ABSTRACT
Chronic low grade inflammation is one of the major metabolic disorders in case of obesity and associated pathologies. By its important secretion function, the role of adipose tissue in this metabolic low grade inflammation is well known. Recently, it was demonstrated that the alarmin high mobility group box protein 1 (HMGB1) is involved in obesity-related pathologies by its increased serum levels in obese compared to normal weight individuals, and by its pro-inflammatory effects. However, the role of HMGB1 on adipocytes inflammation is poorly documented and we propose to investigate this point. Primary culture of human subcutaneous adipocytes were performed from human adipose tissue samples. Cells were treated with recombinant HMGB1 with/without anti-TLR4 antibody and inhibitors of NF-κB and P38 MAPK. Supernatants were collected for IL-6 and MCP-1 ELISA. HMGB1 initiates Toll-like receptor 4 (TLR4)-dependent activation of inflammation through the downstream NF-κB and P38 MAPK signaling pathway to upregulate the secretion of the pro-inflammatory cytokine IL-6. HMGB1 has pro-inflammatory effects on adipocytes. This reinforces the role of TLR4 in adipose tissue inflammation and antagonizing the HMGB1 inflammatory pathway could bring on new therapeutic targets to counteract obesity-associated pathologies.
Introduction
Obesity is recognized as a global pandemic disease. It is defined as an abnormal or excessive fat accumulation that may impair health and increases the risk of many diseases such as diabetes, cardiovascular diseases, stroke and even cancer. One of the characteristics of obesity and associated pathologies is the chronic low grade inflammation defined by increased serum levels of pro-inflammatory molecules.Citation1
High mobility group box protein 1 (HMGB1) is an abundant non-histone nuclear binding protein expressed in all cell types, except erythocytes. Under normal conditions, HMGB1 maintains DNA structure, repair, recombination and facilitates gene transcription. However under inflammation and stress, HMGB1 translocates from nucleus to the cytoplasm to be actively or passively released to promote inflammation.Citation2
It is demonstrated that HMGB1 protein expression is higher in adipose tissue from obese persons than in normal-weight persons.Citation3 In vitro studies show that in human adipose tissue, adipose stromal cells (ASCs) derived from the stromal vascular fraction (SVF) are able to actively secrete HMGB1 during inflammation, whereas adipocytes are not.Citation3 Nevertheless, adipocytes could respond to this local danger signal, as HMGB1 induces IL-6 secretion in pre-adipocyte SW872 cell cultures.Citation4 This possible “paracrine” role of HMGB1 on human adipocyte has so far not been much studied. Moreover, it is established that disulfide HMGB1 acts via the TLR4 pathway to induce cytokine production in most of cell types, while other studies demonstrate that RAGE-dependant processes are involved in the HMGB1-regulation of adipocytes and that this pathway is TLR4-independent.Citation4 The HMGB1 inflammatory signaling pathway in adipose tissue remains unclear and the aim of this present work was to investigate the role of the disulfide HMGB1 isoform on human adipose tissue inflammation via a hypothetical direct effect on adipocytes. In addition, we aimed to investigate receptor and intracellular signaling pathways requirements for such a putative process.
Materials and methods
Reagents
Collagenase (NB4, 0.12 PZU/mg) was from SERVA. All reagents for cell culture were purchased from PAN Biotech except insulin (Umuline Rapide, Lilly). Ringer Lactate was obtained from B. Braun. Recombinant HMGB1 was produced as previously described in E. coli expression system followed by Triton X-114 treatment for LPS removal.Citation5 Antibody against TLR4 was from Hycult Biotechnologies (clone HTA125, Clinisciences). NF-κB inhibitor (6-amino-4-(4-phenoxyphenylethylamino)-quinazoline) was from Calbiochem. P38 MAP-kinase inhibitor (SB202190) was from Sigma.
Origin of human adipose tissue samples and harvesting protocol
Subcutaneous (abdominal, buttocks, hips and thighs) adipose tissue samples were obtained from 6 normal weight women (aged from 34 to 50 years, mean 42.5 years; mean body mass index = 25.1 kg/m2) undergoing liposuction, performed under general anesthesia for aesthetic reasons. Apart from hormonal contraception, the subjects were not receiving any treatment with prescribed medication at the time of liposuction. Fat tissue was harvested mechanically with a vacuum pump after infiltration of a tumescence solution (40 mL lidocaine 2% + adrenalin 1 mg/L for 1 L Ringer Lactate) for local anesthesia. Study was approved by the Reunion Island ethics committee for biomedical research. Samples were handled within an hour after the end of the surgery.
Human adipocytes isolation and primary culture
After washing, adipose tissue sample was digested for 30 min at 37°C in Ringer Lactate containing 0.5 mg/mL collagenase. The floating cells (adipocytes) were rinsed in Ringer Lactate and plated in medium 199 supplemented with 1% fetal bovine serum (FBS), 2 g/L glucose, 2 mM L-glutamine, 8 µg/mL biotin, 4 µg/mL pantothenate and 66 nM insulin, 5 mg/mL amphotericin B, 0.2 mg/mL streptomycin and 200 U/mL penicillin. For secretion analysis, 200 µL of cells (approximately 30 000 cells) were plated in 24-well plates with 300 µL of complemented media. For antibody blocking, 50 µL of cells (approximately 7 500 cells) were plated in 96-w plates with 125 µL media. Cells were then maintained at 37°C in 5% CO2 for a period of 24 hours prior to the experiments.
IL-6 and MCP-1 ELISA
Following treatment, media samples were collected and assayed for IL-6 and MCP-1 (eBioscience), according to the manufacturer's instructions. ELISA sensitivity: 2 pg/mL for IL-6; 6 pg/mL for MCP-1.
Statistics
All values are measured as mean ± SEM. Statistical analysis was performed using Graph pad PRISM 5 software (Windows). Differences were analyzed by T-test () or ANOVA one-way analysis of variance and Dunnett's multiple comparisons post-test ().
Figure 1. Pro-inflammatory effects of HMGB1 on adipocytes. For each experiment, human adipose tissue was harvested to extract adipocytes. Cells were treated with disulfide HMGB1 (2.5 µg/mL) for 24h. Supernatants were collected and IL-6 (A) and MCP-1 (B) secretion was determined by ELISA. Results are expressed in % ± SEM normalized to control cells (n = 6 for each condition; P < 0.0001 (***) compared to appropriate control cells, using T-test).
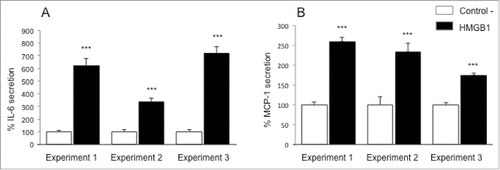
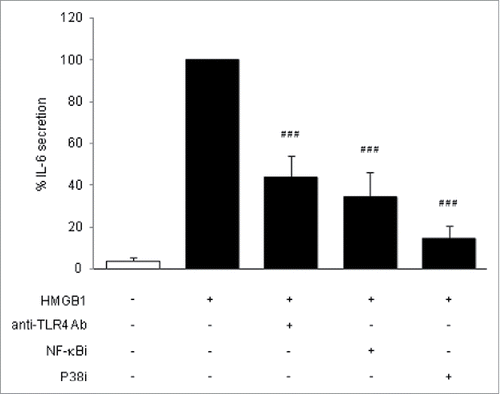
Figure 2. Signaling pathways of HMGB1. Human adipocytes were pretreated for 1 h with anti-TLR4 blocking antibody (20 µg/mL), with NF-κB inhibitor (1 μM) or with P38 MAPK inhibitor (5 μM) and then treated with disulfide HMGB1 for 24 h (2.5 µg/mL). IL-6 secretion was determined by ELISA from adipocytes supernatants, expressed in % normalized to HMGB1-treated cells. Results are shown as mean ± SEM of 3 independent experiments (n = 6 for each condition per experiment; P < 0.0001 (###) compared to HMGB1-treated cells, using one-way ANOVA and Dunnett's post-test).
Results
Adipose tissue is composed of different cell types, among which mature adipocytes are abundant and known to secrete many pro-inflammatory molecules, notably through activation of TLR4, which is expressed and functional on these cells.Citation6
To determine whether HMGB1 could act in a paracrine manner on adipocytes to trigger inflammation, we analyzed the secretion of the pro-inflammatory adipokine IL-6 and the chemokine MCP-1 after incubation of adipocytes with 2.5 μg/mL of recombinant disulfide HMGB1, the TLR4 ligand isoform (). In these experiments, we used LPS at 50 pg/mL as a positive control of inflammation (data not shown). The pro-inflammatory effect of HMGB1 was seen after a culture period of 24 h with an important increase of IL-6 and MCP-1 release from the adipocytes. Depending on the experiment, an increase from 2 to 7-fold was observed.
We then studied signaling pathways mediated the pro-inflammatory effects of HMGB1 on adipocytes. First we checked if TLR4 was involved. When adipocytes were co-incubated with HMGB1 and a TLR4-blocking antibody, there was a significant half-decrease of IL-6 secretion compared to HMGB1-exposed cells cultured without antibodies (). This result indicated that TLR4 was, at least, one of the receptors involved in the secretion of pro-inflammatory IL-6 from adipocytes following HMGB1 treatment. Next, we focused on the intracellular signaling pathways by using NF-κB and P38 MAPK inhibitors. These inhibitors have no intrinsic effects on cell death and IL-6 secretion (data not shown) and were previously controlled for their efficiency.Citation7 A strong and significant inhibition of HMGB1-induced IL-6 secretion was shown when NF-κB and P38 MAPK inhibitors were co-incubating with HMGB1, which indicates the involvement of NF-κB and P38 MAPK in the disulfide HMGB1 signaling ().
Discussion
The presence of increased extracellular HMGB1 levels has been reported in obesity and associated pathologies but only few studies have been carried out to analyze a possible causality link between adipose tissue inflammation and HMGB1.
Many studies have shown the positive correlation between circulating HMGB1 levels and BMICitation8-10 and it has been also reported that the expression of HMGB1 in adipose tissue is altered in case of obesity.Citation3,8,11 This difference in HMGB1 expression has been attributed to inflammation insofar as it triggers HMGB1 secretion from cells derived from the stromal fraction of adipose tissue (ASCs), whereas mature adipocytes do not contribute to this secretion.Citation3
Here we investigated the effects of exogenous disulfide HMGB1 on cultured primary human adipocytes and our results prove that HMGB1 may contribute to chronic and low grade inflammation by its effect on adipocytes, as it has been demonstrated in SW872 pre-adipocytes.Citation4 This idea is a novel one because it was previously anticipated that HMGB1 induces its pro-inflammatory action on adipose tissue via effects on infiltrated macrophages.Citation12 Our data provide information that strengthens the functional role of the adipocyte itself in the low grade inflammation, via HMGB1.
It is now established that the different HMGB1 redox isoforms utilize different reciprocal cell receptors to mediate their functions. Disulfide HMGB1 mediates its effects via the TLR4/MD-2 complex.Citation13 A recent study using SW872 pre-adipocyte cell cultures showed that HMGB1 of undefined redox state bounds to RAGE but not to TLR4 and induced IL-6 and MCP-1 secretion.Citation4 Contrary to this study, we here showed that TLR4 is, at least, one of the receptors involved in the secretion of IL-6 from mature adipocytes following HMGB1 stimulation. This discrepancy may be explained by the difference regarding cell types used in the studies (SW872 human preadipocyte cell line versus human primary culture of mature adipocyte) or regarding HMGB1 isoform added to the cell cultures.
However, we don't get a complete inhibition with the anti-TLR4 antibody used, and beyond technical explanations such as concentration or incubation time, it could also mean that additional receptors might be involved in collaboration with TLR4.Citation14,15
In the same model, it has been reported that following TLR4 activation with LPS, the production of pro-inflammatory cytokines such as TNFα is dependent of NF-κB and P38 MAPK.Citation7 Our study therefore presents many similarities between LPS and HMGB1 signaling.
Several reports confirm that HMGB1 is released in excess in adipose tissue in obese individuals. However, there are presently no studies of the redox state of this extracellular HMGB1. This point is crucial, since the redox isoform determines the function of HMGB1.Citation16 Fully reduced HMGB1 is a chemotactic factor, disulfide HMGB1 a cytokine-inducing factor while sulfonyl HMGB1 has no known biological function. Studies to elucidate these processes in adipose tissue may help to figure out novel therapeutic interventions based on controlling the redox states of HMGB1.
Conclusions
This study demonstrates that the cytokine-inducing isoform of HMGB1 promotes pro-inflammatory IL-6 secretion from human mature primary adipocytes via the activation of TLR4 and the downstream molecules NF-κB and P38 MAPK. Thus, in this study, we highlight the key role of adipocytes in the pathogenic mechanism of obesity.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
We are grateful to José Gonçalves M.D, Olivier Hulard M.D, David Valenti M.D and Pierre Delarue M.D for the collection of adipose tissue samples and their cooperation in the study. We thank the Clinifutur group, and the personnel from Les Orchidées and St-Vincent clinics. We also thank Brice Nativel, Wildriss Viranaicken and Thomas Iwema for the preliminary biochemical and cellular tests on rHMGB1. We thank Aurélie Catan for her help in laboratory handlings. We are grateful to the Félix Guyon University Hospital for its material support.
Funding
This work was supported by the University of Reunion Island. A-L.V-L has a grant from FedEBS (Fédération Environnement Biodiversité), University of Reunion Island and M.K.G has a PhD scholarship from Regional Council of Reunion Island.
References
- Scarpellini E, Tack J. Obesity and metabolic syndrome: an inflammatory condition. Digestive Dis 2012; 30:148-53; PMID:22722429; http://dx.doi.org/10.1159/000336664
- Chen G, Ward MF, Sama AE, Wang H. Extracellular HMGB1 as a proinflammatory cytokine. J Interferon Cytokine Res: Off J Int Soc Int Cytokine Res 2004; 24:329-33; PMID:15212706
- Gunasekaran MK, Viranaicken W, Girard AC, Festy F, Cesari M, Roche R, Hoareau L. Inflammation triggers high mobility group box 1 (HMGB1) secretion in adipose tissue, a potential link to obesity. Cytokine 2013; 64:103-11; PMID:23938155; http://dx.doi.org/10.1016/j.cyto.2013.07.017
- Nativel B, Marimoutou M, Thon-Hon VG, Gunasekaran MK, Andries J, Stanislas G, Planesse C, Da Silva CR, Césari M, Iwema T, et al. Soluble HMGB1 is a novel adipokine stimulating IL-6 secretion through RAGE receptor in SW872 preadipocyte cell line: contribution to chronic inflammation in fat tissue. PloS One 2013; 8:e76039; PMID:24073286; http://dx.doi.org/10.1371/journal.pone.0076039
- Li J, Wang H, Mason JM, Levine J, Yu M, Ulloa L, Czura CJ, Tracey KJ, Yang H. Recombinant HMGB1 with cytokine-stimulating activity. J Immunol Methods 2004; 289:211-23; PMID:15251426; http://dx.doi.org/10.1016/j.jim.2004.04.019
- Bes-Houtman S, Roche R, Hoareau L, Gonthier MP, Festy F, Caillens H, Gasque P, Lefebvre d'Hellencourt C, Cesari M. Presence of functional TLR2 and TLR4 on human adipocytes. Histochem Cell Biol 2007; 127:131-7; PMID:16988837; http://dx.doi.org/10.1007/s00418-006-0230-1
- Hoareau L, Bencharif K, Rondeau P, Murumalla M, Ravanan P, Tallet F, Delarue P, Cesari M, Roche R, Festy F. Signaling pathways involved in LPS induced TNFalpha production in human adipocytes. J Inflamm 2010; 7-1; PMID:20181058
- Guzman-Ruiz R, Ortega E, Rodriguez A, Vazquez-Martinez R, Diaz-Ruiz A, Garcia-Navarro S, Giralt M, Garcia-Rios A, Cobo-Padilla D, Tinahones FJ, et al. Alarmin high-mobility group B1 (HMGB1) is regulated in human adipocytes in insulin resistance and influences insulin secretion in beta-cells. Int J Obesity 2014; 38:1545-54; PMID:24577317; http://dx.doi.org/10.1038/ijo.2014.36
- Arrigo T, Chirico V, Salpietro V, Munafo C, Ferrau V, Gitto E, Lacquaniti A, Salpietro C. High mobility-group protein B1: a new biomarker of metabolic syndrome in obese children. Eur Endocrinol / Eur Federatf Endocrine Soc 2013; 168:631-8; PMID:23384711
- Giaccobe A, Grasso R, Imbesi G, Salpietro CD, Grasso L, Lagana AS, Triolo O, Di Benedetto A. High mobility group protein B1: a new biomarker of obesity in pregnant women? Gynecol Endocrinol: Off J Int Soc Gynecol Endocrinol 2014; 1-3
- Lappalainen T, Kolehmainen M, Schwab U, Pulkkinen L, de Mello VD, Vaittinen M, Laaksonen DE, Poutanen K, Uusitupa M, Gylling H. Gene expression of FTO in human subcutaneous adipose tissue, peripheral blood mononuclear cells and adipocyte cell line. J Nutrigenet Nutrigenomics 2010; 3:37-45; PMID:20948226; http://dx.doi.org/10.1159/000320732
- Wagner M. A dangerous duo in adipose tissue: high-mobility group box 1 protein and macrophages. Yale J Biol Med 2014; 87:127-33; PMID:24910558
- Yang H, Wang H, Ju Z, Ragab AA, Lundback P, Long W, Valdes-Ferrer SI, He M, Pribis JP, Li J, et al. MD-2 is required for disulfide HMGB1-dependant TLR4 signaling. J Exp Med 2015; 212:5-14; PMID:25559892; http://dx.doi.org/10.1084/jem.20141318
- Schiraldi M, Raucci A, Munoz LM, Livoti E, Celona B, Venereau E, Apuzzo T, De Marchis F, Pedotti M, Bachi A, et al. HMGB1 promotes recruitment of inflammatory cells to damage tissues by forming a complex with CXCL12 and signaling via CXCR4. J Exp Med 2012; 209:551-63; PMID:22370717; http://dx.doi.org/10.1084/jem.20111739
- Nogueira-Machado JA, Volpe CM, Veloso CA, Chaves MM. HMGB1, TLR and RAGE: a functional tripod that leads to diabetic inflammation. Expert Opin Therap Targets 2011; 15:1023-35; PMID:21585289; http://dx.doi.org/10.1517/14728222.2011.575360
- Yang H, Lundback P, Ottosson L, Erlandsson-Harris H, Venereau E, Bianchi ME, Al-Abed Y, Andersson U, Tracey KJ, Antoine DJ. Redox modification of cysteine residues regulates the cytokine activity of high mobility group box-1 (HMGB1). Mol Med 2012; 18:250-9; PMID:22105604; http://dx.doi.org/10.2119/molmed.2011.00389
