ABSTRACT
Excess calories stored in white adipose tissue (WAT) could be reduced either through the activation of brown adipose tissue (BAT) or the development of brown-like cells (“beige” or “brite”) in WAT, a process named “browning.” Calorie dissipation in brown and beige adipocytes might rely on the induction of uncoupling protein 1 (UCP1), which is absent in white fat cells. Any increase in UCP1 is commonly considered as the trademark of energy expenditure. The intracellular events involved in the recruitment process of beige precursors were extensively studied lately, as were the effectors, hormones, cytokines, nutrients and drugs able to modulate the route of browning and theoretically affect fat mass in rodents and in humans. The aim of this review is to update the characterization of the extracellular effectors that induce UCP1 in WAT and potentially provoke calorie dissipation. The potential influence of metabolic cycling in energy expenditure is also questioned.
Introduction
To combat the obesity epidemic, both a drastic reduction in energy supply and an elevation of energy expenditure are considered as highly suitable therapies. Brown adipose tissue (BAT) has been considered for years to be the adequate tissue that should be activated for dissipating as heat the excess of energy stored in white adipose tissue (WAT), although translation from rodents to humans might not be obvious.Citation1 Thanks to the pioneering work of Ricquier and Kader in 1976, and of Nicholls' team in 1978, it is now of common knowledge that the process of energy expenditure as heat requires - at least - the presence in brown adipocytes of a protein with a peculiar function, the uncoupling protein 1 (UCP1).Citation2,3 In coupled mitochondria, protons of the respiratory chain reintegrate the matrix while activating the ATP synthase, hence producing ATP. In brown adipocytes, UCP1 acts as a proton channel that diverts the proton flux, thereby increasing the rate of respiratory chain, without ATP synthesis.Citation4 The representation of an unfettered runaway engine, resulting in heat production, can be used to illustrate such a process.
Since BAT is now recognized as being present in adult humans, several studies have been undertaken to develop strategies for increasing the proportion of this tissue versus WAT in overweight or obese subjects. An opposite strategy, i.e. a reduction in WAT browning, has been recently proposed to fight cachexia in patients suffering from cancer.Citation5 The animal models of choice are rodents in which interscapular brown fat is easily localized and dissected. The original studies on the activation of this tissue were focused on the mechanisms by which cold exposure of the animals would induce its development and UCP1 expression. This process involves β 3 adrenergic receptors, cAMP production and lipolysis. Remarkable advancement arose from the discovery of Loncar and of Cousin et al. who showed that islands of adipocytes with a brown phenotype are present in WAT and that cold exposure or adrenergic stimulation induces UCP1 in these islands.Citation6,7 Since then, the concept of WAT browning has emerged and the literature on the subject has tremendously increased. Cells resembling brown adipocytes in WAT were further described and named “brite” for “brown-in-white” or “beige” adipocytes.Citation8-10
Mechanistic sketch of browning and UCP1 induction
The question of the existence of either a common precursor or two different ones for white and brown adipocytes has been raised long ago and remains open, although significant progress has been made lately with the introduction of the browning concept.Citation11 The present view is that white, beige and brown adipocytes arise from distinct precursors, based notably on the occurrence or not of Myf5, although a white/brown transdifferentiation may occur.Citation12 With the use of clonally derived adult human brown adipocytes Shinoda et al. identified molecular markers of UCP1-positive cells: the potassium channel K3 (KCNK3) and mitochondrial tumor suppressor 1 (MTUS1).Citation13 These two genes were found to mark beige adipocyte differentiation and thermogenic function.Citation13 An interesting and well-documented review article was published on this topic lately.Citation14 However, the specific marker of the brown phenotype remains UCP1 because this protein is remarkably not expressed in white adipocytes or in any other tissue.
An abundant literature can be found on the process of preadipocyte differentiation using various types of cultured cells, whether primary or established lines. Like in these past studies, the present ones on the browning process have established that a number of intracellular mediators act as facilitators. Among these mediators can be found the zinc finger protein PR domain containing 16 (PRDM16).Citation15
However results in this field should be taken with caution to avoid associating markers of differentiation with effectors of UCP1 induction once cells are stabilized in their differentiated state. For instance, the role of the co-activator peroxisome proliferator activated receptor gamma co-activator 1 α (PGC-1α), as a specific marker for brown and beige adipocytes, is not obvious since it is also present in WAT.Citation16,17 PGC-1α is not only involved in UCP1 gene regulation but acts also as a transcriptional co-activator for general mitochondriogenesis.Citation17 Although PRDM16 is clearly established as a marker of the beige lineage, its direct role as a transcriptional inducer of the UCP1 gene is unclear. In mice with an adipocyte-specific deletion of PRDM16, cold-induced UCP1 gene expression remained effective in BAT while it was not in subcutaneous WAT.Citation18 This result indicated that brown and beige adipocytes were probably metabolically different. In contrast, a report from Thorens' laboratory points to Plac8 – an activator of C/EBP-ß gene transcription – as a selective inducer of brown adipocyte differentiation, because genetic ablation of this gene leads to obvious functional defects only in BAT.Citation19 Additionally, another protein – zinc finger protein 516 ; Zfp516 – is a recently discovered transcriptional activator of the UCP1 gene.Citation20 However, Zfp516 acts both in brown and beige adipocytes as an inducer of UCP1 gene expression in response to cold or β-agonists, hence showing no cell-type specificity. Other nuclear proteins can exert repressing effects of the beiging program. This could be the case for instance of the receptor-interacting protein 140 (RIP140), a transcriptional co-regulator, that appears to act as a co-repressor involved in the repression of the expression of marker genes of browning, including PRDM16 and UCP1.Citation21 In addition to research aimed at delineating potentially active proteins, an exponentially growing field of interest is currently studying the involvement of miRNAs in the browning process, trying to identify which of these ones are required and which others act as negative regulators. For instance, miRNA-155 was shown to repress browning, while the miRNA-26 family appeared to act mainly as promoters of brown adipocyte characteristics during adipocyte differentiation.Citation22,23
The recent demonstration that polymorphism in the fat mass- and obesity-associated (FTO) locus was associated with obesity provoked an emulation for studies related to WAT browning. While Tews et al. showed that FTO deficiency induced UCP1 in both gonadal and inguinal WAT, Claussnitzer et al. and Stratigopoulos et al. found that the FTO hypomorphism acted as a repressor of mitochondrial thermogenesis and caused obesity in mice.Citation24-26 Therefore, the differential impact of FTO on WAT browning and obesity remains to be clarified.
Physiological stimuli and extracellular effectors that affect browning
To approach the mechanisms by which hormones, nutrients and drugs affect browning, a couple of notions should be kept in mind. Some agents would induce differentiation of precursors toward a brown phenotype, whereas others would stimulate the thermogenic capacity and augment UCP1 expression in brown adipocytes that would preexist in WAT. In addition, the observed effects could be either direct or indirect on adipose tissue. For instance, the in vivo effects of cold, orexin or leptin and insulin appear to be central ones whereas melatonin can exert both central and peripheral thermogenic actions and serotonin acts specifically on adipocytes (see below). The use of either differentiated brown adipocytes or adipose tissue explants are scarce and, most often, studies have been carried out on cell systems of differentiation.
A non-exhaustive and still growing series of extracellular effectors able to modulate WAT browning are detailed below and are depicted in .
Figure 1. Schematic representation of extracellular effectors able to modulate white adipose tissue browning. Stimulatory and inhibitory effects on the browning process, schematized by increased uncoupling protein 1 (UCP1) expression in adipose tissue (AT) are represented respectively in green and red arrows. Yellow cells depict “white” adipocytes and gray cells illustrate beige adipocytes with increased mitochondria content. Blood vessels and other cells present in AT (preadipocytes, fibroblasts, macrophages, lymphocytes) are also shown. BAIBA, β -aminoisobutyric acid; FGF21, fibroblast growth factor 21; IL, interleukin; GDF, growth and differentiation factor; BMP, bone morphogenic protein; T3, trioodothyronine; ANP, atrial natriuretic peptide; GLP1, glucagon-like peptide 1; KLF, kruppel-like factor; PG, prostaglandin; CLA, conjugated trans-10, cis-12 fatty acid; ARG, arginine; CIT, citrulline; TGF, transforming growth factor
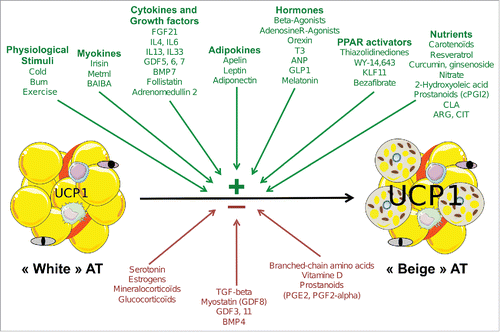
Cold, burn and exercise
Cold exposure is responsible for both shivering and non-shivering thermogenesis, the latter involving BAT activation.Citation27 Cold activates cutaneous thermal sensory receptors that signal the hypothalamus to activate the sympathetic nerve that locally discharges norepinephrine directly to BAT.Citation28 The innervation that has been observed in BAT-like arrangements of WAT from cold-adapted rats could also be a major determinant of UCP1 expression.Citation6 Such an effect is indirect, although UCP1 induction was also observed when adipocytes are directly submitted to a cooler environment, suggesting that these cells can sense temperature to activate thermogenesis.Citation29 Cold also induces in mice a microbiota remodeling that could be an important contributor of beige fat formation and energy expenditure.Citation30 Other recent publications have confirmed the role of microbiota in this process.Citation31
WAT browning under cold exposure together with the observed increase in glucose tolerance can therefore be considered as beneficial.Citation32 A contradiction in the field came from the recent work of Li et al. showing that mice deficient in Gs-α both in BAT and WAT adipocytes had improved insulin sensitivity and glucose metabolism although BAT activation and the browning process were ablated.Citation33 Remarkably, these mice did not develop obesity when fed a high-fat diet (HFD), showing that neither thermogenesis in BAT nor in beige adipocytes were required to stabilize fat mass.Citation33
In contrast to cold exposure, severe burn injury induces subcutaneous fat browning.Citation34 This effect, however, could be more related to a deletorious effect of the elevation of energy expenditure developed by burn patients.
The demonstration that exercise can contribute to WAT browning comes from the pioneering works of Xu el al.Citation35 and of Cao L. et al.Citation36 Xu et al. reported that mice trained on a treadmill for 8 weeks had a greater number of brown adipocyte precursor cells in BAT and an increase in UCP1 expression in WAT. In addition, this exercise alleviated the deleterious effects of HFD. In the Cao study, mice held in cages with free access to running wheels for a 4-weeks voluntary activity, presented a significant, although modest, increase in UCP1 mRNA level in retroperitoneal WAT, together with other markers of beige adipocytes. When such mice were maintained in an enriched environment (a complex physical and social housing, including running wheels), UCP1 gene expression was strongly up-regulated and a decreased adiposity was observed. The hypothalamic brain-derived neurotrophic factor (BDNF) overexpression could be the mediator of the effect of this enriched environment on WAT browning and energy expenditure.Citation36
Another interesting observation came from the work of Casteilla's group who showed that lactate, that is notably produced by muscle during exercise, was a strong inducer of the remodeling of WAT toward browning, probably via a change in intracellular redox state.Citation14,37 Actually, pyruvate, lactate and ketone bodies activate the Fibroblast growth factor 21 (FGF21) regulatory pathway, which was previously established as a strong inducer of UCP1 expression in fat tissues by Spiegelman's laboratory.Citation38,39
Cytokines, growth factors and hormones
Cytokines and growth factors
FGF21 is mainly produced in adipose tissues and in the liver. This growth factor could be an important regulator of metabolic processes, particularly for WAT browning.Citation38 A more recent report indicated that the FGF21-induced energy dissipation might not be related to UCP1, because it occurred in UCP1-/- mice.Citation40 However, a study from Klaus' group specified that in FGF21 -/- mice, the browning process cannot occur, but demonstrated also that in genetically engineered mice expressing UCP1 in skeletal muscles and having a deletion of the FGF21 gene, resistance to HFD-induced obesity and glycemic control were still efficient, strongly suggesting that « improved glycemic control and insulin sensitivity induced by muscle mitochondrial stress occurred independent of FGF21 » as stressed by the authors.Citation41
Interleukin-6 (IL6) is produced in large quantities by muscle during exercise and daily injections of IL6 to mice increased WAT UCP1.Citation42 The serum concentration of other muscle-secreted myokines is also raised during training. Boström et al. demonstrated that Irisin, a type I membrane protein that is processed proteolytically, was regulated by PGC-1α and had powerful effects on WAT browning, both in culture and in vivo, at the nanomolar concentration.Citation43 Further works supported the role of this newly identified hormone.Citation44 Another hormone, called meteorin-like (Metrnl), was recently put to the fore front by Spiegelman's laboratory. It was shown that the level of circulating Metrnl raised after an acute bout of exercise and caused an increase in whole-body energy expenditure, associated with the browning of white fat depots.Citation45 Metrnl action is indirect, recruiting interleukin-4 (IL4)- and interleukin-13 (IL13)-producing eosinophils into WAT.Citation45 This discovery sustains the recent observation from Chawla's group about the importance of alternative macrophage activation in cold-induced thermogenesis.Citation46 In the same vein, IL33 activates UCP1 expression in WAT, in relation with the presence of group 2 innate lymphoid cells in the tissue, where such cells may act to limit fat mass gain.Citation47 Last but not least, a recent work identified β -aminoisobutyric acid (BAIBA) as a novel myokine that was augmented during exercise and shown able to increase the expression of brown adipocyte-specific genes in WAT.Citation48
Koncarevic et al. established that TGF-β family regulated thermogenesis in primary adipocyte cultures.Citation49 A review article on this topic was recently published by Singh et al.Citation50 The TGF-β superfamily members indeed appear to play important either negative or positive roles in WAT browning. This family includes over 30 members of cytokines such as activins, bone morphogenetic proteins (BMP) and GDFs. While BMP4 was shown to play merely an inhibitory role on brown fat differentiation and UCP1 expression, BMP7 was an inducer of this pathway.Citation51 With these effectors here again, the inducing effect on UCP1 can be somewhat different when applied to preadipocytes and to mature adipocytes since UCP1 was not affected by BMP7 in differentiated cells of a murine brown preadipocyte cell line.Citation52 While GDF5 overexpression exerted a positive action on brite cell development and UCP1 expression in inguinal subcutaneous WAT, GDF3 and 11 inhibited browning.Citation53 Follistatin, which is a neutralizing protein for several members of the TGF-β superfamily, upregulated UCP1 in WAT.Citation50 The pathway by which these factors transfer their signal to cells involved the activin receptor type IIB (ActRIIB) and smad transcription factors.Citation50
Some cytokines are specifically synthetized and produced by adipocytes and thus named adipokines. Among these, apelin promoted browning both on short-term and long-term treatments in vivo and in cultured cells.Citation54 This observation is surprising because apelin expression is stimulated by insulin and it increases in obesity.Citation54,55 The proposed hypothesis that a beneficial feedback control occurs on adipocyte metabolism is under debate.Citation54 A parallel can be made with the situation encountered with leptin. The serum concentration of this hormone also increases during overweight and obesity. Commins et al. reported that in leptin-treated ob/ob mice, UCP1 was induced in BAT and WAT.Citation56 This view was recently challenged by Nedergaard's laboratory who described that the injection of leptin to mice had no effect on BAT metabolism and thermogenesis.Citation57 However, Tiganis' team demonstrated earlier that intra-hypothalamic administration of leptin to mice resulted, several days later, in an augmented amount of WAT UCP1.Citation58 Interestingly, the co-infusion of insulin and leptin sound highly efficient on UCP1 induction. Authors concluded that these hormones activated proopiomelanocortin neurons to promote WAT browning and reduce adiposity.Citation58 Hence, such experiments suggested that leptin was efficient here via its central action. Whether this hormone can also act directly on adipocytes to induce UCP1 will await further investigations. One of the potential mechanisms could be a leptin-induced stimulation of the thyroid axis resulting in an increased triiodothyronine (T3) serum concentration that would augment thermogenesis.Citation59
Since adiponectin KO mice that were maintained in a cold environment were resistant to browning and because chronic cold exposure of normal mice induced adiponectin accumulation in subcutaneous WAT, Hui et al. suggested that this adipokine played a key role in this process.Citation60 The link made between adiponectin expression, M2 macrophage proliferation and WAT browning led the authors to propose the existence of a crosstalk between innate immunity and adaptive thermogenesis.Citation60
Hormones
Norepinephrine is a well-known physiological effector of non-shivering thermogenesis, notably in response to cold. The exact mechanism by which this process occurs is however not clearly established. Would cold induce the differentiation of brown precursors or augments UCP1 synthesis - hence the thermogenic capacity - in dormant brown adipocytes, or both? The specific β-receptor of brown adipocytes is the β-3 isotype and several lines of evidence indicated that this receptor mediated UCP1 induction in response to specific agonists, via cAMP production.Citation61 However, norepinephrine is not a selective ligand for β-3 receptors and can also act via β-1 receptors present in precursor cells, with a possible participation in browning.Citation62 A more recent report by Jimenez et al. indicated that deletion of β-3 adrenoceptor in mice depressed the occurrence of brown adipocytes in WAT.Citation63
In the search of BAT therapies, Gnad et al. made a very interesting discovery about adenosine and adenosine receptors.Citation64 Adenosine has been known for long as an inhibitor of lipolysis in WAT, acting to reduce the sensitivity to catecholamines. This action holds also true in brown adipocytes from hamsters or rats. In contrast to these studies, low adenosine concentrations increased lipolysis in human brown adipocytes.Citation64 This is due to the preponderance of A2a receptors (A2aR) in brown adipocytes whereas the anti-lipolytic effect of adenosine is the result of abundant A1 receptors in WAT. Furthermore, specific A2aR agonists or the injection of lentiviral vectors expressing A2aR into murine white adipocytes induced UCP1 and browning. Hence, pharmacological agonists of A2R could be promising drugs for stimulating energy expenditure.
T3 was early described as an inducer of UCP1 gene transcription in BAT.Citation59 T3 receptor agonists are browning agents in subcutaneous WAT and the thermogenic action of T3 depends on the presence of UCP1.Citation65-67
Orexin is another CNS-derived hormone that has an important role on BAT development.Citation68 A subpopulation of orexin neurons project to BAT sympathetic premotor neurons. In cultured cells from orexin-null mice, a defect in the differentiation of brown preadipocytes was observed.Citation68 Some BAT premotor neurons are also glutaminergic and increase the thermogenic activity, while others can release serotonin (5-HT) to interact with 5-HT1A receptors and reduce thermogenesis.Citation69 However, it seems that more than neuronal-derived effects on BAT, local adipose production of 5-HT affects thermogenesis.Citation70 Mice with an inducible adipocyte-selective knock out of the tryptophan hydroxylase - Tph1- gene, that encodes one of the 5-HT-producing enzymes, exhibited a phenotype similar to that obtained with mice in which 5-HT synthesis is inhibited pharmacologically.Citation71 Thus, the local production of 5-HT is important in that case. The potential direct effect of orexin on WAT browning will require further investigations.
Some steroids play interesting roles in that context. The administration of tamoxifen, an antagonist for estrogen receptors, to mice caused browning of subcutaneous AT and increased UCP-1 expression, hence suggesting that the estrogen receptors were involved in this process.Citation72 Mineralocorticoids and glucocorticoids opposed isoproterenol-induced activation of BAT.Citation73 In addition, glucocorticoids (dexamethasone) down-regulated BAT UCP1 expression and inhibited the browning process both in vivo in mice and in vitro, on stromal vascular cells from human WAT.Citation74 This negative action could be mediated by a microRNA named miR-27b and could explain the observed positive correlation between glucocorticoid treatment and weight gain. The mineralocorticoid receptor (MR) is also implicated in the negative effect of these hormones on WAT browning and UCP1 induction, because MR antagonists markedly increased UCP1 levels in visceral and inguinal fat depots.Citation75,76
Three other hormones were recently demonstrated as WAT browning agents, the glucagon-like peptide 1 (GLP-1), the atrial natriuretic peptide (ANP), and melatonin.Citation77-79 Melatonin can be taken for several weeks with no reported side effects and therefore constitute a potential interesting compound when energy expenditure is the target.
Activators of peroxisome proliferator activated receptors (PPARs)
Since their initial discovery in 1990, PPARs have been the subject of a large number of studies, among which their involvement in adipose tissue development and metabolism has been put to the forefront.Citation80-82 Particularly, the gamma isoform of PPARs has been early considered as essential to preadipocyte differentiation. Thiazolidinediones (TZD) are selective PPAR-gamma ligands and activators. Following the initial demonstration by Foellmi-Adams et al. that the selective PPAR-gamma activator pioglitazone exerted a direct effect on BAT cells in vitro by increasing UCP1 mRNA and protein levels, Moller's group found that in vivo administration of TZD to rats for 14 days resulted in UCP1 up-regulation.Citation83,84 Although these drugs can activate UCP1 gene transcription via a PPAR element (PPRE) present in the promoter of the gene, their potential browning effect is probably linked to their capacity to stimulate preadipocyte differentiation toward a beige phenotype as reported previously.Citation85,86 In line with this concept, Seale's laboratory demonstrated that the early B cell factor-2 (Ebf2) recruited PPAR-gamma to its brown-selective binding sites, including the UCP1 gene.Citation87 Ebf2-null mice were completely devoid of brown characters, in link with a loss of expression of UCP1, PRDM16 and Cidea mRNA. Conversely, white adipocytes transduced with Ebf2 presented a rise in the expression of UCP1, PRDM16, PGC1-α or Cidea, thus suggesting the browning role of this transcription factor. Hence, Ebf2 acts ahead of the other actors of the browning process. In contrast, other determinants intervene downstream of PPAR-gamma, like the Kruppel-like factor 11 (KLF11), identified by Mandrup and colleagues as a PPAR-gamma-induced transcription factor that sustained the acquisition of a beige adipocyte gene program in a human adipocyte cell line, the hMADS.Citation88 Once established, this KLF11-dependent differentiation program was maintained in a rosiglitazone-independent manner. The in vivo relevance of the importance of KLF11 in browning remains to be demonstrated.
The PPRE of the UCP1 gene also binds PPAR-α and a short (6h) exposure of mice to Wy 14,643, a selective PPAR-α ligand, resulted in a large induction of UCP1 mRNA in BAT.Citation85 Although WY-14,643 and bezafibrate stimulate mitochondrial biogenesis and UCP1 expression in cultured models of human and mouse white adipocytes, the formal demonstration that PPAR-α agonists can induce in vivo browning remains an opened question.Citation85 For PPAR effects, the co-activator PGC-1α appeared to be an essential player for both mitochondriogenesis and the observed concomitant upregulation of the UCP1 gene.Citation16,89 The potential relative contributions of diverse PPAR isoforms in the browning process have been nicely reviewed lately.Citation66
Nutrients
A series of nutrients or of compounds that can be given as food supplements or derive from them – carotenoids and fucoxanthine, resveratrol, curcumin, conjugated linoleic acids (CLA), ketone bodies - have the potency to stimulate UCP1 expression in BAT. Most of these compounds have now been described as browning agents. Another compound, namely nitrate, can also play such a role.Citation90 Inorganic nitrate is given to humans for its action on blood pressure.Citation91 Because of its effect on the reduction of body fat, Roberts et al. made the hypothesis that it could induce WAT browning and showed that this was indeed the case in treated rodents via a regulation involving cGMP.Citation90
The impact of carotenoids - including β-carotenes, retinoids and fucoxanthines - on WAT browning was analyzed lately in an exhaustive review article.Citation66 The stimulation of browning was associated to the beneficial effect of these compounds on adiposity. Carotenoids are natural liposoluble pigments from plants, alguae and certain bacteria. Some carotenoids, like β-carotene can give birth to vitamin A and must be provided from fruits and vegetables because humans cannot synthetize them. These compounds are therefore of wide interest as potential energy dissipators. In contrast to vitamin A, 1,25-dihydroxyvitamin D3 (vitamin D) appears to favor WAT mass expansion and to directly suppress UCP1 expression.Citation92
Another natural compound -resveratrol- isolated from grapes and other food vegetables was extensively studied because of its multiple beneficial effects, including its protective action on the development of obesity and insulin resistance.Citation66 A recent report from Wang et al. indicated that resveratrol induced brown-like adipocyte formation in inguinal WAT of mice and that this process required AMPkinase activation.Citation93 These authors suggest that the beneficial anti-obesity action of this polyphenol could rely on its browning ability.Citation93 Curcumin, a popular asian spice with weight loss and anti-diabetic properties, and ginsenoside isolated from ginseng, were also shown to stimulate UCP1 in cultured adipocytes.Citation94,95 In addition, curcumin administered to mice by gavage for 50 days induced the browning of subcutaneous WAT.Citation96
Selective FAs could be of interest to reduce obesity and potentially increase energy expenditure. Oudart et al. originally demonstrated that rats fed a high-fat diet enriched with omega-3 FAs developed mitochondrial biogenesis and thermogenesis in BAT, together with increased GDP binding as UCP1 detection.Citation97 Further work on this topic from Kopecky's and Escriba's groups demonstrated that neither omega-9 nor omega-3 FAs could affect UCP-1 in BAT or WAT.Citation98,99 In contrast, the omega-6 FA arachidonic acid and its derivatives influenced either positively (cPGI2) or negatively (PGE2 and PGF2-α) UCP1 induction in the hMADs preadipocyte human cell line.Citation100,101 A synthetic FA named 2-Hydroxyoleic acid, was found able to strongly stimulate UCP-1 selectively in retroperitoneal WAT from treated rats.Citation99 Additional work from the same laboratory demonstrated that this FA derivative presented interesting properties as a potential therapeutic agent, both in obesity and cancer. Another group of dietary FAs, the conjugated trans-10, cis-12 fatty acids (CLAs), also has the ability to reduce fat mass.Citation66,102,103 WAT browning could be implicated in such an effect of CLA. In addition to selective FAs, hepatic FA metabolites like the β-hydroxy butyrate derivative, induced UCP-1 mRNA in cultured adipocytes from mice.Citation37
Specific emphasis on amino acids
It is known for years that calorie restriction augments life span and reduces fat mass. A series of experiments were then focused on the effect that a selective restriction of essential amino-acids, like methionine or leucine, with limited changes in energy intake, would have on energy wasting. In both cases, energy expenditure was augmented with a rise in BAT UCP1 mRNA, linked to CNS activation.Citation104,105 Further studies from Guo's laboratory demonstrated that deficiency in other branched-chain amino acids (valine or isoleucine) exerted effects similar to those of leucine deprivation on reducing fat mass and UCP1 induction in BAT.Citation106 However, specifically in the study of Hasek et al. about methionine restriction, a clear increase in epididymal WAT browning was observed as soon as 2 weeks post diet.Citation105 Although authors suggest that such effects of amino acid deprivation are probably central, therefore affecting WAT indirectly, the underlying mechanisms remain to be determined. Nevertheless, long-lasting deficiencies in essential amino acids cannot be a therapeutic issue because of the damage they would probably cause in humans.
In an opposite way, the chronic intake of protein-rich diets have been tested as weight reducing therapies.Citation107 However controversial results have been obtained, depending on the length of the diet and the animal. For instance, Klaus' group observed that the administration to rats of a high-protein, low-fat diet for 8 weeks, stimulated energy expenditure, oxygen consumption and UCP1 in BAT.Citation108 In contrast, the same group reported that, when given to mice for 12 weeks, a similar high-protein diet did not affect energy homeostasis and UCP1 expression.Citation109 Interestingly, a leucine-supplemented diet had no effect either.Citation104 The browning induction of such diets remain to be investigated.
A special emphasis was put on arginine (ARG) and citrulline (CIT). In 2005, Wu's laboratory reported that dietary ARG supplementation was beneficial to glucose homeostasis and reduced fat mass in Zucker diabetic fatty rats.Citation110 Further work from the same group showed that dietary ARG supplementation provoked muscle gain, reduced body WAT, improved insulin sensitivity and markedly increased BAT mass in rats.Citation111 No evidence of ARG-induced increase in UCP1 either in BAT or in WAT was provided yet.Citation112 The situation is controversial with that observed in mice, in which ARG supplementation did not affect BAT mass.Citation113 Once again here, rats and mice behave differently and the reasons for such a discrepancy are not clearly established, although the possibility that it arises from different thermoneutral temperatures between rats and mice cannot be ruled out.Citation27,114 Although controversial effects have been described concerning ARG effects on muscle performance in humans, this amino-acid has become widely used as a food supplement in athletes.
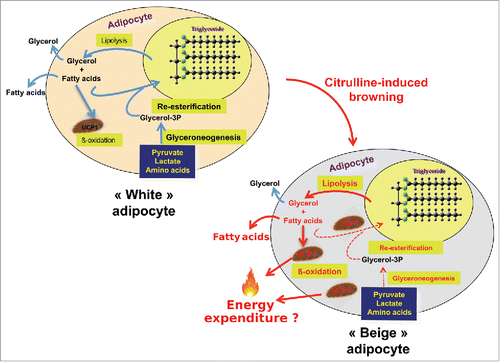
Like ARG, CIT is also a safe food supplement. CIT is an amino acid that is not used for protein synthesis. CIT is an intermediary product in nitric oxide (NO) synthesis, requiring the presence of argininosuccinate synthase (ASS) and argininosuccinate lyase (ASL) in addition to NO synthases. In rats and pigs, dietary intake of either ARG or CIT stimulated muscle protein synthesis.Citation115 Actually, CIT seemed more efficient than ARG, probably because of a better absorption in the gut and because it was shown to escape hepatic uptake, in contrast to ARG.Citation116 CIT was thus proposed as a food supplement during aging and in sports to improve muscle mass and function.Citation117,118 Based on the initial observation by Cynober's laboratory that a diet enriched with CIT for 3 months in old rats provoked an increase in muscle mass together with a large decrease in intra-abdominal WAT mass, Moinard and coll. further studied the effect that such a diet had on muscle metabolism.Citation119 To start deciphering the mechanisms involved in the WAT-reducing action of CIT, we used explants of retroperitoneal (RET) WAT from rats that we maintained in culture under controlled conditions. A 24h treatment with CIT markedly induced lipolysis and β-oxidation while it reduced glyceroneogenesis and FA re-esterification in explants from control and HFD-fed young rats.Citation120 Similar data were obtained on RET explants from old rats, except that β-oxidation remained unaffected by CIT.Citation121 Further experiments showed that both in RET and peri-epidymal (EPI) explants, two typical WAT depots, from young rats, CIT raised UCP1 mRNA and protein amount.Citation122 Of peculiar interest was the observation that WAT from old rats was not responsive.Citation122 The reasons for this age-related difference is unknown at present. From these results obtained with explants from young rats, we can conclude that CIT acts directly on WAT and relates probably on a « white to beige » transdifferentiation rather than the differentiation of brown precursors. This browning effect, and the observed increase in lipolysis and β-oxidation, point to CIT as an exquisite agent that could be used for energy expenditure and fat mass reduction ().
Figure 2. Schematic representation of citrulline effects on rat adipocyte metabolism and on browning. Modifications are represented with red arrows. Arrow thickness illustrates the intensity of the effects. In response to citrulline (CIT), lipolysis is activated in explants from rat white adipose tissue, with a rise in the phosphorylation of hormone-sensitive lipaseCitation120 and a drastic decrease in glyceroneogenesis and fatty acid re-esterification, leading to increased fatty acid release. CIT also induces the β-oxidative capacity of the cells together with the uncoupling-protein 1 (UCP1).Citation120,122 The observed up-regulation of mitochondrial transcription factor A gene expression indicates that mitochondriogenesis could occur.Citation122
Metabolic cycling
The observation that mice lacking UCP1 still regulated their body temperature when they were acclimated to 4°C indicated that other mechanisms were involved in thermal homeostasis and thus potentially in energy dissipation.Citation123 One of the proposed mechanisms is metabolic cycling. Since the influence of metabolic cycling in relation to energy expenditure as heat in WAT and BAT has been exhaustively reviewed recently by Flachs et al.,Citation98 this mechanism is given below in outline only. Specifically, we have decided to emphasize the points for which our opinion differs from that developed in the above-cited 2013 review article.
The story starts with the pioneering work of Vaughan and Steinberg demonstrating a general correlation between the rates of TAG breakdown and re-synthesis in WAT.Citation124 Some of the FAs produced by lipolysis was re-esterified back to TAG, establishing the TAG/FA cycle. As early as in 1976, Newsholme and Crabtree wrote: “Recently, the role of substrate cycles in heat generation has attracted considerable attention.”Citation125 The idea is that heat is generated either directly or indirectly from ATP hydrolysis. Knowing that for each ATP synthesized and hydrolyzed, about 18 kcal of heat are produced when the fuel substrate is triglyceride; it becomes clear that the TAG/FA cycle can generate heat.
The FA re-esterification process of the TAG/FA cycle that occurs spontaneously during lipolysis, requires the presence of glycerol-3P which, under fasting conditions or epinephrine exposure can barely come from glucose or from lipolytic glycerol because glucose transport and glycolysis are strongly reduced and because glycerol kinase is only weakly expressed in WAT. Under such conditions, the metabolic pathway involved in glycerol-3P synthesis is glyceroneogenesis as defined about 45 years ago by Hanson and colleagues as the de novo synthesis of glyerol-3P from pyruvate, lactate or amino acids.Citation126,127 Since then, the estimate of FA re-esterification was considered to be in direct relation with glyceroneogenesis, which is evaluated either by tritiated water or by the incorporation of 14C from [1-14C]-pyruvate into TAG. The key enzyme in this process was demonstrated long ago to be the cytosolic isoform of phosphoenolpyruvate carboxykinase (PEPCK-C).Citation128 The existence of the TAG/FA cycle was demonstrated in BAT by Newsholme's and Migliorini's laboratories.Citation129,130 It was then further assessed that, in rat BAT, glyceroneogenesis was up-regulated by fasting, hyperprotein-fed or cafeteria-fed diets.Citation129,131-133 Beta-agonists increased TAG/FA cycle in both BAT and WAT.Citation134,135 This β-agonist effect was exerted directly in adipocytes and stimulated PEPCK-C gene transcription and protein expression.Citation136
Newsholme's laboratory originally made the interesting observation that the TAG/FA cycle was activated by cold-exposure in BAT but not in WAT.Citation130 They concluded that BAT was the more important site of thermogenesis in response to cold. However, the cold-induced WAT browning could be associated with an increase in FA re-esterification that could have been masked because of the weak amount of beige cells that differentiate in WAT, together with the existence of the TAG/FA cycle in all white adipocytes.
What are the physiological and pharmacological situations that could stimulate the TAG/FA cycle to potentially favor calorie dissipation? As mentioned above, fasting is the physiological state of excellence that promotes both lipolysis and FA re-esterification. Part of the lipolytic FAs are released into the blood and serve as energy substrates for β-oxidation in demanding tissues. In WAT, another part of lipolytic FAs are directly β-oxidized, therefore producing ATP used as energy for adipocytes, including the operation of the TAG/FA cycle.
Fasting and lipolytic situations should induce per se a reduction in WAT mass. However, obese individuals or rodents present a chronic elevated level of FAs, at least because of a mass effect and probably also as a result of the insulin-resistant state frequently observed in this pathophysiological state.Citation137 To reduce WAT mass, it would then be beneficial to stimulate lipolysis and to reduce FA re-esterification, resulting in an overall shrinkage of the adipocyte TAG droplets. On the one hand, any decrease in FA re-esterification would accelerate the emptying of excess TAG. On the other hand, any increase in FA re-esterification would slow-down the process of overall lipolysis. The hypothesis developped by Flachs et al. is that such a stimulation of the TAG/FA cycle would be made possible because of the concommitent induction of β-oxidation that would provide the required ATP.Citation98
Thiazolidinediones (TZDs) are the most potent inducers of glyceroneogenesis, FA re-esterification and PEPCK induction in WAT.Citation138-141 These drugs also augmented the acyl-CoA dehydrogenase activity in mouse WAT independent of PPAR-α, suggesting that β-oxidation was upregulated.Citation142 Hence, TZD treatment lowers circulating FA levels and ameliorates glucose utilization. However, the lack of decrease in adiposity in TZD-treated animals or humans is in contradiction with the simple hypothesis that targeting the TAG/FA cycle would favor calorie dissipation. In addition, transgenic mice with a selective overexpression of PEPCK-C in adipose tissue became obese, showing that the overproduction of PEPCK-C, together with its deregulated expression due, in this study, to the strong FABP4 gene promoter, favored fat mass development.Citation143 Another argument against the involvement of the TAG/FA cycle in energy dissipation comes from the effects of glucocorticoids: these hormones induced lipolysis and reduced PEPCK-C gene transcription in WAT, thus favoring FA release, although they induced weight gain.Citation135,136,144
We earlier demonstrated that the regulation of adipocyte PEPCK-C expression and glyceroneogenesis was a key type-2 diabetes mellitus (T2DM) target and that the stimulation of this pathway by TZDs induced a reduction of circulating diabetogenic FAs.Citation138,145,146 In contrast, glucocorticoids reduce PEPCK-C and favor insulin-resistance (see above). The cataplerotic action of PEPCK-C could also be a mechanism by which excess FAs are re-used for TAG synthesis, thus reducing their involvement in the development of an insulin-resistance state.Citation145,147,148 It can be speculated that increased TCA cycle intermediate concentrations would result in the reduction of tricarboxylic acid (TCA) cycling, fatty acid oxidation and mitochondrial red-ox state.Citation147 The importance of PEPCK-C and glyceroneogenesis in T2DM found confirmation in a recent Claussnitzer manuscript's.Citation149 In the same vein, we showed that polyunsaturated long-chain FAs were strong inducers of PEPCK-C gene expression in rodent and human WAT as well as in cultured adipocytes.Citation141,150 For the same reasons as depicted above, the omega-3 stimulation of the TAG/FA cycle is probably not at the origin of weight loss. The demonstration that Omega-3 FAs induced β-oxidation in WAT and in cultured adipocytes of the 3T3-L1 line could be the alternative mechanism by which these FAs augmented energy expenditure and protected against accumulation of body fat independent of UCP1 and cold-induced thermogenesis.Citation98
As mentioned above, CIT stimulated lipolysis and FA β-oxidation while reducing glyceroneogenesis and PEPCK-C in WAT explants whether rats were either fed a standard or a high-fat diet ().Citation120 These observations are in line with the TAG-lowering action of CIT in WAT but, in that case, the absence of PEPCK-linked cataplerosis might be compensated by an increase in α-ketoglutarate efflux for generating glutamate, although this hypothesis requires further investigation.
In the absence of UCP1, other sources of energy dissipation mechanisms were proposed to be either the G3P shuttle or Ca2+ cycling.Citation152,153 The G3P shuttle is probably not involved because mice with deletions both in the mitochondrial glycerol-3-P dehydrogenase and UCP1 genes can still had increased energy expenditure thus suggesting the existence of other thermogenic mechanisms.Citation152 The Ca2+ cycling hypothesis is still faint and requires further work for clarification. More recently, using quantitative proteomics, Kazak et al. identified components of arginine-dependent creatine and proline metabolism that were reproducibly enriched in beige AT mitochondria relative to BAT.Citation154 It is known for long that in rat BAT, the activity levels of creatine kinase is stimulated by cold acclimation, a situation that should result in an augmentation of creatine levels. Several reports indicated that the pharmacological reduction of the intracellular levels of creatine and phosphocreatine using ß- guanidinopropionic acid (ß-GPA) in many tissues induced weight reduction.Citation155 Kazak et al. used a much lower dose of ß-GPA that, when administered intraperitonally to mice during cold exposure for 4 days, affected neither body weight nor fat-pad weight, when compared to cold exposure alone.Citation154 They showed that ß-GPA opposed the β-adrenergic stimulation of thermogenesis. These data plus a series of other arguments conducted the authors to propose that a futile cycle of creatine metabolism participated in energy expenditure and thermal homeostasis. If it were so, it would be of great interest to depict inducers of this pathway that could be safely given to individuals for stimulating their calorie expenditure and weight loss. However, i) the manipulation of this metabolic pathway with drugs appears to be complex and ii) long-term treatment with high doses of ß-GPA results in weight loss while lower doses of the same drug has no weight effect.Citation154
Taken together, these data indicate that the external control of the above-described metabolic cycles could be of interest but it appears that their manipulation have not been yet proven to favor sufficient energy dissipation for allowing any loss of WAT mass.
Conclusions
A series of extracellular effectors have proven beneficial for UCP1 induction in WAT, hence potentially important for energy dissipation and fat mass reduction, although the link between the two is almost neither directly investigated. On a practical viewpoint, a large number of these agents could have pleiotropic effects and provoke health problem, at least if administered on a long-term basis. Others, like melatonin, carotenoids, resveratrol, curcumin or ginsenosid can be currently taken without known side effects. In addition to these nutriments, ARG or CIT are already used as dietary supplement, especially in sport, against fatigue and were shown to reduce fat mass, at least in rodents. When administered to humans, ARG is metabolized in large proportion in the liver whereas CIT crosses the splanchnic area and is fully available for other tissues. CIT could thus be the adequate amino acid for the induction of WAT browning and energy expenditure, with the ability to reducing fat mass in addition to its well-known positive effect on muscle mass.
Abbreviations
| A2aR | = | adenosine 2a receptors |
| ANP | = | atrial natriuretic peptide |
| ARG | = | L-arginine |
| BAIBA | = | β -aminoisobutyric acid |
| β-GPA | = | β-guanidinopropionic acid |
| BAT | = | brown adipose tissue |
| BMP | = | bone morphogenic protein |
| CIT | = | L-citrulline |
| CLA | = | conjugated trans-10, cis-12 fatty acid |
| Cidea | = | cell death activator-A |
| Ebf2 | = | early B cell factor-2 |
| EPI | = | peri-epididymal |
| FA | = | fatty acids |
| FGF21 | = | fibroblast growth factor 21 |
| GDF | = | growth and differentiation factor |
| GLP1 | = | glucagon-like peptide 1 |
| HFD | = | high-fat diet |
| IL | = | interleukin |
| KLF | = | kruppel-like factor |
| MR | = | mineralocorticoid receptor |
| PG | = | prostaglandin |
| PEPCK-C | = | cytosolic phosphoenolpyruvate carboxykinase |
| PGC-1α | = | peroxisome proliferator-activated receptor gamma co-activator 1α |
| PPAR | = | peroxisome proliferator-activated receptor |
| PPRE | = | PPAR element |
| RET | = | retroperitoneal |
| T2DM | = | type-2 diabetes mellitus |
| T3 | = | triiodothyronine |
| TCA | = | tricarboxylic acid |
| TAG | = | triacylglycerol |
| TGF | = | transforming growth factor |
| TZD | = | thiazolidinediones |
| UCP1 | = | uncoupling protein 1 |
| WAT | = | white adipose tissue |
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- Warner A, Mittag J. Breaking BAT: can browning create a better white? J Endocrinol 2016; 228:R19-29; PMID:26450134
- Heaton GM, Wagenvoord RJ, Kemp A, Nicholls DG. Brown-adipose-tissue mitochondria: photoaffinity labelling of the regulatory site of energy dissipation. Eur J Biochem FEBS 1978; 82:515-21; http://dx.doi.org/10.1111/j.1432-1033.1978.tb12045.x
- Ricquier D, Kader JC. Mitochondrial protein alteration in active brown fat: a soidum dodecyl sulfate-polyacrylamide gel electrophoretic study. Biochem Biophys Res Commun 1976; 73:577-83; PMID:1008874; http://dx.doi.org/10.1016/0006-291X(76)90849-4
- Nicholls DG, Locke RM. Thermogenic mechanisms in brown fat. Physiol Rev 1984; 64:1-64; PMID:6320232
- Petruzzelli M, Schweiger M, Schreiber R, Campos-Olivas R, Tsoli M, Allen J, Swarbrick M, Rose-John S, Rincon M, Robertson G, et al. A switch from white to brown fat increases energy expenditure in cancer-associated cachexia. Cell Metab 2014; 20:433-47; PMID:25043816; http://dx.doi.org/10.1016/j.cmet.2014.06.011
- Cousin B, Cinti S, Morroni M, Raimbault S, Ricquier D, Pénicaud L, Casteilla L. Occurrence of brown adipocytes in rat white adipose tissue: molecular and morphological characterization. J Cell Sci 1992; 103(Pt 4):931-42; PMID:1362571
- Loncar D. Convertible adipose tissue in mice. Cell Tissue Res 1991; 266:149-61; PMID:1747909; http://dx.doi.org/10.1007/BF00678721
- Petrovic N, Walden TB, Shabalina IG, Timmons JA, Cannon B, Nedergaard J. Chronic peroxisome proliferator-activated receptor gamma (PPARgamma) activation of epididymally derived white adipocyte cultures reveals a population of thermogenically competent, UCP1-containing adipocytes molecularly distinct from classic brown adipocytes. J Biol Chem 2010; 285:7153-64; PMID:20028987; http://dx.doi.org/10.1074/jbc.M109.053942
- Waldén TB, Hansen IR, Timmons JA, Cannon B, Nedergaard J. Recruited vs. nonrecruited molecular signatures of brown, “brite,” and white adipose tissues. Am J Physiol Endocrinol Metab 2012; 302:E19-31; http://dx.doi.org/10.1152/ajpendo.00249.2011
- Wu J, Boström P, Sparks LM, Ye L, Choi JH, Giang AH, Khandekar M, Virtanen KA, Nuutila P, Schaart G, et al. Beige adipocytes are a distinct type of thermogenic fat cell in mouse and human. Cell 2012; 150:366-76; PMID:22796012; http://dx.doi.org/10.1016/j.cell.2012.05.016
- Smorlesi A, Frontini A, Giordano A, Cinti S. The adipose organ: white-brown adipocyte plasticity and metabolic inflammation. Obes Rev Off J Int Assoc Study Obes 2012; 13 Suppl 2:83-96; http://dx.doi.org/10.1111/j.1467-789X.2012.01039.x
- Bartelt A, Heeren J. Adipose tissue browning and metabolic health. Nat Rev Endocrinol 2014; 10:24-36; PMID:24146030; http://dx.doi.org/10.1038/nrendo.2013.204
- Shinoda K, Luijten IHN, Hasegawa Y, Hong H, Sonne SB, Kim M, Xue R, Chondronikola M, Cypess AM, Tseng YH, et al. Genetic and functional characterization of clonally derived adult human brown adipocytes. Nat Med 2015; 21:389-94; PMID:25774848; http://dx.doi.org/10.1038/nm.3819
- Jeanson Y, Carrière A, Casteilla L. A New Role for Browning as a Redox and Stress Adaptive Mechanism? Front Endocrinol 2015; 6:158
- Seale P, Kajimura S, Yang W, Chin S, Rohas LM, Uldry M, Tavernier G, Langin D, Spiegelman BM. Transcriptional control of brown fat determination by PRDM16. Cell Metab 2007; 6:38-54; PMID:17618855; http://dx.doi.org/10.1016/j.cmet.2007.06.001
- Tiraby C, Tavernier G, Lefort C, Larrouy D, Bouillaud F, Ricquier D, Langin D. Acquirement of brown fat cell features by human white adipocytes. J Biol Chem 2003; 278:33370-6; PMID:12807871; http://dx.doi.org/10.1074/jbc.M305235200
- Villena JA. New insights into PGC-1 coactivators: redefining their role in the regulation of mitochondrial function and beyond. FEBS J 2015; 282:647-72; PMID:25495651; http://dx.doi.org/10.1111/febs.13175
- Cohen P, Levy JD, Zhang Y, Frontini A, Kolodin DP, Svensson KJ, Lo JC, Zeng X, Ye L, Khandekar MJ, et al. Ablation of PRDM16 and beige adipose causes metabolic dysfunction and a subcutaneous to visceral fat switch. Cell 2014; 156:304-16; PMID:24439384; http://dx.doi.org/10.1016/j.cell.2013.12.021
- Jimenez-Preitner M, Berney X, Uldry M, Vitali A, Cinti S, Ledford JG, Thorens B. Plac8 is an inducer of C/EBPβ required for brown fat differentiation, thermoregulation, and control of body weight. Cell Metab 2011; 14:658-70; PMID:21982742; http://dx.doi.org/10.1016/j.cmet.2011.08.008
- Dempersmier J, Sambeat A, Gulyaeva O, Paul SM, Hudak CSS, Raposo HF, Kwan HY, Kang C, Wong RHF, Sul HS. Cold-inducible Zfp516 activates UCP1 transcription to promote browning of white fat and development of brown fat. Mol Cell 2015; 57:235-46; PMID:25578880; http://dx.doi.org/10.1016/j.molcel.2014.12.005
- Kiskinis E, Chatzeli L, Curry E, Kaforou M, Frontini A, Cinti S, Montana G, Parker MG, Christian M. RIP140 represses the “brown-in-white” adipocyte program including a futile cycle of triacylglycerol breakdown and synthesis. Mol Endocrinol Baltim Md 2014; 28:344-56; http://dx.doi.org/10.1210/me.2013-1254
- Chen Y, Siegel F, Kipschull S, Haas B, Fröhlich H, Meister G, Pfeifer A. miR-155 regulates differentiation of brown and beige adipocytes via a bistable circuit. Nat Commun 2013; 4:1769; PMID:23612310; http://dx.doi.org/10.1038/ncomms2742
- Karbiener M, Pisani DF, Frontini A, Oberreiter LM, Lang E, Vegiopoulos A, Mössenböck K, Bernhardt GA, Mayr T, Hildner F, et al. MicroRNA-26 family is required for human adipogenesis and drives characteristics of brown adipocytes. Stem Cells Dayt Ohio 2014; 32:1578-90; http://dx.doi.org/10.1002/stem.1603
- Tews D, Fischer-Posovszky P, Fromme T, Klingenspor M, Fischer J, Rüther U, Marienfeld R, Barth TF, Möller P, Debatin KM, et al. FTO deficiency induces UCP-1 expression and mitochondrial uncoupling in adipocytes. Endocrinology 2013; 154:3141-51; PMID:23751871; http://dx.doi.org/10.1210/en.2012-1873
- Claussnitzer M, Dankel SN, Kim KH, Quon G, Meuleman W, Haugen C, Glunk V, Sousa IS, Beaudry JL, Puviindran V, et al. FTO obesity variant circuitry and adipocyte browning in humans. N Engl J Med 2015; 373:895-907; PMID:26287746; http://dx.doi.org/10.1056/NEJMoa1502214
- Stratigopoulos G, Burnett LC, Rausch R, Gill R, Penn DB, Skowronski AA, LeDuc CA, Lanzano AJ, Zhang P, Storm DR, et al. Hypomorphism of Fto and Rpgrip1l causes obesity in mice. J Clin Invest 2016; 126(5):1897-910[ cited 2016 Apr 26]; PMID:27064284; http://dx.doi.org/10.1172/JCI85526
- Cannon B, Nedergaard J. Nonshivering thermogenesis and its adequate measurement in metabolic studies. J Exp Biol 2011; 214:242-53; PMID:21177944; http://dx.doi.org/10.1242/jeb.050989
- Morrison SF, Madden CJ, Tupone D. Central control of brown adipose tissue thermogenesis. Front Endocrinol 2012; 3:00005
- Ye L, Wu J, Cohen P, Kazak L, Khandekar MJ, Jedrychowski MP, Zeng X, Gygi SP, Spiegelman BM. Fat cells directly sense temperature to activate thermogenesis. Proc Natl Acad Sci U S A 2013; 110:12480-5; PMID:23818608; http://dx.doi.org/10.1073/pnas.1310261110
- Chevalier C, Stojanović O, Colin DJ, Suarez-Zamorano N, Tarallo V, Veyrat-Durebex C, Rigo D, Fabbiano S, Stevanović A, Hagemann S, et al. Gut microbiota orchestrates energy homeostasis during cold. Cell 2015; 163:1360-74; PMID:26638070; http://dx.doi.org/10.1016/j.cell.2015.11.004
- Suárez-Zamorano N, Fabbiano S, Chevalier C, Stojanović O, Colin DJ, Stevanović A, Veyrat-Durebex C, Tarallo V, Rigo D, Germain S, et al. Microbiota depletion promotes browning of white adipose tissue and reduces obesity. Nat Med 2015; 21:1497-501; http://dx.doi.org/10.1038/nm.3994
- Wang TY, Liu C, Wang A, Sun Q. Intermittent cold exposure improves glucose homeostasis associated with brown and white adipose tissues in mice. Life Sci 2015; 139:153-9; PMID:26281919; http://dx.doi.org/10.1016/j.lfs.2015.07.030
- Li YQ, Shrestha YB, Chen M, Chanturiya T, Gavrilova O, Weinstein LS. Gsα deficiency in adipose tissue improves glucose metabolism and insulin sensitivity without an effect on body weight. Proc Natl Acad Sci U S A 2016; 113:446-51; PMID:26712027; http://dx.doi.org/10.1073/pnas.1517142113
- Patsouris D, Qi P, Abdullahi A, Stanojcic M, Chen P, Parousis A, Amini-Nik S, Jeschke MG. Burn Induces Browning of the Subcutaneous White Adipose Tissue in Mice and Humans. Cell Rep 2015; 13:1538-44; PMID:26586436; http://dx.doi.org/10.1016/j.celrep.2015.10.028
- Xu X, Ying Z, Cai M, Xu Z, Li Y, Jiang SY, Tzan K, Wang A, Parthasarathy S, He G, et al. Exercise ameliorates high-fat diet-induced metabolic and vascular dysfunction, and increases adipocyte progenitor cell population in brown adipose tissue. Am J Physiol Regul Integr Comp Physiol 2011; 300:R1115-1125; PMID:21368268; http://dx.doi.org/10.1152/ajpregu.00806.2010
- Cao L, Choi EY, Liu X, Martin A, Wang C, Xu X, During MJ. White to brown fat phenotypic switch induced by genetic and environmental activation of a hypothalamic-adipocyte axis. Cell Metab 2011; 14:324-38; PMID:21907139; http://dx.doi.org/10.1016/j.cmet.2011.06.020
- Carrière A, Jeanson Y, Berger-Müller S, André M, Chenouard V, Arnaud E, Barreau C, Walther R, Galinier A, Wdziekonski B, et al. Browning of white adipose cells by intermediate metabolites: an adaptive mechanism to alleviate redox pressure. Diabetes 2014; 63:3253-65; http://dx.doi.org/10.2337/db13-1885
- Fisher FM, Kleiner S, Douris N, Fox EC, Mepani RJ, Verdeguer F, Wu J, Kharitonenkov A, Flier JS, Maratos-Flier E, et al. FGF21 regulates PGC-1α and browning of white adipose tissues in adaptive thermogenesis. Genes Dev 2012; 26:271-81; PMID:22302939; http://dx.doi.org/10.1101/gad.177857.111
- Jeanson Y, Ribas F, Galinier A, Arnaud E, Ducos M, Andre M, Chenouard V, Villarroya F, Casteilla L, Carriere A. Lactate induces FGF21 expression in adipocytes through a p38-MAPK pathway. Biochem J 2016; 473(6):685-92; PMID:26769382; http://dx.doi.org/10.1042/BJ20150808
- Véniant MM, Sivits G, Helmering J, Komorowski R, Lee J, Fan W, Moyer C, Lloyd DJ. Pharmacologic Effects of FGF21 are independent of the “Browning” of White Adipose Tissue. Cell Metab 2015; 21:731-8; http://dx.doi.org/10.1016/j.cmet.2015.04.019
- Ost M, Coleman V, Voigt A, van Schothorst EM, Keipert S, van der Stelt I, Ringel S, Graja A, Ambrosi T, Kipp AP, et al. Muscle mitochondrial stress adaptation operates independently of endogenous FGF21 action. Mol Metab 2016; 5:79-90; PMID:26909316; http://dx.doi.org/10.1016/j.molmet.2015.11.002
- Knudsen JG, Murholm M, Carey AL, Biensø RS, Basse AL, Allen TL, Hidalgo J, Kingwell BA, Febbraio MA, Hansen JB, et al. Role of IL-6 in exercise training- and cold-induced UCP1 expression in subcutaneous white adipose tissue. PloS One 2014; 9:e84910; PMID:24416310; http://dx.doi.org/10.1371/journal.pone.0084910
- Boström P, Wu J, Jedrychowski MP, Korde A, Ye L, Lo JC, Rasbach KA, Boström EA, Choi JH, Long JZ, et al. A PGC1-α-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature 2012; 481:463-8; PMID:22237023; http://dx.doi.org/10.1038/nature10777
- Jedrychowski MP, Wrann CD, Paulo JA, Gerber KK, Szpyt J, Robinson MM, Nair KS, Gygi SP, Spiegelman BM. Detection and Quantitation of Circulating Human Irisin by Tandem Mass Spectrometry. Cell Metab 2015; 22:734-40; PMID:26278051; http://dx.doi.org/10.1016/j.cmet.2015.08.001
- Rao RR, Long JZ, White JP, Svensson KJ, Lou J, Lokurkar I, Jedrychowski MP, Ruas JL, Wrann CD, Lo JC, et al. Meteorin-like is a hormone that regulates immune-adipose interactions to increase beige fat thermogenesis. Cell 2014; 157:1279-91; PMID:24906147; http://dx.doi.org/10.1016/j.cell.2014.03.065
- Nguyen KD, Qiu Y, Cui X, Goh YPS, Mwangi J, David T, Mukundan L, Brombacher F, Locksley RM, Chawla A. Alternatively activated macrophages produce catecholamines to sustain adaptive thermogenesis. Nature 2011; 480:104-8; PMID:22101429; http://dx.doi.org/10.1038/nature10653
- Brestoff JR, Kim BS, Saenz SA, Stine RR, Monticelli LA, Sonnenberg GF, Thome JJ, Farber DL, Lutfy K, Seale P, et al. Group 2 innate lymphoid cells promote beiging of white adipose tissue and limit obesity. Nature 2015; 519:242-6; PMID:25533952; http://dx.doi.org/10.1038/nature14115
- Roberts LD, Boström P, O'sullivan JF, Schinzel RT, Lewis GD, Dejam A, Lee YK, Palma MJ, Calhoun S, Georgiadi A, et al. β-Aminoisobutyric acid induces browning of white fat and hepatic β-oxidation and is inversely correlated with cardiometabolic risk factors. Cell Metab 2014; 19:96-108; PMID:24411942; http://dx.doi.org/10.1016/j.cmet.2013.12.003
- Koncarevic A, Kajimura S, Cornwall-Brady M, Andreucci A, Pullen A, Sako D, Kumar R, Grinberg AV, Liharska K, Ucran JA, et al. A novel therapeutic approach to treating obesity through modulation of TGFβ signaling. Endocrinology 2012; 153:3133-46; PMID:22549226; http://dx.doi.org/10.1210/en.2012-1016
- Singh R, Braga M, Pervin S. Regulation of brown adipocyte metabolism by myostatin/follistatin signaling. Front Cell Dev Biol 2014; 2:60; PMID:25364764
- Tseng YH, Kokkotou E, Schulz TJ, Huang TL, Winnay JN, Taniguchi CM, Tran TT, Suzuki R, Espinoza DO, Yamamoto Y, et al. New role of bone morphogenetic protein 7 in brown adipogenesis and energy expenditure. Nature 2008; 454:1000-4; PMID:18719589; http://dx.doi.org/10.1038/nature07221
- Townsend KL, An D, Lynes MD, Huang TL, Zhang H, Goodyear LJ, Tseng YH. Increased mitochondrial activity in BMP7-treated brown adipocytes, due to increased CPT1- and CD36-mediated fatty acid uptake. Antioxid Redox Signal 2013; 19:243-57; PMID:22938691; http://dx.doi.org/10.1089/ars.2012.4536
- Hinoi E, Nakamura Y, Takada S, Fujita H, Iezaki T, Hashizume S, Takahashi S, Odaka Y, Watanabe T, Yoneda Y. Growth differentiation factor-5 promotes brown adipogenesis in systemic energy expenditure. Diabetes 2014; 63:162-75; PMID:24062245; http://dx.doi.org/10.2337/db13-0808
- Than A, He HL, Chua SH, Xu D, Sun L, Leow MK-S, Chen P. Apelin Enhances Brown Adipogenesis and Browning of White Adipocytes. J Biol Chem 2015; 290:14679-91; PMID:25931124; http://dx.doi.org/10.1074/jbc.M115.643817
- Boucher J, Masri B, Daviaud D, Gesta S, Guigné C, Mazzucotelli A, Castan-Laurell I, Tack I, Knibiehler B, Carpéné C, et al. Apelin, a newly identified adipokine up-regulated by insulin and obesity. Endocrinology 2005; 146:1764-71; PMID:15677759; http://dx.doi.org/10.1210/en.2004-1427
- Commins SP, Watson PM, Padgett MA, Dudley A, Argyropoulos G, Gettys TW. Induction of uncoupling protein expression in brown and white adipose tissue by leptin. Endocrinology 1999; 140:292-300; PMID:9886838
- Fischer AW, Hoefig CS, Abreu-Vieira G, de Jong JMA, Petrovic N, Mittag J, Cannon B, Nedergaard J. Leptin Raises Defended Body Temperature without Activating Thermogenesis. Cell Rep 2016; 14:1621-31; PMID:26876182; http://dx.doi.org/10.1016/j.celrep.2016.01.041
- Dodd GT, Decherf S, Loh K, Simonds SE, Wiede F, Balland E, Merry TL, Münzberg H, Zhang ZY, Kahn BB, et al. Leptin and insulin act on POMC neurons to promote the browning of white fat. Cell 2015; 160:88-104; PMID:25594176; http://dx.doi.org/10.1016/j.cell.2014.12.022
- Ukropec J, Anunciado RVP, Ravussin Y, Kozak LP. Leptin is required for uncoupling protein-1-independent thermogenesis during cold stress. Endocrinology 2006; 147:2468-80; PMID:16469807; http://dx.doi.org/10.1210/en.2005-1216
- Hui X, Gu P, Zhang J, Nie T, Pan Y, Wu D, Feng T, Zhong C, Wang Y, Lam KSL, et al. Adiponectin Enhances Cold-Induced Browning of Subcutaneous Adipose Tissue via Promoting M2 Macrophage Proliferation. Cell Metab 2015; 22:279-90; PMID:26166748; http://dx.doi.org/10.1016/j.cmet.2015.06.004
- Nisoli E, Tonello C, Landi M, Carruba MO. Functional studies of the first selective β 3-adrenergic receptor antagonist SR 59230A in rat brown adipocytes. Mol Pharmacol 1996; 49:7-14; PMID:8569714
- Bronnikov G, Houstĕk J, Nedergaard J. Beta-adrenergic, cAMP-mediated stimulation of proliferation of brown fat cells in primary culture. Mediation via β 1 but not via β 3 adrenoceptors. J Biol Chem 1992; 267:2006-13; PMID:1346138
- Jimenez M, Barbatelli G, Allevi R, Cinti S, Seydoux J, Giacobino JP, Muzzin P, Preitner F. Beta 3-adrenoceptor knockout in C57BL/6J mice depresses the occurrence of brown adipocytes in white fat. Eur J Biochem FEBS 2003; 270:699-705; http://dx.doi.org/10.1046/j.1432-1033.2003.03422.x
- Gnad T, Scheibler S, von Kügelgen I, Scheele C, Kilić A, Glöde A, Hoffmann LS, Reverte-Salisa L, Horn P, Mutlu S, et al. Adenosine activates brown adipose tissue and recruits beige adipocytes via A2A receptors. Nature 2014; 516:395-9; PMID:25317558; http://dx.doi.org/10.1038/nature13816
- Alvarez-Crespo M, Csikasz RI, Martínez-Sánchez N, Diéguez C, Cannon B, Nedergaard J, López M. Essential role of UCP1 modulating the central effects of thyroid hormones on energy balance. Mol Metab 2016; 5(4):271-82; PMID:27069867; http://dx.doi.org/10.1016/j.molmet.2016.01.008
- Bonet ML, Oliver P, Palou A. Pharmacological and nutritional agents promoting browning of white adipose tissue. Biochim Biophys Acta 2013; 1831:969-85; PMID:23246573; http://dx.doi.org/10.1016/j.bbalip.2012.12.002
- Lin JZ, Martagón AJ, Cimini SL, Gonzalez DD, Tinkey DW, Biter A, Baxter JD, Webb P, Gustafsson JÅ, Hartig SM, et al. Pharmacological Activation of Thyroid Hormone Receptors Elicits a Functional Conversion of White to Brown Fat. Cell Rep 2015; 13:1528-37; PMID:26586443; http://dx.doi.org/10.1016/j.celrep.2015.10.022
- Sellayah D, Bharaj P, Sikder D. Orexin is required for brown adipose tissue development, differentiation, and function. Cell Metab 2011; 14:478-90; PMID:21982708; http://dx.doi.org/10.1016/j.cmet.2011.08.010
- Tupone D, Madden CJ, Morrison SF. Autonomic regulation of brown adipose tissue thermogenesis in health and disease: potential clinical applications for altering BAT thermogenesis. Front Neurosci 2014; 8:1-14; PMID:24478622; http://dx.doi.org/10.3389/fnins.2014.00014
- Oh C-M, Namkung J, Go Y, Shong KE, Kim K, Kim H, Park BY, Lee HW, Jeon YH, Song J, et al. Regulation of systemic energy homeostasis by serotonin in adipose tissues. Nat Commun 2015; 6:6794; PMID:25864946; http://dx.doi.org/10.1038/ncomms7794
- Crane JD, Palanivel R, Mottillo EP, Bujak AL, Wang H, Ford RJ, Collins A, Blümer RM, Fullerton MD, Yabut JM, et al. Inhibiting peripheral serotonin synthesis reduces obesity and metabolic dysfunction by promoting brown adipose tissue thermogenesis. Nat Med 2015; 21:166-72; PMID:25485911; http://dx.doi.org/10.1038/nm.3766
- Hesselbarth N, Pettinelli C, Gericke M, Berger C, Kunath A, Stumvoll M, Blüher M, Klöting N. Tamoxifen affects glucose and lipid metabolism parameters, causes browning of subcutaneous adipose tissue and transient body composition changes in C57BL/6NTac mice. Biochem Biophys Res Commun 2015; 464:724-9; PMID:26164229; http://dx.doi.org/10.1016/j.bbrc.2015.07.015
- Viengchareun S, Penfornis P, Zennaro MC, Lombès M. Mineralocorticoid and glucocorticoid receptors inhibit UCP expression and function in brown adipocytes. Am J Physiol Endocrinol Metab 2001; 280:E640-649; PMID:11254472
- Kong X, Yu J, Bi J, Qi H, Di W, Wu L, Wang L, Zha J, Lv S, Zhang F, et al. Glucocorticoids transcriptionally regulate miR-27b expression promoting body fat accumulation via suppressing the browning of white adipose tissue. Diabetes 2015; 64:393-404; PMID:25187367; http://dx.doi.org/10.2337/db14-0395
- Armani A, Cinti F, Marzolla V, Morgan J, Cranston GA, Antelmi A, Carpinelli G, Canese R, Pagotto U, Quarta C, et al. Mineralocorticoid receptor antagonism induces browning of white adipose tissue through impairment of autophagy and prevents adipocyte dysfunction in high-fat-diet-fed mice. FASEB J Off Publ Fed Am Soc Exp Biol 2014; 28:3745-57
- Pisani DF, Beranger GE, Corinus A, Giroud M, Ghandour RA, Altirriba J, Chambard J-C, Mazure NM, Bendahhou S, Duranton C, et al. The K+ channel TASK1 modulates β-adrenergic response in brown adipose tissue through the mineralocorticoid receptor pathway. FASEB J Off Publ Fed Am Soc Exp Biol 2016; 30:909-22
- Beiroa D, Imbernon M, Gallego R, Senra A, Herranz D, Villarroya F, Serrano M, Fernø J, Salvador J, Escalada J, et al. GLP-1 agonism stimulates brown adipose tissue thermogenesis and browning through hypothalamic AMPK. Diabetes 2014; 63:3346-58; PMID:24917578; http://dx.doi.org/10.2337/db14-0302
- Bordicchia M, Liu D, Amri E-Z, Ailhaud G, Dessì-Fulgheri P, Zhang C, Takahashi N, Sarzani R, Collins S. Cardiac natriuretic peptides act via p38 MAPK to induce the brown fat thermogenic program in mouse and human adipocytes. J Clin Invest 2012; 122:1022-36; PMID:22307324; http://dx.doi.org/10.1172/JCI59701
- Jiménez-Aranda A, Fernández-Vázquez G, Campos D, Tassi M, Velasco-Perez L, Tan DX, Reiter RJ, Agil A. Melatonin induces browning of inguinal white adipose tissue in Zucker diabetic fatty rats. J Pineal Res 2013; 55:416-23
- Bastie C, Holst D, Gaillard D, Jehl-Pietri C, Grimaldi PA. Expression of peroxisome proliferator-activated receptor PPARdelta promotes induction of PPARgamma and adipocyte differentiation in 3T3C2 fibroblasts. J Biol Chem 1999; 274:21920-5; PMID:10419513; http://dx.doi.org/10.1074/jbc.274.31.21920
- Issemann I, Green S. Activation of a member of the steroid hormone receptor superfamily by peroxisome proliferators. Nature1990; 347:645-50; PMID:2129546; http://dx.doi.org/10.1038/347645a0
- Tontonoz P, Kim JB, Graves RA, Spiegelman BM. ADD1: a novel helix-loop-helix transcription factor associated with adipocyte determination and differentiation. Mol Cell Biol 1993; 13:4753-9; PMID:8336713; http://dx.doi.org/10.1128/MCB.13.8.4753
- Foellmi-Adams LA, Wyse BM, Herron D, Nedergaard J, Kletzien RF. Induction of uncoupling protein in brown adipose tissue. Synergy between norepinephrine and pioglitazone, an insulin-sensitizing agent. Biochem Pharmacol 1996; 52:693-701; PMID:8765467; http://dx.doi.org/10.1016/0006-2952(96)00345-0
- Kelly LJ, Vicario PP, Thompson GM, Candelore MR, Doebber TW, Ventre J, Wu MS, Meurer R, Forrest MJ, Conner MW, et al. Peroxisome proliferator-activated receptors gamma and α mediate in vivo regulation of uncoupling protein (UCP-1, UCP-2, UCP-3) gene expression. Endocrinology 1998; 139:4920-7; PMID:9832429
- Barbera MJ, Schluter A, Pedraza N, Iglesias R, Villarroya F, Giralt M. Peroxisome proliferator-activated receptor α activates transcription of the brown fat uncoupling protein-1 gene. A link between regulation of the thermogenic and lipid oxidation pathways in the brown fat cell. J Biol Chem 2001; 276:1486-93; PMID:11050084; http://dx.doi.org/10.1074/jbc.M006246200
- del Mar Gonzalez-Barroso M, Pecqueur C, Gelly C, Sanchis D, Alves-Guerra MC, Bouillaud F, Ricquier D, Cassard-Doulcier AM. Transcriptional activation of the human ucp1 gene in a rodent cell line. Synergism of retinoids, isoproterenol, and thiazolidinedione is mediated by a multipartite response element. J Biol Chem 2000; 275:31722-32; PMID:10921912; http://dx.doi.org/10.1074/jbc.M001678200
- Rajakumari S, Wu J, Ishibashi J, Lim HW, Giang AH, Won KJ, Reed RR, Seale P. EBF2 Determines and Maintains Brown Adipocyte Identity. Cell Metab 2013; 17:562-74; PMID:23499423; http://dx.doi.org/10.1016/j.cmet.2013.01.015
- Loft A, Forss I, Siersbæk MS, Schmidt SF, Larsen A-SB, Madsen JGS, Pisani DF, Nielsen R, Aagaard MM, Mathison A, et al. Browning of human adipocytes requires KLF11 and reprogramming of PPARγ superenhancers. Genes Dev 2015; 29:7-22; PMID:25504365; http://dx.doi.org/10.1101/gad.250829.114
- Puigserver P, Wu Z, Park CW, Graves R, Wright M, Spiegelman BM. A cold-inducible coactivator of nuclear receptors linked to adaptive thermogenesis. Cell 1998; 92:829-39; PMID:9529258; http://dx.doi.org/10.1016/S0092-8674(00)81410-5
- Roberts LD, Ashmore T, Kotwica AO, Murfitt SA, Fernandez BO, Feelisch M, Murray AJ, Griffin JL. Inorganic nitrate promotes the browning of white adipose tissue through the nitrate-nitrite-nitric oxide pathway. Diabetes 2015; 64:471-84; PMID:25249574; http://dx.doi.org/10.2337/db14-0496
- Kapil V, Milsom AB, Okorie M, Maleki-Toyserkani S, Akram F, Rehman F, Arghandawi S, Pearl V, Benjamin N, Loukogeorgakis S, et al. Inorganic nitrate supplementation lowers blood pressure in humans: role for nitrite-derived NO. Hypertension 2010; 56:274-81; PMID:20585108; http://dx.doi.org/10.1161/HYPERTENSIONAHA.110.153536
- Wong KE, Szeto FL, Zhang W, Ye H, Kong J, Zhang Z, Sun XJ, Li YC. Involvement of the vitamin D receptor in energy metabolism: regulation of uncoupling proteins. Am J Physiol Endocrinol Metab 2009; 296:E820-828; PMID:19176352; http://dx.doi.org/10.1152/ajpendo.90763.2008
- Wang S, Liang X, Yang Q, Fu X, Rogers CJ, Zhu M, Rodgers BD, Jiang Q, Dodson MV, Du M. Resveratrol induces brown-like adipocyte formation in white fat through activation of AMP-activated protein kinase (AMPK) α1. Int J Obes 2005; 39:967-76http://dx.doi.org/10.1038/ijo.2015.23
- Lone J, Choi JH, Kim SW, Yun JW. Curcumin induces brown fat-like phenotype in 3T3-L1 and primary white adipocytes. J Nutr Biochem 2016; 27:193-202; PMID:26456563; http://dx.doi.org/10.1016/j.jnutbio.2015.09.006
- Mu Q, Fang X, Li X, Zhao D, Mo F, Jiang G, Yu N, Zhang Y, Guo Y, Fu M, et al. Ginsenoside Rb1 promotes browning through regulation of PPARγ in 3T3-L1 adipocytes. Biochem Biophys Res Commun 2015; 466:530-5; PMID:26381176; http://dx.doi.org/10.1016/j.bbrc.2015.09.064
- Wang S, Wang X, Ye Z, Xu C, Zhang M, Ruan B, Wei M, Jiang Y, Zhang Y, Wang L, et al. Curcumin promotes browning of white adipose tissue in a norepinephrine-dependent way. Biochem Biophys Res Commun 2015; 466:247-53; PMID:26362189; http://dx.doi.org/10.1016/j.bbrc.2015.09.018
- Oudart H, Groscolas R, Calgari C, Nibbelink M, Leray C, Le Maho Y, Malan A. Brown fat thermogenesis in rats fed high-fat diets enriched with n-3 polyunsaturated fatty acids. Int J Obes Relat Metab Disord J Int Assoc Study Obes 1997; 21:955-62; http://dx.doi.org/10.1038/sj.ijo.0800500
- Flachs P, Rossmeisl M, Kuda O, Kopecky J. Stimulation of mitochondrial oxidative capacity in white fat independent of UCP1: a key to lean phenotype. Biochim Biophys Acta 2013; 1831:986-1003; PMID:23454373; http://dx.doi.org/10.1016/j.bbalip.2013.02.003
- Vögler O, López-Bellan A, Alemany R, Tofé S, González M, Quevedo J, Pereg V, Barceló F, Escriba PV. Structure-effect relation of C18 long-chain fatty acids in the reduction of body weight in rats. Int J Obes 2008; 32:464-73; http://dx.doi.org/10.1038/sj.ijo.0803768
- Ghandour RA, Giroud M, Vegiopoulos A, Herzig S, Ailhaud G, Amri EZ, Pisani DF. IP-receptor and PPARs trigger the conversion of human white to brite adipocyte induced by carbaprostacyclin. Biochim Biophys Acta 2016; 1861:285-93; http://dx.doi.org/10.1016/j.bbalip.2016.01.007
- Pisani DF, Ghandour RA, Beranger GE, Le Faouder P, Chambard JC, Giroud M, Vegiopoulos A, Djedaini M, Bertrand-Michel J, Tauc M, et al. The ω6-fatty acid, arachidonic acid, regulates the conversion of white to brite adipocyte through a prostaglandin/calcium mediated pathway. Mol Metab 2014; 3:834-47; PMID:25506549; http://dx.doi.org/10.1016/j.molmet.2014.09.003
- Shen W, Baldwin J, Collins B, Hixson L, Lee KT, Herberg T, Starnes J, Cooney P, Chuang C-C, Hopkins R, et al. Low level of trans-10, cis-12 conjugated linoleic acid decreases adiposity and increases browning independent of inflammatory signaling in overweight Sv129 mice. J Nutr Biochem 2015; 26:616-25; PMID:25801353; http://dx.doi.org/10.1016/j.jnutbio.2014.12.016
- Wendel AA, Purushotham A, Liu LF, Belury MA. Conjugated linoleic acid induces uncoupling protein 1 in white adipose tissue of ob/ob mice. Lipids 2009; 44:975-82; PMID:19779754; http://dx.doi.org/10.1007/s11745-009-3348-9
- Cheng Y, Meng Q, Wang C, Li H, Huang Z, Chen S, Xiao F, Guo F. Leucine deprivation decreases fat mass by stimulation of lipolysis in white adipose tissue and upregulation of uncoupling protein 1 (UCP1) in brown adipose tissue. Diabetes 2010; 59:17-25; PMID:19833890; http://dx.doi.org/10.2337/db09-0929
- Hasek BE, Stewart LK, Henagan TM, Boudreau A, Lenard NR, Black C, Shin J, Huypens P, Malloy VL, Plaisance EP, et al. Dietary methionine restriction enhances metabolic flexibility and increases uncoupled respiration in both fed and fasted states. Am J Physiol Regul Integr Comp Physiol 2010; 299:R728-739; PMID:20538896; http://dx.doi.org/10.1152/ajpregu.00837.2009
- Du Y, Meng Q, Zhang Q, Guo F. Isoleucine or valine deprivation stimulates fat loss via increasing energy expenditure and regulating lipid metabolism in WAT. Amino Acids 2012; 43:725-34; PMID:22016194; http://dx.doi.org/10.1007/s00726-011-1123-8
- Marsset-Baglieri A, Fromentin G, Tomé D, Bensaid A, Makkarios L, Even PC. Increasing the protein content in a carbohydrate-free diet enhances fat loss during 35% but not 75% energy restriction in rats. J Nutr 2004; 134:2646-52; PMID:15465761
- Petzke KJ, Friedrich M, Metges CC, Klaus S. Long-term dietary high protein intake up-regulates tissue specific gene expression of uncoupling proteins 1 and 2 in rats. Eur J Nutr 2005; 44:414-21; PMID:15602629; http://dx.doi.org/10.1007/s00394-004-0545-4
- Noatsch A, Petzke KJ, Millrose MK, Klaus S. Body weight and energy homeostasis was not affected in C57BL/6 mice fed high whey protein or leucine-supplemented low-fat diets. Eur J Nutr 2011; 50:479-88; PMID:21170537; http://dx.doi.org/10.1007/s00394-010-0155-2
- Fu WJ, Haynes TE, Kohli R, Hu J, Shi W, Spencer TE, Carroll RJ, Meininger CJ, Wu G. Dietary L-arginine supplementation reduces fat mass in Zucker diabetic fatty rats. J Nutr 2005; 135:714-21; PMID:15795423
- Jobgen W, Meininger CJ, Jobgen SC, Li P, Lee MJ, Smith SB, Spencer TE, Fried SK, Wu G. Dietary L-arginine supplementation reduces white fat gain and enhances skeletal muscle and brown fat masses in diet-induced obese rats. J Nutr 2009; 139:230-7; PMID:19106310; http://dx.doi.org/10.3945/jn.108.096362
- Wu Z, Satterfield MC, Bazer FW, Wu G. Regulation of brown adipose tissue development and white fat reduction by L-arginine. Curr Opin Clin Nutr Metab Care 2012; 15:529-38; PMID:23075933; http://dx.doi.org/10.1097/MCO.0b013e3283595cff
- Clemmensen C, Madsen AN, Smajilovic S, Holst B, Bräuner-Osborne H. L-Arginine improves multiple physiological parameters in mice exposed to diet-induced metabolic disturbances. Amino Acids 2012; 43:1265-75; PMID:22200933; http://dx.doi.org/10.1007/s00726-011-1199-1
- Tian XY, Ganeshan K, Hong C, Nguyen KD, Qiu Y, Kim J, Tangirala RK, Tonotonoz P, Chawla A. Thermoneutral housing accelerates metabolic inflammation to potentiate atherosclerosis but not insulin resistance. Cell Metab 2016; 23:165-78; PMID:26549485; http://dx.doi.org/10.1016/j.cmet.2015.10.003
- Jonker R, Engelen MPKJ, Deutz NEP. Role of specific dietary amino acids in clinical conditions. Br J Nutr 2012; 108Suppl 2:S139-148; http://dx.doi.org/10.1017/S0007114512002358
- Moinard C, Cynober L. Citrulline: A New player in the control of nitrogen homeostasis. J Nutr 2007; 137:1621S-5S; PMID:17513438
- Osowska S, Duchemann T, Walrand S, Paillard A, Boirie Y, Cynober L, Moinard C. Citrulline modulates muscle protein metabolism in old malnourished rats. Am J Physiol Endocrinol Metab 2006; 291:E582-586; PMID:16608884; http://dx.doi.org/10.1152/ajpendo.00398.2005
- Sureda A, Pons A. Arginine and citrulline supplementation in sports and exercise: ergogenic nutrients? Med Sport Sci 2012; 59:18-28; PMID:23075551; http://dx.doi.org/10.1159/000341937
- Moinard C, Le Plenier S, Noirez P, Morio B, Bonnefont-Rousselot D, Kharchi C, Ferry A, Neveux N, Cynober L, Raynaud-Simon A. Citrulline supplementation induces changes in body composition and limits age-related metabolic changes in healthy male rats. J Nutr 2015; 145:1429-37; PMID:26019250; http://dx.doi.org/10.3945/jn.114.200626
- Joffin N, Jaubert AM, Durant S, Bastin J, De Bandt JP, Cynober L, Moinard C, Coumoul X, Forest C, Noirez P. Citrulline reduces glyceroneogenesis and induces fatty acid release in visceral adipose tissue from overweight rats. Mol Nutr Food Res 2014; 58:2320-30; PMID:25271764; http://dx.doi.org/10.1002/mnfr.201400507
- Joffin N, Jaubert AM, Durant S, Bastin J, De Bandt JP, Cynober L, Moinard C, Forest C, Noirez P. Citrulline induces fatty acid release selectively in visceral adipose tissue from old rats. Mol Nutr Food Res 2014; 58:1765-75; PMID:24913603; http://dx.doi.org/10.1002/mnfr.201400053
- Joffin N, Jaubert AM, Bamba J, Barouki R, Noirez P, Forest C. Acute induction of uncoupling protein 1 by citrulline in cultured explants of white adipose tissue from lean and high-fat-diet-fed rats. Adipocyte 2015; 4:129-34; PMID:26167416; http://dx.doi.org/10.4161/21623945.2014.989748
- Golozoubova V, Cannon B, Nedergaard J. UCP1 is essential for adaptive adrenergic nonshivering thermogenesis. Am J Physiol Endocrinol Metab 2006; 291:E350-357; PMID:16595854; http://dx.doi.org/10.1152/ajpendo.00387.2005
- Vaughan M, Steinberg D. Effect of hormones on lipolysis and esterification of free fatty acids during incibation of adipose tissue in vitro. J Lipid Res 1963; 4:193-9; PMID:14168151
- Newsholme EA, Crabtree B. Substrate cycles in metabolic regulation and in heat generation. Biochem Soc Symp 1976; 41:61-109; PMID:184791
- Hanson RW, Ballard FJ. The relative significance of acetate and glucose as precursors for lipid synthesis in liver and adipose tissue from ruminants. Biochem J 1967; 105:529-36; PMID:5583995; http://dx.doi.org/10.1042/bj1050529
- Reshef L, Hanson RW, Ballard FJ. Glyceride-glycerol synthesis from pyruvate. Adaptive changes in phosphoenolpyruvate carboxykinase and pyruvate carboxylase in adipose tissue and liver. J Biol Chem 1969; 244:1994-2001; PMID:5781996
- Yang J, Kalhan SC, Hanson RW. What is the metabolic role of phosphoenolpyruvate carboxykinase? J Biol Chem 2009; 284:27025-9; PMID:19636077; http://dx.doi.org/10.1074/jbc.R109.040543
- Brito MN, Brito NA, Brito SR, Moura MA, Kawashita NH, Kettelhut IC, Migliorini RH. Brown adipose tissue triacylglycerol synthesis in rats adapted to a high-protein, carbohydrate-free diet. Am J Physiol 1999; 276:R1003-1009; PMID:10198378
- Brooks BJ, Arch JR, Newsholme EA. Effect of some hormones on the rate of the triacylglycerol/fatty-acid substrate cycle in adipose tissue of the mouse in vivo. Biosci Rep 1983; 3:263-7; PMID:6860784; http://dx.doi.org/10.1007/BF01122458
- Chaves VE, Frasson D, Martins-Santos MES, Navegantes LCC, Galban VD, Garófalo MAR, Kettelhut IC, Migliorini RH. Fatty acid synthesis and generation of glycerol-3-phosphate in brown adipose tissue from rats fed a cafeteria diet. Can J Physiol Pharmacol 2008; 86:416-23; PMID:18641690; http://dx.doi.org/10.1139/Y08-052
- Festuccia WTL, Kawashita NH, Garofalo MAR, Moura MAF, Brito SRC, Kettelhut IC, Migliorini RH. Control of glyceroneogenic activity in rat brown adipose tissue. Am J Physiol Regul Integr Comp Physiol 2003; 285:R177-182; PMID:12793997; http://dx.doi.org/10.1152/ajpregu.00713.2002
- Festuccia WTL, Guerra Sá R, Kawashita NH, Garófalo MAR, Evangelista EA, Rodrigues V, Kettelhut IC, Migliorini RH. Expression of glycerokinase in brown adipose tissue is stimulated by the sympathetic nervous system. Am J Physiol Regul Integr Comp Physiol 2003; 284:R1536-1541; PMID:12736183; http://dx.doi.org/10.1152/ajpregu.00764.2002
- Moura MAF, Festuccia WTL, Kawashita NH, Garófalo MAR, Brito SRC, Kettelhut IC, Migliorini RH. Brown adipose tissue glyceroneogenesis is activated in rats exposed to cold. Pflüg Arch Eur J Physiol 2005; 449:463-9; http://dx.doi.org/10.1007/s00424-004-1353-7
- Reshef L, Hanson RW. The interaction of catecholamines and adrenal corticosteroids in the induction of phosphopyruvate carboxylase in rat liver and adipose tissue. Biochem J 1972; 127:809-18; PMID:4342497; http://dx.doi.org/10.1042/bj1270809
- Franckhauser S, Antras-Ferry J, Robin P, Robin D, Granner DK, Forest C. Expression of the phosphoenolpyruvate carboxykinase gene in 3T3-F442A adipose cells: opposite effects of dexamethasone and isoprenaline on transcription. Biochem J 1995; 305 (Pt 1):65-71; PMID:7826355; http://dx.doi.org/10.1042/bj3050065
- Boden G. Free fatty acids-the link between obesity and insulin resistance. Endocr Pract Off J Am Coll Endocrinol Am Assoc Clin Endocrinol 2001; 7:44-51
- Beale EG, Hammer RE, Antoine B, Forest C. Disregulated glyceroneogenesis: PCK1 as a candidate diabetes and obesity gene. Trends Endocrinol Metab TEM 2004; 15:129-35; PMID:15046742; http://dx.doi.org/10.1016/j.tem.2004.02.006
- Glorian M, Duplus E, Beale EG, Scott DK, Granner DK, Forest C. A single element in the phosphoenolpyruvate carboxykinase gene mediates thiazolidinedione action specifically in adipocytes. Biochimie 2001; 83:933-43; PMID:11728630; http://dx.doi.org/10.1016/S0300-9084(01)01343-8
- Leroyer SN, Tordjman J, Chauvet G, Quette J, Chapron C, Forest C, Antoine B. Rosiglitazone controls fatty acid cycling in human adipose tissue by means of glyceroneogenesis and glycerol phosphorylation. J Biol Chem 2006; 281:13141-9; PMID:16524879; http://dx.doi.org/; http://dx.doi.org/10.1074/jbc.M512943200
- Tordjman J, Chauvet G, Quette J, Beale EG, Forest C, Antoine B. Thiazolidinediones block fatty acid release by inducing glyceroneogenesis in fat cells. J Biol Chem 2003; 278:18785-90; PMID:12644461; http://dx.doi.org/10.1074/jbc.M206999200
- Goetzman ES. The regulation of acyl-CoA dehydrogenases in adipose tissue by rosiglitazone. Obes Silver Spring Md 2009; 17:196-8; http://dx.doi.org/10.1038/oby.2008.467
- Franckhauser S, Muñoz S, Pujol A, Casellas A, Riu E, Otaegui P, Su B, Bosch F. Increased fatty acid re-esterification by PEPCK overexpression in adipose tissue leads to obesity without insulin resistance. Diabetes 2002; 51:624-30; PMID:11872659; http://dx.doi.org/10.2337/diabetes.51.3.624
- Geer EB, Islam J, Buettner C. Mechanisms of glucocorticoid-induced insulin resistance: focus on adipose tissue function and lipid metabolism. Endocrinol Metab Clin North Am 2014; 43:75-102; PMID:24582093; http://dx.doi.org/10.1016/j.ecl.2013.10.005
- Beale EG, Harvey BJ, Forest C. PCK1 and PCK2 as candidate diabetes and obesity genes. Cell Biochem Biophys 2007; 48:89-95; PMID:17709878; http://dx.doi.org/10.1007/s12013-007-0025-6
- Cadoudal T, Blouin JM, Collinet M, Fouque F, Tan GD, Loizon E, Beale EG, Frayn KN, Karpe F, Vidal H, et al. Acute and selective regulation of glyceroneogenesis and cytosolic phosphoenolpyruvate carboxykinase in adipose tissue by thiazolidinediones in type 2 diabetes. Diabetologia 2007; 50:666-75; PMID:17242918; http://dx.doi.org/10.1007/s00125-006-0560-5
- Burgess SC, Hausler N, Merritt M, Jeffrey FMH, Storey C, Milde A, Koshy S, Lindner J, Magnuson MA, Malloy CR, et al. Impaired tricarboxylic acid cycle activity in mouse livers lacking cytosolic phosphoenolpyruvate carboxykinase. J Biol Chem 2004; 279:48941-9; PMID:15347677; http://dx.doi.org/10.1074/jbc.M407120200
- Owen OE, Kalhan SC, Hanson RW. The key role of anaplerosis and cataplerosis for citric acid cycle function. J Biol Chem 2002; 277:30409-12; PMID:12087111; http://dx.doi.org/10.1074/jbc.R200006200
- Claussnitzer M, Dankel SN, Klocke B, Grallert H, Glunk V, Berulava T, Lee H, Oskolkov N, Fadista J, Ehlers K, et al. Leveraging cross-species transcription factor binding site patterns: from diabetes risk loci to disease mechanisms. Cell 2014; 156:343-58; PMID:24439387; http://dx.doi.org/10.1016/j.cell.2013.10.058
- Antras-Ferry J, Robin P, Robin D, Forest C. Fatty acids and fibrates are potent inducers of transcription of the phosphenolpyruvate carboxykinase gene in adipocytes. Eur J Biochem FEBS 1995; 234:390-6; http://dx.doi.org/10.1111/j.1432-1033.1995.390_b.x
- Duplus E, Forest C. Is there a single mechanism for fatty acid regulation of gene transcription? Biochem Pharmacol 2002; 64:893-901; PMID:12213584; http://dx.doi.org/10.1016/S0006-2952(02)01157-7
- Anunciado-Koza R, Ukropec J, Koza RA, Kozak LP. Inactivation of UCP1 and the glycerol phosphate cycle synergistically increases energy expenditure to resist diet-induced obesity. J Biol Chem 2008; 283:27688-97; PMID:18678870; http://dx.doi.org/10.1074/jbc.M804268200
- Ukropec J, Anunciado RP, Ravussin Y, Hulver MW, Kozak LP. UCP1-independent thermogenesis in white adipose tissue of cold-acclimated Ucp1-/- mice. J Biol Chem 2006; 281:31894-908; PMID:16914547; http://dx.doi.org/10.1074/jbc.M606114200
- Kazak L, Chouchani ET, Jedrychowski MP, Erickson BK, Shinoda K, Cohen P, Vetrivelan R, Lu GZ, Laznik-Bogoslavski D, Hasenfuss SC, et al. A creatine-driven substrate cycle enhances energy expenditure and thermogenesis in beige fat. Cell 2015; 163:643-55; PMID:26496606; http://dx.doi.org/10.1016/j.cell.2015.09.035
- Oudman I, Clark JF, Brewster LM. The effect of the creatine analogue β-guanidinopropionic acid on energy metabolism: a systematic review. PloS One 2013; 8:e52879; PMID:23326362; http://dx.doi.org/10.1371/journal.pone.0052879
