ABSTRACT
Adipose Tissue (AT) is a complex organ with a crucial regulatory role in energy metabolism and in the development of obesity and the Metabolic Syndrome (MS). Modified responses and the metabolism of hormones have been observed in visceral adiposity during obesity, specifically as related with cortisone. The objective of this study was to assess, in the 3T3-L1 adipocyte cell line, the short-term effect of cortisone on the expression of 11β-Hydroxysteroid dehydrogenase 1 (Hsd1), which is responsible for activation of cortisone into cortisol, and for Aquaporin 7 (Aqp7), involved in glycerol transport through the cell membrane. Total RNA (tRNA) and complementary DNA (cDNA) were obtained from cell samples treated with cortisone (0.1, 1, and 10 μM) during different times (0, 5, 10, 15, and 20 min, and 48 h) to quantify the expression of the aforementioned genes by real time PCR employing MnSOD and Ppia as housekeeping genes. There was a time-dependent response of Aqp7, a dose-dependent response of Hsd1, and an increase observed in the expression of both genes during min 1 of treatment (5- and 6-fold, respectively), followed by a decrease during the following 5–10 min (P < 0.05). With the 1-μM cortisone treatment, both genes showed cubic tendencies in their expression; the Hsd1 tendency is described by the equation y = 0.18×3−1.65×2+3.59x+1.31, while the Aqp7 tendency is described by y = 0.33×3–2.67×2+4.93x+1.84. There are immediate and quantitatively important actions of cortisone on the expression of Aqp7 and Hsd1 in 3T3-L1 adipocytes.
Introduction
Obesity has become the major health challenge worldwide. In its most simplified version, obesity is an imbalance in Adipose Tissue (AT) with a principal rate of synthesis of triacylglycerols (TAG) compared with their degradation. TAG accumulation in visceral deposits (omental and mesenteric), identified as central obesity, confers an increased risk for triggering metabolic diseases,Citation1-3 regardless of total TAG accumulation in the body, and even the accumulation of subcutaneous TAG deposits, considered protective.Citation2-4 The similarity between the metabolic abnormalities observed in central obesity and the presence of an excess of glucocorticoids (GC) as in, for example, Cushing's syndrome, have led to the proposal that an alteration in the metabolism or in the action of GC on visceral AT plays an essential role in the pathogenesis of metabolic diseases associated with central obesity.Citation5 Paradoxically, under physiological conditions, the action of GC promotes lipolysis, thus the release of glycerol and fatty acids from AT.Citation6,7 While excess GC does cause obesity and diabetes, circulating GC levels are normal in obese subjects.Citation8 This led to the knowledge that the physiological action of GC on AT depends, in part, on their circulating levels and also, and importantly, on their tissue activation prior to impacting on their receptor, which occurs through 11β-Hydroxysteroid dehydrogenase 1 (Hsd1), the latter converting inactive cortisone into active cortisol.Citation9-11 The Matsuzaki et al.Citation12 experiment solved this puzzle: it is the overexpression of Hsd1 that results in obesity and the Metabolic Syndrome (MS). In turn, knockout (KO) of Hsd1 protects against the obesity and hyperglycemia induced with a high-fat diet.Citation13
A protein that competes with HSD1 in its ability to regulate the metabolism of TAG in AT is aquaporin 7 (AQP7).Citation14,15 The latter is member of the AQP family, that mobilizes H2O and metabolites through the cell membranes; AQP7 is localized in the membranes of adipocytes and mobilizes H2O and glycerol through the membranes.Citation16,17 In Aqp7 KO mice, increases have been demonstrated of intracellular glycerol content and TAG, adipocyte growth, and obesity and Insulin Resistance (IR) appear.Citation16,18,19 Conversely, overexpression of Aqp7 in 3T3-L1 cells stimulates the release of glycerol, glucose consumption, and the expression of phosphorylated protein kinase B (PKB-p), a well-accepted indicator for insulin sensitivityCitation20 and the attenuation of IR.Citation21 Moreover, it is known that dexamethasone (DXM), a synthetic steroid capable of inducing IR,Citation15 downregulatesAqp7Citation15 and that is necessary for 3T3-L1 differentiation. In particular, obesity can be caused by Hsd1 overexpressionCitation12 or Aqp7 KO,Citation16 and viceversa, Aqp7 overexpression protects against obesityCitation1,2 as well as against Hsd1 KO.Citation13
The aim of this study was to compare the action of cortisone on the expression of the two proteins analyzed previously: HSD1 and AQP7. With the intention of controlling the largest number of variables inherent to the study, it was decided to carry out the experiment in vitro in 3T3-L1preadipocytes after inducing their differentiation into adipocytes, and to explore the immediate responses to cortisone addition. Our working hypothesis comprised that cortisone favored Hsd1 expression and reduced Aqp7 expression in this manner, by means of two complementary mechanisms for strengthening the metabolism of pre-diabetic adipocytes. The results showed, contrary to our hypothesis, the ability of cortisone to induce immediate and nearly parallel mRNA (mRNA) expression for both of the genes-under-study.
Results
Preadipocyte differentiation
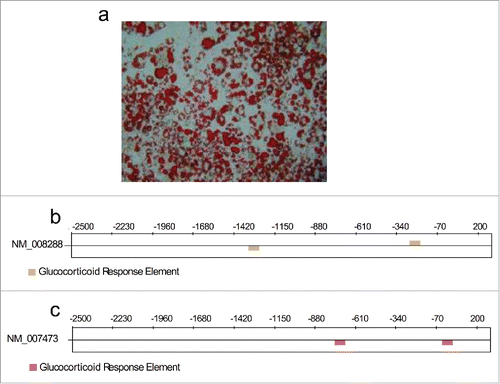
Cultured 3T3-L1 cells were induced as described in Methods, and includes Oil Red O-stained dishes to get an idea of adipose conversion efficiency from the adipocytes utilized.
Glucocorticoid response elements (GRE) for Hsd1 and Aqp7genes
The promoter sequences for the genes encoding for Hsd1 and Aqp7 were analyzed with the TFM-Explorer computer software to search for GREs. This software identified two potential response elements in each gene at positions −1320 and −253 for Hsd1 () and at −748 and −29 for Aqp7 (). The presence of these GREs confirmed that cortisone cancertainly regulate the expression of the genes studied and justified the experiments performed in this work.
Time-dependent effects of cortisone on the expression of mRNA for Hsd1 and Aqp7 in 3T3-L1 differentiated adipocytes
The differentiated 3T3-L1 cells used herein to test the effects of the cortisone are depicted in . To test the metabolic response on the expression of Hsd1 and Aqp7, an experiment employing three different concentrations of cortisone (0.1, 1, and 10 μM) during six different time intervals (1, 5, 10, 15, 20 min, and 48 h) was performed. The cortisone treatments led to an immediate and significant increase (P<0.05) in a waved manner in mRNA expression for both genes during the experiment (). The expression of the Hsd1 mRNA that increased during min 1 was observed with 0.1, 1, and 10 µM cortisone, but the response was greater with the 0.1- and 1-μM treatments, with 4.5- and 4.8-fold induction, respectively. It is noteworthy that the three concentrations exhibited a similar waved manner (as depicted in ), and it should be noted that the response with 0.1 and 1 μM after 20 min returned to control values, while mRNA expression with 10 μM was diminished (P < 0.05).
Figure 2. (a) Expression levels for Hsd1 in 3T3-L1 adipocytes. Lines show the relative expression behavior of mRNA during the first 20 min of the experiment for treatments with 0 μM (continuous line), 0.1 μM (dotted line), 1 μM (dashed line), and 10 μM (double line) cortisone, P < 0.05. (b) Expression levels for Aqp7 in 3T3-L1 adipocytes. Lines show the relative expression behavior of mRNA during the first 20 min for treatments with 0μM (continuous line), 0.1 μM (dotted line), and 1μM (double line). Data for 10 μM are not shown. All error bars represent ± SEM, P < 0.05 (n = 3).
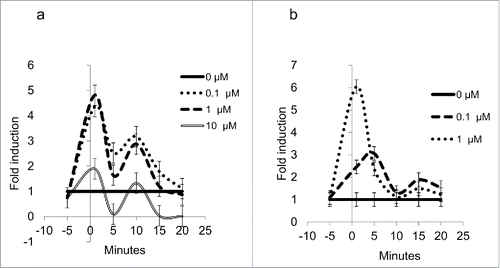
In the case of Aqp7 (), mRNA expression was increased 6-fold during min 1 with 1-μM cortisone and 3-fold after min 5 with 0.1-μM cortisone, but similar expressions of mRNA after min 5 until the end of the experiment were observed with both treatments (P < 0.05). Quantification of mRNA relative expression by quantitative Polymerase Chain Reaction (qPCR) was not possible with the 10-μM cortisone treatment, and an increase in amplification cycles was considered unnecessary, because the results would not have been veracious.
There are three aspects of the results included in that attract attention: a) the rapid response of 1-µM cortisone, a minute lag for the 5-fold increase in Hsd1 mRNA, and the 6-fold increase for Aqp7 mRNA (); b) the strong tendency to return to mRNA baseline values within the next 5 min for Hsd1 () and within 10 min for Aqp7 (); and c) the rough parallelism of both mRNA in response to the hormone treatment. Responses to 0.1 µM cortisone, as compared with 1 µM cortisone, were similar for Hsd1 () and smaller for Aqp7 ().
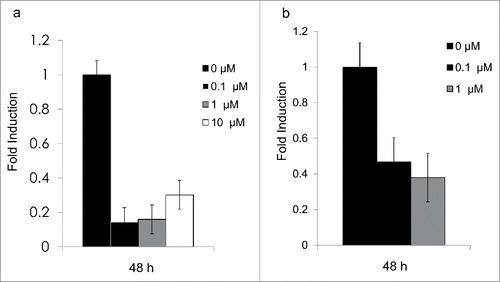
After 48 h, mRNA expression for both genes was repressed by all treatments (0.2- and 0.4-fold induction, respectively) ().
Figure 3. (a) Expression levels for Hsd1 in 3T3-L1 adipocytes. Bars show the relative expression of mRNA after 48 h for treatments with 0 μM, 0.1 μM, 1 μM, and 10 μM cortisone, P < 0.05. (b) Expression levels for Aqp7 in 3T3-L1 adipocytes. Bars show the relative expression of mRNA after 48 h for treatments with 0μM, 0.1 μM, and 1μM cortisone. The 10 µM treatment revealed undetectable levels for theAQP7 gene, but no signs of cell-increased mortality nor detachment. All error bars represent mean ± SEM, P < 0.05 (n = 3).
Expression of mRNA for Hsd1 and Aqp7
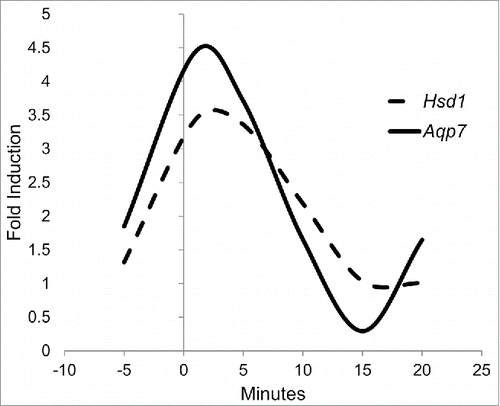
After analyzing the results for Hsd1 and Aqp7, their behaviors were compared and adjusted to a quadratic response curve. mRNA expression for Hsd1 and Aqp7 under treatment with 1 μM cortisone was adjusted with correlations of 0.5 and 0.39, respectively. Although these are not strong correlations, it is noteworthy that mRNA expression for both genes demonstrated an imminent increase at the beginning of, and a repression late in, the experiment. For the 1-μM cortisone treatment, both protein expressions exhibited cubic tendencies: the Hsd1 tendency is described by the equation y = 1.31+3.59x-1.65×2+0.18×3, while the Aqp7 tendency is characterized by y = 1.84+4.93x-2.67×2+0.33×3 (). This mathematical adjustment underlies the fact that the kinetic effect of cortisone during the time employed is not linear and that this is both time- and dose-dependent.
Figure 4. 3T3-L1 cell line precursors were differentiated and subsequently treated during 0, 5, 10, 15 and 20 min with different cortisone concentrations (0, 0.1, 1, and 10 µM). Because 1 µM exhibited the greatest impact on the relative expression of both genes, we provide a quadratic response curve for HSD1 expression (dashed line) and AQP7 (continuous line). Data represent mean P < 0.05 (n = 3).
Glucose and glycerol quantification during the treatment time
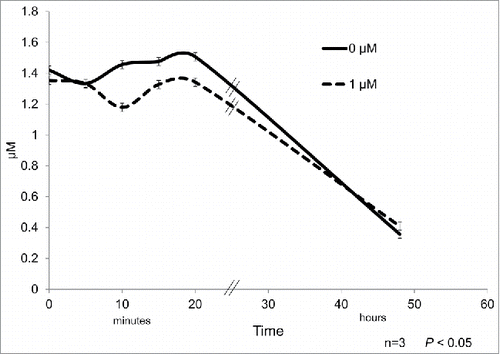
The treatment with the highest expression of mRNA (1μM) was considered for quantification of glucose and free glycerol in the culture medium. The glucose concentration in the culture media was found to have a minor and transitory decrease after 10 and 20 min of cortisone treatment with respect to control samples ().
Figure 5. 3T3-L1 cell line precursors were differentiated and subsequently treated during 0, 5, 10, 15, and 20 min with 1 µM cortisone. Glucose was quantified and the different time points of the treatment for controls (continuous line) and differentiated adipocytes (dashed line). Data represent mean ± SEM, P < 0.05 (n = 3).
There were no significant differences between glycerol concentrations in the culture medium throughout the experiment as compared with control samples (data not shown).
Discussion
The anti-inflammatory action of glucocorticoids is well known; however, under certain pathological circumstances, such as MS, they can favor inflammation. For this reason, only after knowing the non-pathologic genomic response of Hsd1 and Aqp7 to glucocorticoids/cortisone, it would be possible to determine whether their modified expressions comprise a cause or a consequence of the disease.Citation7,22,23 Therefore, this study was performed on differentiated adipocytes derived from a non-obese source after confirming the possible role of cortisone in regulating the expression of these genes through the two GREs predicted in each of their promoters. Even when some authors have reported changes in the expression of either Hsd1Citation24 or some aquaporinsCitation25-28 in response to glucocorticoid stimuli, no reports have been found that link both of the genes of this research to the MS.
An immediate increase in DNA transcription after appropriate prokaryote cell stimulation is frequently reported. Scarce information is available for eukaryotic cells; yeast subjected to chemical stress demonstrated two upregulated genes that appeared extremely rapidly: between 30 s and 2 min after their stimulation,Citation29 and about 7% of genes from the yeast genome were induced >5-fold after 10 min in a medium saline shock.Citation30 Our results demonstrated an immediate cortisone-mediated mRNA increase in both enzymes studied: HSD1 and AQP7 (), which are two leading proteins of lipid metabolism and which have been related with obesity. Such an increase was followed, at once, by fast mRNA degradation of both genes, subsequently demonstrating a second wave of mRNA synthesis and degradation, and also for both genes. Interestingly, mathematical response-curve analysis showed a cubic tendency for the 0.1- and 1-µM treatments (). Such changes in genes expression are unrelated with glucose and free glycerol quantification in the cultured medium (). To date, to the best of our knowledge, no studies have reported minute-dependent modifications in the expression of these proteins in response to cortisone. All of these responses render our experimental model an excellent one for considering emerging principles or mRNA production and degradation dynamics in eukaryotic cells: first, upon proper stimulation, an increase in mRNA synthesis might occur at <1 min (); second, coupling between transcription and mRNA decay are connected in some fashion in a so-called “circular process;”Citation31 third, changes in degradation rates are important for shaping sharp, “peaked” responses;Citation32 and fourth, coupling between transcription and mRNA decay most frequently accompanies a cellular process that involves, among others, the response to cellular stress,Citation32 as the present case might represent.
An alternative to understanding the actions of cortisol at higher doses might be an eventual “cortisone-resistance” phenomenon. This is a well-characterized phenomenon in lymphocytesCitation33 and it is observed when concentrations of corticoid hormones exceed the physiological concentrations and when the bulk of cells expires.Citation34 Therefore, a cortisone-induced toxicity at 10 µM cannot be discarded.
Balachandran et al.Citation35 performed, with the HSD1 enzyme, a similar experiment in vivo and in vitro, finding a diminished and an increased response in expression, respectively; however, it is important to consider that the treatment time was different (6 and 48 h). Also, the in vitro experiment was performed in an isolated environment, while the experiment performed in vivo preserved the adipose niche. This suggests that it may be necessary to evaluate the acute response in the isolated adipocytes of healthy and obese individuals, and also the in vivo response, in order to be able to determine whether the increase is a consequence of the hypertrophy and could exert influence the development and progression of the different, but related, diseases due to the genomic effect.
Because the presence of AQP(s) and their functions in the cell are of recent discovery, available information on these is limited. In the case of Aqp7, studies are focused on its expression and translation in MS (tissue-specific) and how these are modified during the differentiation process. As in the case of Hsd1, preadipocytes lack the expression of mRNA and protein for Aqp7. Its messenger is first detected after 6 days of differentiation, and the protein appears to be increased and maintained in the mature adipocytes.Citation15,36
This study suggests an important hormone response, which would be worthwhile to explore in greater depth, and the study also questions the real, fast regulation mediated by corticosteroids that favors a dramatic increase in expression during short time periods and the purpose of this. We have now identified an important effect on genomic responses mediated by GC, and its impact on metabolic complications is our new assignment. Response during short intervals should be explored in metabolically compromised cells in order to determine the role of cortisone in metabolism and the pathogenesis of obesity in adipose tissue.
Methods
Identification of GRE for Hsd1 and Aqp7genes
The promoters for the genes encoding for Hsd1(ID 15483, Ref sequence NM_008288) and Aqp7(ID 11832, Ref sequence NM_007473)were analyzed using the Transcription Factor Matrix-Explorer (TFM-E) from LIFL (Laboratoired'InformatiqueFondamentale de Lille, Université Lille, FR; http://bioinfo.lifl.fr/TFM).Citation37 The sequences corresponding to the region between +200 and −2500 bpwere examined with the TRANSFAC-JASPAR matrices database and then searched for the GRE.
3T3-L1 cell culture
The 3T3-L1 preadipocyte cell line was cultured in DMEM (Gibco, Life Technologies, 11995-065) containing 10% Fetal Bovine Serum (FBS; Gibco, 16000-044) and 1% Penicillin/Streptomycin (Gibco, 15140-122). Cells were seeded at a density of 1 × 104 per ml. On day 2 post-confluence, the differentiation process was induced with a 2-day treatment, changing the medium to DMEM supplemented with 5 µg/ml insulin (Gibco, A11382IJ), 0.25 µM dexamethasone (DXM; Sigma-Aldrich, D4902), and 0.5 mM isobutylmethylxanthine (IBMX; Gibco, PHZ1124). Differentiation was followed by a single-day treatment without DXM and IBMX and 4 additional days without insulin. FBS and antibiotics were included during the entire differentiation process. To ensure that differentiation was properly complied with, oil-red O staining was performed as previously reported by Nicholson et al.Citation22
Cell treatment
Differentiated cells in 6 well-plates (procedure described previously) were incubated in a restricted medium for 2 h (DMEM and Penicillin/Streptomycin). After restriction, the cells were treated with three different concentrations (0.1, 1, and 10 μM) of cortisone (Sigma-Aldrich, C2755) during 5 different time periods (5, 10, 15, and 20 min, and 48 h). The treatment medium was collected and utilized to identify time-dependent changes through quantification of glucose and free glycerol using the glucose oxidase-peroxidase method and the free glycerol determination kit (Sigma-Aldrich, FG0100-1KT), respectively. To maintain control of the starting point, cells were harvested with no treatment (illustrated as −5-min timing in ), and these harvested cells were employed as the starting point for all treatments, because increased expressions were detected immediately after treatment.
RNA isolation
After the culture treatment, total RNA was isolated employing the TRIzol reagent (Life Technologies, 15596018) according to the manufacturer's instructions and stored at −70°C. The RNA obtained was quantified with a NanoDrop 1000 spectrophotometer (Thermo Fisher Scientific) at 260 nm, and purity was assessed utilizing 260:280 ratios. RNA integrity was confirmed by electrophoresis in a 1% agarose gel.
cDNA synthesis
After evaluating the concentration and purity of the RNA extractions, 1 µg RNA was taken from each sample to perform the transcription of a cDNA (cDNA) brand, with oligo dT15 and Superscript II Reverse Transcriptase (Life Technologies, 18064071). To avoid genomic DNA contamination, RNA samples were pre-treated with DNAse (Roche, 776785).
Real-time PCR (qPCR)
Specific primers for the genes Hsd1, Aqp7, MnSOD, and Ppia were designed using Oligo7 software (). PCR products were sequenced to verify primer-specific identity. Quantitative RNA expression for Aqp7 and Hsd1 was determined utilizing a StepOne Real-Time PCR System (Applied Biosystems) and the LightCyclerFastStart DNA Master Sybr Green I kit (Roche Applied Science, 12 239 264 001). The program was performed as follows: denaturation at 96°C for 10 min, followed by 40 cycles of 1 s at 95°C for denaturation, 10 s at 65°C for primer alignment, and 10 s at 72°C for elongation; the melting curve started at 60°C for 1 min and ended with 15 s at 95°C. Subsequently, the relative abundance of mRNA was calculated by normalization to MnSOD and Ppia [best housekeeping gene candidates suggested by NormFinder (MDL, Aarhus, Denmark)] according to the 2−ΔΔCT method.
Table 1. Primers for qPCR.
Special care was taken to validate the data presented in : a) each experiment was performed three times with the coordinated participation of three experienced technicians; b) systematic experimental control was included 5 min prior to the addition of cortisone; c) in all cases, two genes were included to normalize the relative abundance of mRNA; d) mRNA controls values remained virtually unchanged; and e) the dispersion of individual experimental values was small and this was reflected in small standard errors.
Glucose quantification in the culture medium
For glucose quantification in the medium, the glucose oxidase-peroxidase method was employed.Citation38 This assay consists of the synthesis of H2O2 from glucose by the glucose-oxidase enzyme. Thishydrogen peroxide reacts with ortho-Dianisidine and peroxidase to yield a colored product, which is subsequently quantified in a spectrophotometer (Agilent 8453) at a 540-nm wavelength.
Free glycerol quantification in the culture medium
Glycerol quantification was performed following the protocol suggested by the free glycerol determination kit (Sigma-Aldrich). For this assay, the free glycerol present in the sample was phosphorylated and subsequently oxidized to produce H2O2. As in the glucose quantification assay, the reagents produced a quinoneimine dye that can be detected and measured at a 540-nm wavelength in a spectrophotometer (Agilent).
Statistical analysis
The experiment comprised a completely randomized design with a 4 × 6 factorial arrangement (treatment per time). The results were analyzed with the SAS general linear model (SAS Institute), and least-square-means comparison testing was performed.
Abbreviations
| Aqp7 | = | Aquaporin 7 |
| AT | = | Adipose Tissue |
| GC | = | Glucocorticoids |
| GRE | = | Glucocorticoid Response Elements |
| Hsd1 | = | 11β-Hydroxysteroid dehydrogenase type 1 |
| IR | = | Insulin Resistance |
| MS | = | Metabolic Syndrome |
| TAG | = | Triacylglycerols |
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
We are grateful to the Proteogenomics Unit (INB-UNAM) for its technical assistance and to Yumi Ghinis and Maggie Brunner for revising the English language of the manuscript.
Funding
T. Quesada thanks CONACYT (México) for a scholarship at FESC-UNAM. The authors received a research grant from PAPIIT-UNAM (IN218112).
References
- Björntorp P. Metabolic implications of body fat distribution. Diabetes Care 1991; 14(12):1132-43; PMID:1773700; http://dx.doi.org/10.2337/diacare.14.12.1132
- Lee MJ, Wu Y, Fried SK. Adipose tissue heterogeneity: implication of depot differences in adipose tissue for obesity complications. Mol Aspects Med 2013; 34(1):1-11; PMID:23068073; http://dx.doi.org/10.1016/j.mam.2012.10.001
- Tchernof A, Després JP. Pathophysiology of human visceral obesity: an update. Physiol Rev 2013; 93(1):359-404; PMID:23303913; http://dx.doi.org/10.1152/physrev.00033.2011
- Lee MJ, Pramyothin P, Karastergiou K, Fried SK. Deconstructing the roles of glucocorticoids in adipose tissue biology and the development of central obesity. Biochem Biophys Acta 2014; 1842(3):473-81
- Bujalska IJ, Kumar S, Stewart PM. Does central obesity reflect “Cushing's disease of the omentum”? Lancet 1997; 349(9060):1210-3; PMID:9130942; http://dx.doi.org/10.1016/S0140-6736(96)11222-8
- Xu C, He J, Jiang H, Zu L, Zhai W, Pu S, Xu G. Direct effect of glucocorticoids on lipolysis in adipocytes. Mol Endocrinol 2009; 23(8):1161-70; PMID:19443609; http://dx.doi.org/10.1210/me.2008-0464
- Peckett AJ, Wright DC, Riddell MC. The effects of glucocorticoids on adipose tissue lipid metabolism. Metabolism 2011; 60(11):1500-10; PMID:21864867; http://dx.doi.org/10.1016/j.metabol.2011.06.012
- Wajchenberg BL. Subcutaneous and visceral adipose tissue: their relation to the metabolic syndrome. Endocr Rev 2000; 21(6):697-738; PMID:11133069; http://dx.doi.org/10.1210/edrv.21.6.0415
- Stewart PM. 11 β-Hydroxysteroid dehydrogenase: implications for clinical medicine. Clin Endocrinol (Oxf) 1996; 44(5):493-9
- Staab CA, Maser E. 11-Hydroxysteroid dehydrogenase type 1 is an important regulator at the interface of obesity and inflammation. J Steroid Biochem Mol Biol 2010; 119(1–2):56-72; PMID:20045052; http://dx.doi.org/10.1016/j.jsbmb.2009.12.013
- Chapman K, Holmes M, Seckl J. 11β-hydroxysteroid dehydrogenases: intracellular gate-keepers of tissue glucocorticoid action. Physiol Rev 2013; 93(3):1139-206; PMID:23899562; http://dx.doi.org/10.1152/physrev.00020.2012
- Matsuzaki H, Paterson J, Shinyama H, Morton NM, Mullins JJ, Seckl JR, Flier JS. A transgenic model of visceral obesity and the metabolic syndrome. Science 2001; 294(5549):2166-70; PMID:11739957; http://dx.doi.org/10.1126/science.1066285
- Kotelevtsev Y, Holmes MC, Burchell A, Houston PM, Schmoll D, Jamieson P, Best R, Brown R, Edwards CR, Seckl JR, et al. 11beta Hydroxysteroid dehydrogenase type 1 knockout mice show attenuated glucocorticoid-inducible responses and resist hyperglycemia on obesity or stress. Proc Natl Acad Sci U S A 1997; 94(26):14924-9; PMID:9405715; http://dx.doi.org/10.1073/pnas.94.26.14924
- Kishida K, Kuriyama H, Funahashi T, Shimomura I, Kihara S, Ouchi N, Nishida M, Nishizawa H, Matsuda M, Takahashi M, et al. Aquaporin adipose, a putative glycerol channel in adipocytes. J Biol Chem 2000; 275(27):20896-902; PMID:10777495; http://dx.doi.org/10.1074/jbc.M001119200
- Fasshauer M, Klein J, Lossner U, Klier M, Kralisch S, Paschke R. Suppression of aquaporin adipose gene expression by isoproterenol, TNFalpha, and dexamethasone. Horm Metab Res 2003; 35(4):222-7; PMID:12778365; http://dx.doi.org/10.1055/s-2003-39478
- Maeda N, Funahashi T, Hibuse T, Nagasawa A, Kishida K, Kuriyama H, Nakamura T, Kihara S, Shimomura I, Matsuzawa Y. Adaptation to fasting by glycerol transport through aquaporin 7 in adipose tissue. Proc Natl Acad Sci U S A 2004; 101(51):17801-6; PMID:15591341; http://dx.doi.org/10.1073/pnas.0406230101
- Miyauchi T, Yamamoto H, Abe Y, Yoshida GJ, Rojek A, Sohara E, Uchida S, Nielsen S, Yasui M. Dynamic subcellular localization of aquaporin-7 in white adipocytes. FEBS Lett 2015; 589(5):608-14; PMID:25643985; http://dx.doi.org/10.1016/j.febslet.2015.01.025
- Hara-Chikuma M, Sohara E, Rai T, Ikawa M, Okabe M, Sasaki S, Uchida S, Verkman AS. Progressive adipocyte hypertrophy in aquaporin-7-deficient mice: adipocyte glycerol permeability as a novel regulator of fat accumulation. J Biol Chem 2005; 280(16):15493-6; PMID:15746100; http://dx.doi.org/10.1074/jbc.C500028200
- Hibuse T, Maeda N, Funahashi T, Yamamoto K, Nagasawa A, Mizunoya W, Kishida K, Inoue K, Kuriyama H, Nakamura T, et al. Aquaporin 7 deficiency is associated with development of obesity through activation of adipose glycerol kinase. Proc Natl Acad Sci U S A 2005; 102(31):10993-8; PMID:16009937; http://dx.doi.org/10.1073/pnas.0503291102
- Hajduch E, Litherland GJ, Hundal HS. Protein kinase B (PKB/Akt)- a key regulator of glucose transport? FEBS Lett 2001; 492(3):199-203; PMID:11257494; http://dx.doi.org/10.1016/S0014-5793(01)02242-6
- Shen FX, Gu X, Pan W, Li WP, Li W, Ye J, Yang LJ, Gu XJ, Ni LS. Over-expression of AQP7 contributes to improve insulin resistance in adipocytes. Exp Cell Res 2012; 318(18):2377-84; PMID:22877989; http://dx.doi.org/10.1016/j.yexcr.2012.07.016
- Nicholson AC, Hajjar DP, Zhou X, He W, Gotto AM, Han J. Anti-adipogenic action of pitavastatin occurs through the coordinate regulation of PPARγ and Pref-1 expression. Br J Pharmacol 2007; 151(6):807-15; PMID:17549051; http://dx.doi.org/10.1038/sj.bjp.0707250
- Walker BR. Cortisol- cause and cure for metabolic syndrome? Diabet Med 2006; 23(12):1281-8; PMID:17116176; http://dx.doi.org/10.1111/j.1464-5491.2006.01998.x
- Voice MW, Seckl JR, Edwards CR, Chapman KE. 11 β-hydroxysteroid dehydrogenase type 1 expression in 2S FAZA hepatoma cells is hormonally regulated: a model system for the study of hepatic glucocorticoid metabolism. Biochem J 1996 Jul 15; 317 (Pt 2):621-5; PMID:8713094; http://dx.doi.org/10.1042/bj3170621
- Chen J, Chang LR, Feng G, Lee ST, Hsieh C, Jeng SF, Huang WS. Stress alters the expression of aquaporins in cultured rat intestinal epithelial cells. ExpTher Med 2015; 10(5):1967-72
- Stoenoiu MS, Ni J, Verkaeren C, Debaix H, Jonas JC, Lameire N, Verbavatz JM, Devuyst O. Corticosteroids induce expression of aquaporin-1 and increase transcellular water transport in rat peritoneum. J Am SocNephrol 2003 Mar; 14(3):555-65
- Chen M, Cai H, Klein JD, Laur O, Chen G. Dexamethasone increases aquaporin-2 protein expression in ex vivo inner medullary collecting duct suspensions. Front Physiol 2015 Nov 3; 6:310; PMID:26578982
- Nevoux J, Viengchareun S, Lema I, Lecoq AL, Ferrary E, Lombès M. Glucocorticoids stimulate endolymphatic water reabsorption in inner ear through aquaporin 3 regulation. P Flugers Arch 2015 Sep; 467(9):1931-43
- Lucau-Danila A, Lelandais G, Kozovska Z, Tanty V, Delaveau T, Devaux F, Jacq C. Early expression of yeast genes affected by chemical stress. Mol Cell Biol 2005; 25(5):1860-8; PMID:15713640; http://dx.doi.org/10.1128/MCB.25.5.1860-1868.2005
- Posas F, Chambers JR, Heyman JA, Hoeffler JP, de Nadal E, Ariño J. The transcriptional response of yeast to saline stress. J BiolChem 2000; 275(23):17249-55
- Haimovich G, Medina DA, Causse SZ, Garber M, Millán-Zambrano G, Barkai O, Chávez S, Pérez-Ortín JE, Darzacq X, Choder M. Gene expression is circular: factors for mRNA degradation also foster mRNA synthesis. Cell 2013; 153(5):1000-11; PMID:23706738; http://dx.doi.org/10.1016/j.cell.2013.05.012
- Haimovich G, Choder M, Singer RH, Trcek T. The fate of the messenger is pre-determined: a new model for regulation of gene expression. Biochem Biophys Acta 2013; 1829(6-7):643-53; PMID:23337853
- Shortman K, Mandel T, Andrews P, Scollay R. Are any functionally mature cells of medullary phenotype located in the thymus cortex? Cell Immunol 1985; 93(2):350-63; PMID:3873994; http://dx.doi.org/10.1016/0008-8749(85)90140-6
- Franchini A, Marchesini E, Ottaviani E. Corticosterone 21-acetate in vivo induces acute stress in chicken thymus: cell proliferation, apoptosis and cytokine responses. Histol Histo Pathol 2004; 19(3):693-9
- Balachandran A, Guan H, Sellan M, van Uum S, Yang K. Insulin and dexamethasone dynamically regulate adipocyte 11-Hydroxysteroid dehydrogenase type 1. Endocrinol 2008; 149(8):4069-79; http://dx.doi.org/10.1210/en.2008-0088
- Miranda M, Escoté X, Ceperuelo-Mallafré V, Alcaide MJ, Simón I, Vilarrasa N, Wabitsch M, Vendrell J. Paired subcutaneous and visceral adipose tissue aquaporin-7 expression in human obesity and type 2 diabetes: differences and similarities between depots. J Clin Endocrinol Metab 2010; 95(7):3470-9; PMID:20463097; http://dx.doi.org/10.1210/jc.2009-2655
- Tonon L, Touzet H, Varré JS. TFM-Explorer: mining cis-regulatory regions in genomes. Nucleic Acids Res 2010; 38(Web Server issue):W286-92; PMID:20522509; http://dx.doi.org/10.1093/nar/gkq473
- Keston AS. Colorimetric enzymatic reagents for glucose Abstracts of Papers. 129th Meeting Amer Chem Soc 1956; 31-2C