL, Redfield MM. Trends in prevalence and outcome of heart failure with preserved ejection fraction. N Engl J Med 2006; 355:251-9; PMID:16855265; http://dx.doi.org/10.1056/NEJMoa052256.
- Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE, Jr., Drazner MH, Fonarow GC, Geraci SA, Horwich T, Januzzi JL, et al. 2013 ACCF/AHA guideline for the management of heart failure: executive summary: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation 2013; 128:1810-52; PMID:23741057; http://dx.doi.org/10.1161/CIR.0b013e31829e8807.
- Iacobellis G. Epicardial and pericardial fat: close, but very different. Obesity 2009; 17:625; author reply 6–7; PMID:19322142; http://dx.doi.org/10.1038/oby.2008.575.
- Gaborit B, Venteclef N, Ancel P, Pelloux V, Gariboldi V, Leprince P, Amour J, Hatem SN, Jouve E, Dutour A, et al. Human epicardial adipose tissue has a specific transcriptomic signature depending on its anatomical peri-atrial, peri-ventricular, or peri-coronary location. Cardiovasc Res 2015; 108:62-73; PMID:26239655; http://dx.doi.org/10.1093/cvr/cvv208.
- Doesch C, Haghi D, Fluchter S, Suselbeck T, Schoenberg SO, Michaely H, Borggrefe M, Papavassiliu T. Epicardial adipose tissue in patients with heart failure. J Cardiovasc Magn Reson 2010; 12:40; PMID:20624277; http://dx.doi.org/10.1186/1532-429X-12-40.
- Watanabe K, Kishino T, Sano J, Ariga T, Okuyama S, Mori H, Matsushima S, Ohtsuka K, Ohnishi H, Watanabe T. Relationship between epicardial adipose tissue thickness and early impairment of left ventricular systolic function in patients with preserved ejection fraction. Heart Vessels 2015. http://dx.doi.org/10.1007/s00380-015-0650-8.
- Tipnis SR, Hooper NM, Hyde R, Karran E, Christie G, Turner AJ. A human homolog of angiotensin-converting enzyme. Cloning and functional expression as a captopril-insensitive carboxypeptidase. J Biol Chem 2000; 275:33238-43; PMID:10924499; http://dx.doi.org/10.1074/jbc.M002615200.
- Donoghue M, Hsieh F, Baronas E, Godbout K, Gosselin M, Stagliano N, Donovan M, Woolf B, Robison K, Jeyaseelan R, et al. A novel angiotensin-converting enzyme-related carboxypeptidase (ACE2) converts angiotensin I to angiotensin 1–9. Circ Res 2000; 87:E1-9; PMID:10969042; http://dx.doi.org/10.1161/01.RES.87.5.e1.
- Bader M, Alenina N, Andrade-Navarro MA, Santos RA. MAS and its related G protein-coupled receptors, Mrgprs. Pharmacol Rev 2014; 66:1080-105; PMID:25244929; http://dx.doi.org/10.1124/pr.113.008136.
- Engeli S, Schling P, Gorzelniak K, Boschmann M, Janke J, Ailhaud G, Teboul M, Massiéra F, Sharma AM. The adipose-tissue renin-angiotensin-aldosterone system: role in the metabolic syndrome? Int J Biochem Cell Biol 2003; 35:807-25; PMID:12676168; http://dx.doi.org/10.1016/S1357-2725(02)00311-4.
- Paul M, Poyan Mehr A, Kreutz R. Physiology of local renin-angiotensin systems. Physiol Rev 2006; 86:747-803; PMID:16816138; http://dx.doi.org/10.1152/physrev.00036.2005.
- Yvan-Charvet L, Quignard-Boulange A. Role of adipose tissue renin-angiotensin system in metabolic and inflammatory diseases associated with obesity. Kidney Int 2011; 79:162-8; PMID:20944545; http://dx.doi.org/10.1038/ki.2010.391.
- Jones BH, Standridge MK, Moustaid N. Angiotensin II increases lipogenesis in 3T3-L1 and human adipose cells. Endocrinology 1997; 138:1512-9; PMID:9075710.
- Boschmann M, Ringel J, Klaus S, Sharma AM. Metabolic and hemodynamic response of adipose tissue to angiotensin II. Obes Res 2001; 9:486-91; PMID:11500529; http://dx.doi.org/10.1038/oby.2001.63.
- Yamamoto S, Yancey PG, Zuo Y, Ma LJ, Kaseda R, Fogo AB, Ichikawa I, Linton MF, Fazio S, Kon V. Macrophage polarization by angiotensin II-type 1 receptor aggravates renal injury-acceleration of atherosclerosis. Arterioscler Thromb Vasc Biol 2011; 31:2856-64; PMID:21979434; http://dx.doi.org/10.1161/ATVBAHA.111.237198.
- Zhang JD, Patel MB, Griffiths R, Dolber PC, Ruiz P, Sparks MA, Stegbauer J, Jin H, Gomez JA, Buckley AF, et al. Type 1 angiotensin receptors on macrophages ameliorate IL-1 receptor-mediated kidney fibrosis. J Clin Invest 2014; 124:2198-203; PMID:24743144; http://dx.doi.org/10.1172/JCI61368.
- Souza LL, Costa-Neto CM. Angiotensin-(1–7) decreases LPS-induced inflammatory response in macrophages. J Cell Physiol 2012; 227:2117-22; PMID:21769868; http://dx.doi.org/10.1002/jcp.22940.
- Thatcher SE, Gupte M, Hatch N, Cassis LA. Deficiency of ACE2 in Bone-Marrow-Derived cells increases expression of TNF-α in adipose stromal cells and augments glucose intolerance in obese C57BL/6 mice. Int J Hypertens 2012; 2012:762094; PMID:22518292; http://dx.doi.org/10.1155/2012/762094.
- Patel VB, Bodiga S, Basu R, Das SK, Wang W, Wang Z, Lo J, Grant MB, Zhong J, Kassiri Z, et al. Loss of angiotensin-converting enzyme-2 exacerbates diabetic cardiovascular complications and leads to systolic and vascular dysfunction: a critical role of the angiotensin II/AT1 receptor axis. Circ Res 2012; 110:1322-35; PMID:22474255; http://dx.doi.org/10.1161/CIRCRESAHA.112.268029.
- Patel VB, Bodiga S, Fan D, Das SK, Wang Z, Wang W, Basu R, Zhong J, Kassiri Z, Oudit GY. Cardioprotective Effects Mediated by Angiotensin II Type 1 Receptor Blockade and Enhancing Angiotensin 1–7 in Experimental Heart Failure in Angiotensin-Converting Enzyme 2-Null Mice. Hypertension 2012; 59:1195-203; PMID:22508831; http://dx.doi.org/10.1161/HYPERTENSIONAHA.112.191650.
- Patel VB, Parajuli N, Oudit GY. Role of angiotensin-converting enzyme 2 (ACE2) in diabetic cardiovascular complications. Clin Sci (Lond) 2014; 126:471-82; PMID:24329564; http://dx.doi.org/10.1042/CS20130344.
- Patel VB, Takawale A, Ramprasath T, Das SK, Basu R, Grant MB, Hall DA, Kassiri Z, Oudit GY. Antagonism of angiotensin 1–7 prevents the therapeutic effects of recombinant human ACE2. J Mol Med (Berl) 2015; 93(9):1003-13.
- Patel VB, Zhong JC, Fan D, Basu R, Morton JS, Parajuli N, McMurtry MS, Davidge ST, Kassiri Z, Oudit GY. Angiotensin-converting enzyme 2 is a critical determinant of angiotensin II-induced loss of vascular smooth muscle cells and adverse vascular remodeling. Hypertension 2014; 64:157-64; PMID:24799609; http://dx.doi.org/10.1161/HYPERTENSIONAHA.114.03388.
- Wang W, Patel VB, Parajuli N, Fan D, Basu R, Wang Z, Ramprasath T, Kassiri Z, Penninger JM, Oudit GY. Heterozygote loss of ACE2 is sufficient to increase the susceptibility to heart disease. J Mol Med (Berl) 2014; 92:847-58; PMID:24728465; http://dx.doi.org/10.1007/s00109-014-1149-y.
- Patel VB, Mori J, McLean BA, Basu R, Das SK, Ramprasath T, Parajuli N, Penninger JM, Grant MB, Lopaschuk GD, et al. ACE2 deficiency worsens epicardial adipose tissue inflammation and cardiac dysfunction in response to diet-induced obesity. Diabetes 2015; 65(1):85-95; http://dx.doi.org/10.2337/db15-0399.
- Fujisaka S, Usui I, Bukhari A, Ikutani M, Oya T, Kanatani Y, Tsuneyama K, Nagai Y, Takatsu K, Urakaze M, et al. Regulatory mechanisms for adipose tissue M1 and M2 macrophages in diet-induced obese mice. Diabetes 2009; 58:2574-82; PMID:19690061; http://dx.doi.org/10.2337/db08-1475.
- Cherian S, Lopaschuk GD, Carvalho E. Cellular cross-talk between epicardial adipose tissue and myocardium in relation to the pathogenesis of cardiovascular disease. Am J Physiol Endocrinol Metab 2012; 303:E937-49; PMID:22895783; http://dx.doi.org/10.1152/ajpendo.00061.2012.
- Van Gaal LF, Mertens IL, De Block CE. Mechanisms linking obesity with cardiovascular disease. Nature 2006; 444:875-80; PMID:17167476; http://dx.doi.org/10.1038/nature05487.
- Fontes-Carvalho R, Fontes-Oliveira M, Sampaio F, Mancio J, Bettencourt N, Teixeira M, Rocha Gonçalves F, Gama V, Leite-Moreira A. Influence of epicardial and visceral fat on left ventricular diastolic and systolic functions in patients after myocardial infarction. Am J Cardiol 2014; 114:1663-9; PMID:25306552; http://dx.doi.org/10.1016/j.amjcard.2014.08.037.
- Fitzgibbons TP, Czech MP. Epicardial and perivascular adipose tissues and their influence on cardiovascular disease: basic mechanisms and clinical associations. J Am Heart Assoc 2014; 3:e000582; PMID:24595191; http://dx.doi.org/10.1161/JAHA.113.000582.
- Loot AE, Roks AJ, Henning RH, Tio RA, Suurmeijer AJ, Boomsma F, van Gilst WH. Angiotensin-(1–7) attenuates the development of heart failure after myocardial infarction in rats. Circulation 2002; 105:1548-50; PMID:11927520; http://dx.doi.org/10.1161/01.CIR.0000013847.07035.B9.
- Santos RA, Simoes e Silva AC, Maric C, Silva DM, Machado RP, de Buhr I, Heringer-Walther S, Pinheiro SV, Lopes MT, Bader M, et al. Angiotensin-(1–7) is an endogenous ligand for the G protein-coupled receptor Mas. Proc Natl Acad Sci U S A 2003; 100:8258-63; PMID:12829792; http://dx.doi.org/10.1073/pnas.1432869100.
- Skurk C, Wittchen F, Suckau L, Witt H, Noutsias M, Fechner H, Schultheiss HP, Poller W. Description of a local cardiac adiponectin system and its deregulation in dilated cardiomyopathy. Eur Heart J 2008; 29:1168-80; PMID:18390538; http://dx.doi.org/10.1093/eurheartj/ehn136.
- Dadson K, Liu Y, Sweeney G. Adiponectin action: a combination of endocrine and autocrine/paracrine effects. Front Endocrinol 2011; 2:62; PMID:22649379; http://dx.doi.org/10.3389/fendo.2011.00062.
- Mori J, Patel VB, Ramprasath T, Alrob OA, DesAulniers J, Scholey JW, Lopaschuk GD, Oudit GY. Angiotensin 1–7 mediates renoprotection against diabetic nephropathy by reducing oxidative stress, inflammation, and lipotoxicity. Am J Physiol Renal Physiol 2014; 306:F812-21; PMID:24553436; http://dx.doi.org/10.1152/ajprenal.00655.2013.
- Iacobellis G, Corradi D, Sharma AM. Epicardial adipose tissue: anatomic, biomolecular and clinical relationships with the heart. Nat Clin Pract Cardiovasc Med 2005; 2:536-43; PMID:16186852; http://dx.doi.org/10.1038/ncpcardio0319.
