ABSTRACT
The global epidemic in obesity and metabolic syndrome requires novel approaches to tackle. White adipose tissue, traditionally seen as a passive energy-storage organ, can be induced to take on certain characteristics of brown fat in a process called browning. The “browned” white adipose tissue, or beige fat, is a potential anti-obesity target. Various signaling pathways can enhance browning. Wnt is a key regulator of adipocyte biology, but its role in browning has not been explored. In this study, we found that in primary mouse adipocytes derived from the inguinal depot, Wnt inhibition by both chemical and genetic methods significantly enhanced browning. The effect of Wnt inhibition on browning most likely targets the beige precursor cells in selected adipose depots.
Introduction
Obesity has reached an epidemic level globally. In the simplest sense, obesity arises from an imbalance of energy intake and energy expenditure. Methods that could increase energy expenditure are potential anti-obesity strategies. Adipose tissue is the primary energy storage organ in the body. There are 2 main types of adipose tissue, white adipose tissue (WAT) and brown adipose tissue (BAT). WAT is responsible for storing excess energy as triglycerides for use in times of scarcity. Excess accumulation of WAT leads to obesity and increases the risk for metabolic disorders. On the other hand, BAT, which expresses UCP1 and other thermogenic proteins, has anti-obesity properties as it dissipates energy via non-shivering thermogenesis. This is a mechanism to maintain core body temperature and prevent hypothermia in mammals and human infants. BAT and WAT are different in their functions, anatomical locations, gene expression, and origins.Citation1
A third type of fat, known as beige or brite (brown-in-white) fat, are brown fat-like cells found in certain depots of white fat.Citation2 Beige adipocytes have been traced to have a smooth muscle-like,Citation3 endothelial or perivascular cell origin.Citation4 In response to certain conditions such as cold exposure,Citation5 PPARγ agonists,Citation6 exercise,Citation7or β-adrenergic stimulation,Citation8 beige adipocytes located within white fat are activated in a process known as browning. Once activated, they express UCP1 and some other BAT genes, and they are also capable of thermogenesis.Citation9 Morphologically, they are characterized by multilocular lipid droplets and a high mitochondrial content. Certain depots of WAT, such as the inguinal and retroperitoneal depots, are more susceptible to browning.Citation10
Different signaling pathways and regulators have been shown to have an effect on browning of white fat.Citation11 For example, inhibition of Notch Citation12 and JAK signaling Citation13 have been shown to promote browning in mouse and human white adipocytes, respectively. Recently, it has been shown that Lrp5, a Wnt coreceptor, is a regulator of adipose progenitor biology.Citation14 Adipose progenitors have been postulated to be a source of beige fat through a de novo adipogenesis process,Citation15 although it was also suggested that beige cells could arise from existing mature white adipocytes.Citation16
Wnts are a family of 19 glycoproteins that act in an autocrine and paracrine manner to regulate tissue homeostasis and remodeling. These molecules signal through diverse non-canonical and canonical pathways, the latter of which converges on the transcriptional regulator β-catenin. Wnt signaling is a key regulator of mesenchymal stem cells (MSC) biology and adipogenesis.Citation17 Conditional deletion of β-catenin in mouse uterus leads to a switch from myogenesis to adipogenesis.Citation18 Wnt signaling also has an inhibitory role in both white and brown adipogenesis.Citation19,20 Interestingly, overexpression of Wnt10b under the control of Ucp1 promoter results in BAT gaining molecular and histological features of WAT.Citation20 It has not been explored if altering Wnt signaling in white fat would have any effect on browning. In this current study, we find that in primary adipocytes derived from mouse inguinal fat inhibition of Wnt signaling enhances browning. The effect of Wnt inhibition on browning appears to target adipocyte progenitors rather than mature adipocytes or the lineage-determined preadipocyte population, lending support to the theory that beige fat is derived from de novo differentiation of beige precursors. Furthermore, Wnt inhibition enhanced the thermogenic properties of primary brown adipocytes, but did not induce browning of primary white adipocytes derived from epididymal depots, suggesting that the effect of Wnt inhibition on browning is specific to certain fat depots.
Results
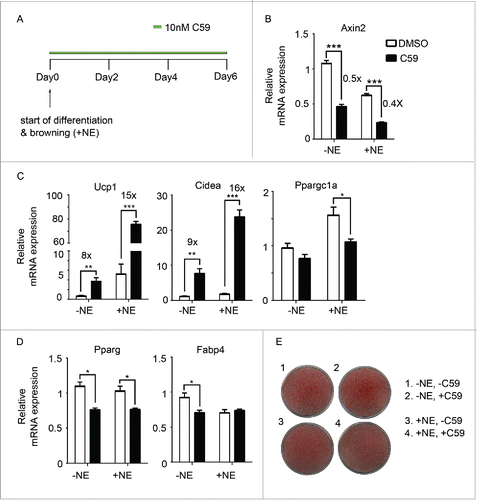
To test if Wnt inhibition would have any effect on browning, the stromal vascular fraction (SVF) from mouse inguinal WAT was isolated and differentiated into mature adipocytes using a standard cocktail (see Methods). Wnt inhibitor C59 or vehicle was added for 6 d from the start of differentiation (). C59 blocks Wnt signaling by inhibiting porcupine (PORCN),Citation21 which is an acyltransferase responsible for palmitoylation of all Wnt proteins.Citation22,23 Inhibiting PORCN blocks Wnt secretion and down-stream Wnt signaling, which was confirmed by the down-regulation of Axin2 () by around 50% both in the presence and absence of norepinephrine. Remarkably, C59 treatment led to increased expression of thermogenic markers (Ucp1 and Cidea), the expression of these 2 genes was increased by more than 15-fold in the presence of norepinephrine (), though the expression of Ppargc1a, an important coregulator in brown fat markers, was not elevated. The induction of the expression of BAT genes occurred in a dose-dependent manner (Supplementary Fig. 1A) and could be observed as early as Day 4 (Supplementary Fig. 1B), though the induction of Ucp1 on the protein level is minimal on Day 6 (Supplementary Fig. 1C). Wnt inhibition has been shown to enhance adipogenesis.Citation19,20 To test if the induction of thermogenic genes is a consequence of enhanced adipogenesis, we checked the expression of Pparg and Fabp4, which did not show any up-regulation upon C59 treatment (). Furthermore, oil red O staining shows similar differentiation efficiency in all groups ().
Figure 1. Inhibition of Wnt signaling pharmacologically enhances browning (A) Experiment schematics of in vitro browning experiment. Primary mouse adipocytes differentiated from the stromal vascular fraction (SVF) of inguinal fat pads were treated with 10 nM of C59 for 6 d from the start of differentiation. Browning was induced by adding 1 µM norepinephrine. (B-D) qPCR analysis of Wnt signaling gene (Axin2) (B), BAT genes (Ucp1, Cidea, Ppargc1a) (C), and pan-adipocyte genes (Pparg, Fabp4) (D) on RNA extracted at Day 6 according to scheme 1(A). Shown is the relative mRNA expression values normalized with the housekeeping gene Rpl23. All data are presented as mean ± SEM by Student's t-test. * p < 0.05, ** p < 0.01 and *** p < 0.001 comparing with control. The fold of induction or inhibition is indicated when the change is significant and relevant. (E) Oil red O staining of primary mouse adipocytes at Day 6.
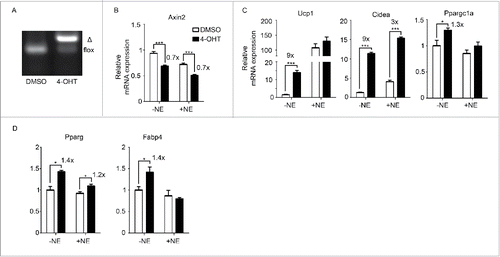
As an alternative approach to inhibiting Porcn and hence Wnt secretion, we isolated SVF from inguinal WAT of Rosa26-CreERT2/Porcn fl/fl mice Citation24-26 and differentiated it into mature adipocytes. The Porcn gene was then excised in vitro by addition of 4-hydroxytamoxifen (4-OHT) (). We verified that Wnt signaling was inhibited as shown by 30% downregulation of Axin2 (). Wnt inhibition as a result of Porcn deletion led to an increased expression of Ucp1, Cidea and Ppargc1a (), though there was also a small but significant induction of Pparg and Fabp4 (). These data suggest that inhibiting Wnt signaling via both pharmacological and genetic approaches enhance expression of key thermogenic genes in primary inguinal adipocytes.
Figure 2. Inhibition of Wnt signaling genetically enhances browning (A) Porcn deletion in Rosa26-CreERT2/Porcnfl/fl mice upon addition of 4-hydroxytamoxifen (4-OHT). Δ: PCR product after Porcn is deleted. (B-D) SVF was isolated from inguinal fat pads of Rosa26-CreERT2/Porcnfl/fl mice and differentiated to adipocytes in the presence or absence of 4-OHT to delete Porcn. RNA was extracted at Day 6 of differentiation. qPCR analysis of Wnt signaling gene (Axin2) (B), BAT genes (Ucp1, Cidea, Ppargc1a) (C), and pan-adipocyte genes (Pparg, Fabp4) (D) on RNA extracted at Day 6 according to scheme 1(A). Shown is the relative mRNA expression values normalized with the housekeeping gene Rpl23. The fold of induction or % of inhibition is indicated when the change is significant and relevant. All data are presented as mean ± SEM by Student's t-test. * p < 0.05, ** p < 0.01 and *** p < 0.001 comparing with the control model.
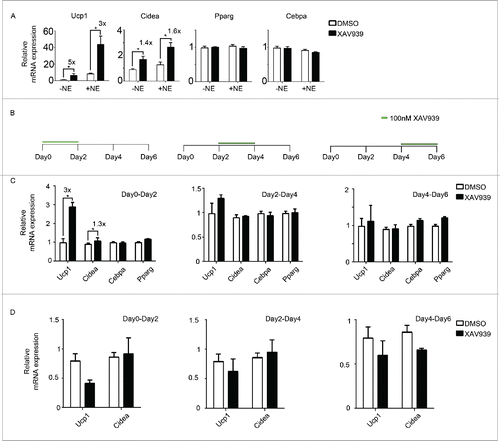
To further dissect the signaling pathway involved, a second Wnt inhibitor was used. XAV939 is a tankyrase inhibitor that specifically targets the canonical Wnt pathway by increasing Axin protein abundance, thereby stimulating β-catenin degradation.Citation27 Similar to C59, XAV939 induced the expression of Ucp1 by 5-fold and that of Cidea by 1.4-fold but not Pparg and Cebpa (), suggesting that the effect of Wnt inhibition on browning works through the canonical Wnt/β-catenin pathway. To further examine what cells are affected as a result of Wnt inhibition, XAV939 was administered for only 2 d starting at 3 different time points during the course of differentiation (). Expression of Ucp1 and Cidea increased by 3-fold and 1.3-fold respectively when XAV939 was administered from Day 0 to Day 2, indicating that the effect of Wnt inhibition on browning is strongest when inhibition takes place early during differentiation (). Wnt inhibition was largely ineffective in inducing BAT genes when it occurred late in differentiation. Similar results were obtained using C59 (data not shown). This suggests that the positive effect of Wnt inhibition on browning most likely targets beige precursor cells rather than mature white adipocytes. We repeated the same experiment in 3T3-L1, a cell line which consists of clonal pre-determined preadipocytes prior to differentiation. There was no induction of thermogenic gene expression in 3T3-L1 upon XAV939 treatment (). This suggests that the beige fat precursors responsive to Wnt inhibition are present in the SVF of primary cells but not in the lineage-predetermined 3T3-L1 cells.
Figure 3. Wnt inhibition likely targets beige precursor cells to enhance browning (A) Primary adipocytes were treated with a second Wnt signaling inhibitor, XAV939. Shown is the qPCR analysis of marker genes after treatment following scheme 1(A). (B) Experiment schematics of in vitro browning experiment using primary adipocytes. Cells were treated with 100 nM XAV939 for only 2 d starting from Day 0, Day 2 or Day 4 during differentiation. RNA was harvested at Day 6 for qPCR analysis. (C) qPCR analysis of markers genes according to experiment plan 3 (B). (D) qPCR analysis of markers genes according to experiment plan 3(B) using 3T3-L1. Shown is the relative mRNA expression values normalized with housekeeping genes Rpl23. The fold of induction is indicated when the change is significant and relevant. All data are presented as mean ± SEM by Student's t-test. * p < 0.05, ** p < 0.01 and *** p < 0.001 comparing with control.
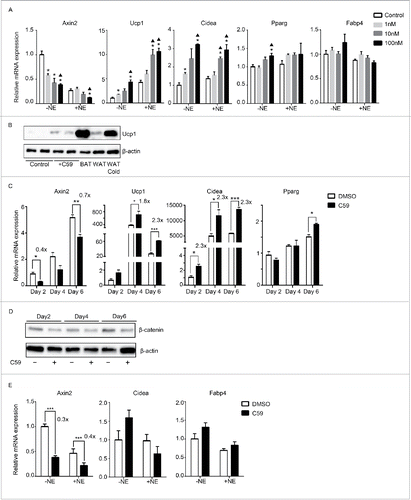
Next, we explored if inhibiting Wnt signaling in primary brown adipocytes would enhance its thermogenic properties. C59 induced Ucp1 and Cidea expression in a dose-responsive manner, while it had little effect on Fabp4 and Pparg expression (). The induction of Ucp1 was also observed at the protein level (). Furthermore, the induction of BAT genes was observed as early as day 4 (Ucp1, 1.8-fold) or even day 2 (Cidea, 2.3-fold) (), suggesting that Wnt inhibition targets precursors or differentiating cells rather than differentiated cells. Downregulation of Wnt signaling by C59 occurred as early as day 2 in both mRNA (Axin2, 60% inhibition) and protein level of β-catenin (). However, Wnt inhibition in primary white adipocytes derived from the SVF of epididymal origin had no effect on browning ().
Figure 4. Wnt inhibition also enhances thermogenesis in primary cells derived from brown adipose tissue but not epididymal adipose tissue (A) Dose-response curve of primary brown adipocytes treated with increasing dose of C59. RNA was extracted at Day 6 for qPCR analysis. Shown is the relative mRNA expression values normalized with housekeeping genes Rpl23. All data are presented as mean ± SEM by Student's t-test. *p < 0.05 comparing with control; ▴p < 0.05 comparing with 1nM. (B) Western Blot analysis on protein extracted at Day 6 from primary brown adipocytes with or without 10 nM C59 treatment. Also shown is protein extracted from brown adipose tissue (BAT), inguinal adipose tissue (WAT) and inguinal adipose tissue from mice underwent 7 d of 5°C cold exposure (WAT cold). (C) Primary brown adipocytes treated with 10 nM of C59 were harvested at 3 different time points (Day 2, Day 4 and Day 6) and subjected to qPCR analysis. (D) Western Blot analysis on protein extracted at Day2, Day4 and Day 6 from primary brown adipocytes during the course of differentiation treated with or without 10 nM C59 (E) Primary white adipocytes derived from epididymal fat pad were subjected to qPCR analysis. All data are presented as mean ± SEM by Student's t-test. *** p < 0.001 comparing with the control model. The fold of induction or inhibition is indicated when the change is significant and relevant.
Discussion
The realization that white fat can take on a brown fat phenotype and function as genuine thermogenic cells opens up the possibility of harnessing this browned white fat to increase energy expenditure and reduce adiposity. Many different treatments, food components, drug substances, transgenes and gene knockouts have been shown to induce white fat browning.Citation2,28 In this study, we investigated if inhibiting Wnt signaling would enhance browning. Using two different small molecule inhibitors of Wnt signaling – one specifically targeting the canonical Wnt pathway – we show in mouse primary inguinal adipocytes that Wnt inhibition led to upregulation of thermogenic genes. Furthermore, the induction and enhancement of browning is most prominent when Wnt signaling is inhibited at the start of differentiation, whereas late inhibition produces little or no effect. This suggests that the effect of Wnt on browning probably targets the beige precursor cells rather than mature white adipocytes. Moreover, the fact that Wnt inhibition has no effect on browning in 3T3-L1 cells suggests that lineage-predetermined white preadipocytes cannot be induced into beige fat upon Wnt inhibition. Interestingly, even primary white cells derived from epididymal fat pad (in contrast to those from inguinal origin) cannot be “browned” upon Wnt inhibition, suggesting that beige precursors likely reside in the heterogeneous SVF of certain fat depots, one example of which is the inguinal depot.
Pericytes have been suggested to be a source of beige precursors.Citation4 Interestingly, it has been shown that Wnt signaling inhibits adipogenic differentiation of pericytes and favors the osteogenic lineage.Citation29 We do not know if the effect of Wnt is cell-autonomous as we could not ascertain the cell type(s) that secrete and receive Wnt. Nevertheless, it is plausible that the immune milieu surrounding inguinal WAT, which has recently been shown to play a major role in browning,Citation30-32 could be a source of Wnt. Wnt could be inhibiting a group of bipotential precursor cells from developing into beige fat. The suppression of Wnt signaling thus abolishes this inhibition, leading to induction of thermogenic genes. Furthermore, Wnt inhibition also has a modest but significant effect on the thermogenic property of primary brown adipocytes derived from the interscapular depot.
In conclusion, our study shows that inhibition of Wnt secretion and downstream Wnt/β-catenin signaling enhances browning in primary inguinal mouse adipocytes. Future directions include identifying the source, identity and target of the Wnts, and the role of Wnt signaling in cell fate determination of beige precursor cells.
Materials and methods
RNA isolation and reverse transcription-PCR
Total RNA was extracted using Qiagen kit. 300ng of RNA was reverse-transcribed to cDNA and used for quantitative PCR using SYBR Green I master mix (Bioline) in HT7900 Real Time PCR System (Applied Biosystems). At least 3 biological replicates were performed for each experiment.
Primary adipocyte culture, differentiation and treatment
Inguinal fat pads from 3-week-old mice were excised, minced and digested in collagenase at 37 °C for 20min. The suspension was then spun at 2000 rpm, washed with PBS, and the pelleted stromal vascular fraction was resuspended in 10 ml of Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% new calf bovine serum (Invitrogen), 100 units/ml penicillin, 100 µg/ml streptomycin and 10 µg/ml of gentamicin (Invitrogen). Medium was changed the next day and then every 2 d. Cells were grown to confluence, and differentiation was initiated at Day0 with DMEM containing 10% fetal bovine serum (FBS), 0.5µM dexamethasone, 850nM insulin, 0.25mM 3-isobutyl-1-methyxanthine and 1µM Rosiglitazone for 2 d. Cells were then incubated in DMEM containing 10% FBS and 850 nM insulin for 2 more days. After Day 4, cells were maintained for 2 more days in DMEM containing 10% FBS. For most experiments, RNA and protein were harvested from mature adipocytes at Day 6.
For primary adipocyte culture involving Rosa26-CreERT2/Porcn fl/fl mice, inguinal fat pad were isolated from 6-month-old mice and cultured as wild-type mice. Deletion of Porcn was initiated by adding 100nM of 4-hydroxytamoxifen (4-OHT) at the start of differentiation.
Western blot
Primary white or brown adipocytes were treated with or without 10nM C59 during the course of differentiation. Cells were lysed in RIPA buffer with protease inhibitor. Proteins were extracted and quantified using Bradford reagent. 30ug of soluble protein was boiled with sample loading buffer for 10min and loaded onto 10% TGX handcast gel (Bio-Rad) for sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). Proteins on the SDS-PAGE gel were then immunoblotted onto PVDF membranes. Primary antibody was then used to probe for Ucp1 (abcam ab10983, 32kDa) and β-catenin (abcam 32572, 92kDa). Membranes were then washed and incubated with horseradish peroxidase-conjugated secondary antibody. Detection of signal was performed using Gel Doc XR system (Bio-rad). Membranes were then stripped for 10 min, re-blocked and incubated with β-actin antibody (Sigma A1978, 43kD) as loading control.
Mouse studies
Mice on the C57BL/6 background were kept at Duke-NUS Medical School animal facilities. Mice were fed a normal chow diet during the course of the study. For cold exposure, 7-week-old male mice were housed at 5°C in a cold chamber (Geneva Scientific) for one week. All mice were sacrificed at 8 weeks of age and interscapular brown adipose tissue and inguinal white adipose tissues were harvested from control and cold-exposed mice and subjected to Western Blot analysis.
Abbreviations
| Axin2 | = | axis inhibition protein 2 |
| BAT | = | brown adipose tissue |
| brite fat | = | brown-in-white fat |
| Cidea | = | Cell death-inducing DFFA-like effector a |
| Fabp4 | = | Fatty acid-binding protein 4 |
| MSC | = | mesenchymal stem cells |
| PORCN | = | Porcupine; Pparg, Peroxisome proliferator-activated receptor gamma |
| SVF | = | stromal vascular fraction |
| UCP1 | = | uncoupling protein 1 |
| WAT | = | White adipose tissue |
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
2015ADIPOCYTE281R1-f05-z-bw.pdf
Download PDF (179.3 KB)Acknowledgments
The authors appreciate the support of Duke-NUS/ SingHealth Academic Medicine Research Institute and the medical editing assistance of Taara Madhavan (Associate, Clinical Sciences, Duke-NUS Graduate Medical School).
References
- Rosen ED, Spiegelman BM. What we talk about when we talk about fat. Cell 2014; 156:20-44; PMID:24439368; http://dx.doi.org/10.1016/j.cell.2013.12.012
- Wu J, Cohen P, Spiegelman BM. Adaptive thermogenesis in adipocytes: Is beige the new brown? Genes Dev 2013; 27:234-50; PMID:23388824; http://dx.doi.org/10.1101/gad.211649.112
- Long JZ, Svensson KJ, Tsai L, Zeng X, Roh HC, Kong X, Rao RR, Lou J, Lokurkar I, Baur W, et al. A Smooth Muscle-Like Origin for Beige Adipocytes. Cell Metab 2014; 19:1-11
- Tran K-V, Gealekman O, Frontini A, Zingaretti MC, Morroni M, Giordano A, Smorlesi A, Perugini J, De Matteis R, Sbarbati A, et al. The vascular endothelium of the adipose tissue gives rise to both white and brown fat cells. Cell Metab 2012; 15:222-9; PMID:22326223; http://dx.doi.org/10.1016/j.cmet.2012.01.008
- Young P, Arch JR, Ashwell M. Brown adipose tissue in the parametrial fat pad of the mouse. FEBS Lett 1984; 167:10-4; PMID:6698197; http://dx.doi.org/10.1016/0014-5793(84)80822-4
- Ohno H, Shinoda K, Spiegelman BM, Kajimura S. PPARγ agonists Induce a White-to-Brown Fat Conversion through Stabilization of PRDM16 Protein. Cell Metab 2012; 15:395-404; PMID:22405074; http://dx.doi.org/10.1016/j.cmet.2012.01.019
- Boström P, Wu J, Jedrychowski MP, Korde A, Ye L, Lo JC, Rasbach KA, Boström EA, Choi JH, Long JZ, et al. A PGC1-α-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature 2012; 481:463-8; PMID:22237023; http://dx.doi.org/10.1038/nature10777
- Cousin B, Cinti S, Morroni M, Raimbault S, Ricquier D, Pénicaud L, Casteilla L. Occurrence of brown adipocytes in rat white adipose tissue: molecular and morphological characterization. J Cell Sci 1992; 103:931-42; PMID:1362571
- Shabalina IG, Petrovic N, de Jong JM, Kalinovich A V, Cannon B, Nedergaard J. UCP1 in brite/beige adipose tissue mitochondria is functionally thermogenic. Cell Rep 2013; 5:1196-203; PMID:24290753; http://dx.doi.org/10.1016/j.celrep.2013.10.044
- Walden TB, Hansen IR, Timmons J a, Cannon B, Nedergaard J. Recruited versus nonrecruited molecular signatures of brown, “brite” and white adipose tissues. Am J Physiol Endocrinol Metab 2011; 302:19-31; http://dx.doi.org/10.1152/ajpendo.00249.2011
- Lo KA, Sun L. Turning WAT into BAT: a review on regulators controlling the browning of white adipocytes. Biosci Rep 2013; 33:711-9; http://dx.doi.org/10.1042/BSR20130046
- Bi P, Shan T, Liu W, Yue F, Yang X, Liang X-R, Wang J, Li J, Carlesso N, Liu X, et al. Inhibition of Notch signaling promotes browning of white adipose tissue and ameliorates obesity. Nat Med 2014; 20:911–918.
- Moisan A, Lee Y-K, Zhang JD, Hudak CS, Meyer C A, Prummer M, Zoffmann S, Truong HH, Ebeling M, Kiialainen A, et al. White-to-brown metabolic conversion of human adipocytes by JAK inhibition. Nat Cell Biol 2014;; PMID:25487280
- Loh NY, Neville MJ, Marinou K, Hardcastle SA, Fielding BA, Duncan EL, McCarthy MI, Tobias JH, Gregson CL, Karpe F, et al. LRP5 Regulates Human Body Fat Distribution by Modulating Adipose Progenitor Biology in a Dose- and Depot-Specific Fashion. Cell Metab 2015; 21:262-72; PMID:25651180; http://dx.doi.org/10.1016/j.cmet.2015.01.009
- Wang QA, Tao C, Gupta RK, Scherer PE. Tracking adipogenesis during white adipose tissue development, expansion and regeneration. Nat Med 2013; 19:1338-44; PMID:23995282; http://dx.doi.org/10.1038/nm.3324
- Barbatelli G, Murano I, Madsen L, Hao Q, Jimenez M, Kristiansen K, Giacobino JP, De Matteis R, Cinti S. The emergence of cold-induced brown adipocytes in mouse white fat depots is determined predominantly by white to brown adipocyte transdifferentiation. Am J Physiol Endocrinol Metab 2010; 298:E1244-53; PMID:20354155; http://dx.doi.org/10.1152/ajpendo.00600.2009
- Christodoulides C, Lagathu C, Sethi JK, Vidal A. Adipogenesis and WNT signalling. Trends Endocrinol Metab 2015; 20:16-24; http://dx.doi.org/10.1016/j.tem.2008.09.002
- Arango N a., Szotek PP, Manganaro TF, Oliva E, Donahoe PK, Teixeira J. Conditional deletion of beta-catenin in the mesenchyme of the developing mouse uterus results in a switch to adipogenesis in the myometrium. Dev Biol 2005; 288:276-83; PMID:16256976; http://dx.doi.org/10.1016/j.ydbio.2005.09.045
- Ross SE. Inhibition of Adipogenesis by Wnt Signaling. Science (80-) 2000; 289:950-3; http://dx.doi.org/10.1126/science.289.5481.950
- Kang S, Bajnok L, Longo KA, Rasmus K, Hansen JB, Kristiansen K, Macdougald OA, Petersen RK. Effects of Wnt Signaling on Brown Adipocyte Differentiation and Metabolism Mediated by PGC-1 α. Mol Cell Biol 2005; 25:1272-82; PMID:15684380; http://dx.doi.org/10.1128/MCB.25.4.1272-1282.2005
- Proffitt KD, Madan B, Ke Z, Pendharkar V, Ding L, Lee MA, Hannoush RN, Virshup DM. Pharmacological inhibition of the Wnt acyltransferase PORCN prevents growth of WNT-driven mammary cancer. Cancer Res 2013; 73:502-7; PMID:23188502; http://dx.doi.org/10.1158/0008-5472.CAN-12-2258
- Najdi R, Proffitt K, Sprowl S, Kaur S, Yu J, Covey TM, Virshup DM, Waterman ML. A uniform human Wnt expression library reveals a shared secretory pathway and unique signaling activities. Differentiation 2012; 84:203-13; PMID:22784633; http://dx.doi.org/10.1016/j.diff.2012.06.004
- Proffitt KD, Virshup DM. Precise regulation of porcupine activity is required for physiological Wnt signaling. J Biol Chem 2012; 287:34167-78; PMID:22888000; http://dx.doi.org/10.1074/jbc.M112.381970
- Biechele S, Cockburn K, Lanner F, Cox BJ, Rossant J. Porcn-dependent Wnt signaling is not required prior to mouse gastrulation. Development 2013; 140:2961-71; PMID:23760955; http://dx.doi.org/10.1242/dev.094458
- Kabiri Z, Greicius G, Madan B, Biechele S, Zhong Z, Zaribafzadeh H, Edison Aliyev J, Wu Y, Bunte R, et al. Stroma provides an intestinal stem cell niche in the absence of epithelial Wnts. Development 2014; 141:2206-15; PMID:24821987; http://dx.doi.org/10.1242/dev.104976
- Kabiri Z, Numata A, Kawasaki A, Blank E, Tenen DG, Virshup DM. Wnts are dispensable for differentiation and self-renewal of adult murine hematopoietic stem cells. Blood 2015; 2402:1086-95; http://dx.doi.org/10.1182/blood-2014-09-598540
- Huang S-MA, Mishina YM, Liu S, Cheung A, Stegmeier F, Michaud GA, Charlat O, Wiellette E, Zhang Y, Wiessner S, et al. Tankyrase inhibition stabilizes axin and antagonizes Wnt signalling. Nature 2009; 461:614-20; PMID:19759537; http://dx.doi.org/10.1038/nature08356
- Bonet ML, Oliver P, Palou A. Pharmacological and nutritional agents promoting browning of white adipose tissue. Biochim Biophys Acta 2012; 5(1831):969–985.
- Kirton JP, Crofts NJ, George SJ, Brennan K, Canfield AE. Wnt/beta-catenin signaling stimulates chondrogenic and inhibits adipogenic differentiation of pericytes: potential relevance to vascular disease? Circ Res 2007; 101:581-9; PMID:17673669; http://dx.doi.org/10.1161/CIRCRESAHA.107.156372
- Nguyen KD, Qiu Y, Cui X, Goh YPS, Mwangi J, David T, Mukundan L, Brombacher F, Locksley RM, Chawla A. Alternatively activated macrophages produce catecholamines to sustain adaptive thermogenesis. Nature 2011; 480:104-8; PMID:22101429; http://dx.doi.org/10.1038/nature10653
- Qiu Y, Nguyen KD, Odegaard JI, Cui X, Tian X, Locksley RM, Palmiter RD, Chawla A. Eosinophils and type 2 cytokine signaling in macrophages orchestrate development of functional beige fat. Cell 2014; 157:1292-308; PMID:24906148; http://dx.doi.org/10.1016/j.cell.2014.03.066
- Petruzzelli M, Schweiger M, Schreiber R, Campos-Olivas R, Tsoli M, Allen J, Swarbrick M, Rose-John S, Rincon M, Robertson G, et al. A Switch from White to Brown Fat Increases Energy Expenditure in Cancer-Associated Cachexia. Cell Metab 2014; 20:433-47; PMID:25043816; http://dx.doi.org/10.1016/j.cmet.2014.06.011
