ABSTRACT
BMP4 has a well-established role in triggering commitment of mesenchymal stem cells into the osteogenic and adipogenic linage. We recently described an additional dual function in adipogenesis: after promoting the formation of both white and brown pre-adipocytes, Bmp4 drives terminal differentiation into mature white rather than brown fat cells. Besides this, Bmp4 seems to have a dual role in metabolism either promoting or repressing oxidative metabolism in a cell context dependent manner.
Bone morphogenic protein 4 (BMP4) is a member of the bone morphogenetic protein family, comprising at least 20 members, which belongs to the transforming growth factor β (TGF-β) superfamily.Citation1,2 Localized on chromosome 14 in both human and mouse genome, BMP4 is highly conserved evolutionary.Citation3 Alternative splicing of its 5′UTR results in 3 transcript variants, all encoding the same peptide: a 46,5 KD preproproptein consisting of an amino-terminal signal domain (aa 1–19), a central domain (aa 20–292) and a carboxyl terminal mature domain (aa 293–408). Following deletion of the signal peptide, the resulting proproteins dimerize. Subsequently, serine endoproteases such as FURIN, PCSK5, PCSK6, and PCSK7 cleave the proprotein at the consensus sequence RXXR to generate the secreted and biologically active form of BMP44,5, which localizes in the extracellular milieu to act in both an autocrine and paracrine manner.Citation6
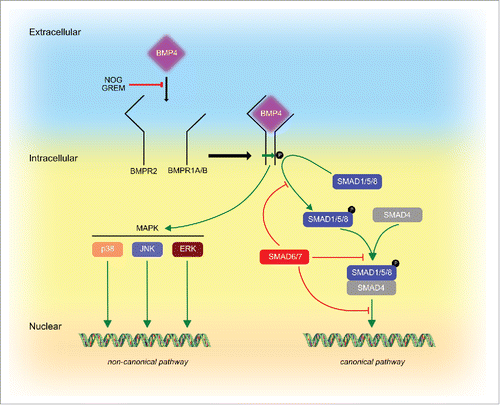
After secretion, BMP4 elicits its function by binding as a dimer to a hetero-oligomeric receptor complex composed by type I and type II serine-threonine kinase receptors.Citation7,8 There are 7 type I receptors, which are referred to as activin-like kinases (ALK1–7) and 3 type II receptors (BMPR2, ACTR2, ACTR2B). BMP4 utilizes ALK3 (BMPR1A) or ALK6 (BMPR1B) in association with BMPR2 to transduce its signal.Citation9 Upon BMP4 binding, type II receptor phosphorylates type I receptor, which in turn leads to phosphorylation of different members of the small mother against decapentaplegic (SMAD) protein family. So far, 8 different SMADs have been identified in mammals and it is believed that BMP4 mainly signals through SMAD 1, 5 and 8, which after forming a complex with the co-SMAD4 can shuttle into the nucleus to modulate gene expressionCitation7 (). Besides the canonical SMAD-dependent signaling pathway, BMP4 can also signal via SMAD-independent pathways to directly induce ERK and p38 MAPKs, PI3K, KFkB, JNK, PKA, PKC and PKD pathways () to regulate cell differentiation, migration, apoptosis and survival.Citation1
Figure 1. BMP4 signaling pathways. The BMP4 signal is transduced by 2 type of serine-threonine kinase receptors, BMPR1A or BMPR1B (type I) and BMPR2 (type II). Upon BMP4 binding, Type II receptor phosphorylates Type I receptor, which in turn leads to phosphorylation of members of the SMAD protein family such as SMAD1, 5, and 8. SMAD1/5/8 can form a complex with SMAD4 to translocate to the nucleus to regulate gene expression. This pathway is termed the canonical SMAD-dependent pathway, which is also regulated by inhibitory SMAD6 and 7 which can interfere with SMAD1/5/8 phosphorylation, SMAD1/5/8-SMAD4 complex formation and shuttling into the nucleus. Furthermore, BMP4 can signal via non-canonical SMAD-independent pathways, which include MAPKs such as p38, ERK and JNK. Both, canonical and non-canonical pathways can be blocked by BMP4 inhibitors such as NOGGIN (NOG) and GREMLIN (GREM).
The BMP4 sequence was first described from a bovine bone preparation,Citation10 afterwards human BMP4 was cloned from a placental cDNA library.Citation11 BMP4 is expressed in developing embryonic tissues and in adult tissues such as spleen, brain, thymus, kidney, lung, hearth, muscle, pancreas, liver, prostate, bladder, stomach.Citation12 Although BMP4 was initially identified due to its ability to promote osteogenesis,Citation13 it is now considered a critical factor not only for embryogenesis and development, but also for the homeostasis of many adult tissues. In this respect, a role for Bmp4 in adipocyte formation and function with potential metabolic effects has recently been described.Citation14-18
Adipogenesis is a multi-step process that can be divided into 2 major events: commitment of mesenchymal stem cells (MSCs) into the adipogenic lineage and terminal differentiation of pre-adipocytes into mature adipocytes.Citation19 Two major kind of adipocytes exists with opposite roles: white adipocytes to store energy in the form of lipids and carbohydrates, and brown adipocytes to dissipate chemical energy in heat to control body weight and temperature.Citation20,21
Initial studies underlined a key role for Bmp4 in triggering adipocyte commitment from MSCs, without investigating whether this would lead to the formation of brown or white adipocyte.Citation14-16 Later, one comprehensive study comparing the effects of different BMPs in the context of brown adipocyte differentiation could show that Bmp4 reduces the expression of Ucp1 mRNA during brown adipogenesis.Citation22 More recent works have contradicted these findings, suggesting that BMP4 can induce brown adipocyte formation in vivo and in vitro.Citation23-25 The in vivo work published by Quian et al. used a murine model for Ap2-mediated overexpression of Bmp4, which led to an increased energy expenditure and an increase in Ucp1 mRNA expression in the inguinal adipose tissue. Interestingly, in their study the authors demonstrated that Ap2-mediated Bmp4 overexpression led to the formation of large lipid droplets and a mostly unilocular phenotype in brown adipose tissue (BAT), reminiscent of a white adipocyte morphology. In the study of Gustafson et al.Citation25 the role of BMP4 in the stromal vascular fraction (SVF) of white adipose tissue (WAT) was investigated. The authors demonstrated that during differentiation of white pre-adipocytes the activity of BMP4 could be blocked by addition of the BMP-inhibitor GREMLIN 1 (GREM1). Furthermore, knock-down of GREM1 or treatment with BMP4, led to the appearance of brown fat cells. These differences are intriguing and might be explained by the fact that GREM1 acts as an inhibitor of BMP7, which has been shown to promote the acquisition of a brown phenotype.Citation22 Furthermore, it is possible that in this particular model the degree of adipocyte formation was altered, which could explain the appearance of more brown adipocytes. A different study by Elsen et al.Citation24 suggested that both BMP4 and BMP7 could promote the acquisition of a brown phenotype. Again, this study focused on SVF from WAT, which might indicate a depot specific function for BMP4. Unfortunately, the authors did not show an induction of UCP1 expression at the protein level, which is important to claim functionality of a brown cell.Citation26 In general, it has to be noted that conclusive functional studies addressing not only expression of functional markers, but also the thermogenic capacity of BMP4/7-derived brown adipocytes are missing.
In our recent work, we studied the role of Bmp4 signaling in adipocyte commitment, terminal differentiation and in mature brown adipocytes.Citation27 We could demonstrate that besides its well-established function in triggering MSCs into the adipogenic lineage, Bmp4 has a secondary function during terminal differentiation. Thus, while Bmp4 can induce commitment of MSCs to pre-adipocytes of both white and brown lineage, Bmp4 signaling during the terminal differentiation phase impairs the acquisition of a mature brown adipocyte phenotype in favor of a more white-like phenotype. Moreover, our in vitro and in vivo data show that exposure of mature brown adipocytes to Bmp4 determines a brown-to-white-like adipocyte shift. Finally, we could demonstrate that Bmp4 can also blunt the formation of “brite” adipocytes, a subpopulation of brown adipocytes that appear in the WAT under certain conditions.Citation28 In line with all these effects, we found that in BAT the levels of BMP4 are significantly lower than in WAT.
There are several possibilities which could explain the different findings of our work,Citation27 or the one from Kahn's group,Citation22 compared with the other studies mentioned above.Citation23-25 One possibility is the use of immortalized pre-adipocytes and MSCs versus the use of a whole SVF from WAT. As BMP4 is a mitogenic growth factor, it is possible that the observed minor upregulation of UCP1 following BMP4 treatment could be the result of an increased number of “brite” cells interspaced in WAT,Citation29,30 which was not studied in any of the works listed above. This is an important issue since Bmp4, as shown by us and others, can induce the commitment of MSCs irrespective of the final lineage. Thus, BMP4 treatment of uncommitted MSCs could lead to a higher rate of adipogenesis and to the increased formation of more adipocytes. Secondly, differences in the differentiation media could account for the divergent results. WeCitation27 and others,Citation31,32 could show that the use of PPARγ agonists such as the thiazolidindiones on WAT-derived SVF is sufficient to promote commitment and/or trans-differentiation to brown-like adipocytes. Thus, the association of these drugs to the mitogenic BMP4 in the differentiation media would definitively account for an increase in the number of brown-like fat cells with consequent detection of higher mitochondria number and expression of brown fat genes.
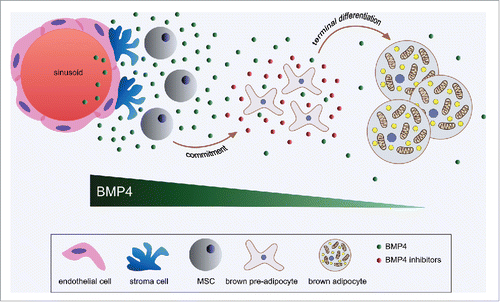
As a morphogen BMP4 is produced from the stem cell niche of many tissuesCitation33 where together with other developmental signals it drives the fate of MSCs. In addition, MSCs can produce BMP414 for paracrine and autocrine effects, which might be important for their self-renewal.Citation34 We could show that Bmp4 is also expressed in brown pre-adipocytes and that its expression is progressively reduced during terminal differentiation. Furthermore, Bmp4 expression is higher in the SVF compared with mature adipocytes indicating that the main source of BMP4 in BAT is probably the sum of the stem cell niche, MSCs and pre-adipocytes. These sources are important for the promotionCitation14-16 and onsetCitation35 of adipogenesis. It is tempting to speculate that the higher Bmp4 levels observed in the committed pre-adipocyte compared with mature fat cells is due to their closer relation to the MSCs, a concept which will have to be addressed in future studies. Interestingly, we observed that when Bmp4 signaling was induced in differentiating brown pre-adipocytes, cells responded by producing several inhibitors of the Bmp4 signaling cascade. This observation is reminiscent of the intestinal mucosa organization, where WNT proteins are produced from the stem cell niche in the crypt to promote proliferation, while in the villus differentiating enterocytes secrete inhibitors of the WNT signaling.Citation28,36 Thus it is possible that BMP4 as a morphogen in the BAT has a decreasing gradient effect from the stem cell niche to mature adipocytes not only due to local diffusion, but also because of the production of BMP4 antagonists from differentiating brown fat cells,Citation37,38 (). It has to be noted that although reduced in mature brown fat cells, we could still detect Bmp4 expression at this stage. Moreover, under cold stimulation the expression of Bmp4 in mature adipocytes was further reduced, which opens the possibility that Bmp4 signaling in BAT has a physiologic role in blocking thermogenesis.
Figure 2. BMP4 in brown adipose tissue. In BAT, BMP4 is enriched in the SVF, where it is likely produced from the endothelial cells of the stem cell niche, MSCs, pre-adipocytes as well as mature fat cells. Upon secretion, BMP4 might elicit an endocrine effect besides to its well-established paracrine and autocrine properties required to trigger commitment of MSCs into the adipogenic lineage and participate to the onset of adipogenesis. During the terminal differentiation phase the expression of BMP4 progressively reduces. Moreover, differentiating brown fat cells can secrete BMP4-inhibitors (e.g., NOGGIN, GREMLIN1, GREMLIN2) in a dose depended manner in response to increasing levels of BMP4. Thus, as a morphogen, BMP4 has a gradient dependent effect from the stem cell niche to mature adipocytes, where its expression is further reduced upon cold stimulation.
In line with our initial observation that Bmp4 is enriched in the SVF of the adipose tissue and the recent finding that adipocyte precursor cells reside in the blood vessel wallCitation39,40 we could demonstrate that in both human and mouse the highest expression of BMP4 is found to be present in endothelial cells. In the past, the Ap2 promoter has been used to generate transgenic animal models with the idea to specifically modulate target gene expression in the adipose tissue. However, a recent study suggests that Ap2 is not specific for the adipocytes, but it is highly expressed also in endothelial cells.Citation41 Indeed, previous transgenic studies based on the Ap2 promoter are now being revisited by the generation of the corresponding Adiponectin promoter-based transgenic model with very different phenotypic outcomes.Citation42,43 In this respect, the metabolic outcome shown in the work of Qian et al.,Citation23 where an Ap2-Bmp4 mouse model was used needs to be re-evaluated as Bmp4 might be produced in various organs from the endothelial cells, and after secretion regulate the function of cells and tissues in a paracrine and maybe also endocrine manner. For instance, the proposed browning effect driven by Bmp4 in WAT can be the consequence of other events, e.g. an indirect effect of Bmp4 at the levels of the central nervous system. In fact, activation of the sympathetic system has been demonstrated to promote browning in WAT.Citation44 In our in vivo experiments reported recently,Citation27 we achieved a selective overexpression of Bmp4 in BAT by controlled administration of adenovirus into the tissue. Using such an approach, we were able to exclude exogenous Bmp4 expression from other tissues such as liver, in line with the observation that circulating levels of Bmp4 were unchanged. Interestingly, selectively overexpression of Bmp4 in the liver leads to the same metabolic phenotype as reported by Qian et al., which furthermore underscores the importance of defining the roles of Bmp4 in an organ context specific manner.Citation23,27
That BMP4 might have also an endocrine effect is suggested by the fact that obese human subjects show increased circulating levels of BMP4.Citation27,45 Also, we found that in diet-induced obesity (DIO) mice (data not shown) the levels of Bmp4 were increased. Based on the results discussed above, it remains however to be established whether these increased levels are a cause or a consequence of obesity. Should the latter be the case, it would need to be investigated if this would have a protective role or if it would further exacerbate the disease state. Also, further studies are required to establish the source of BMP4 under these conditions. Whether a tissue selective approach can be considered to increase or decrease BMP4 will remain to be determined. For example, reduced BMP4 signaling in BAT could increase oxidative metabolism, while in WAT it could promote the recruitment of new cells to stimulate an expansion of WAT based on an increase number of cells that would act as a sink for circulating triglycerides. To design new target strategies for such purposes, a better and more elaborate characterization of the down-stream signaling as well as of the modulatory signaling of BMP4 which might be targeted in a tissue dependent manner will be required. In our opinion it will be important in the future to study factors which inhibit BAT activity with the same attention given to those that promote its activity. Our work, in fact, is one of a fewCitation46,47 that focuses on an endogenous player that impairs BAT formation and activity. Only such an extended approach will allow us to draw a more comprehensive picture of the molecular mechanisms that regulate BAT activity to design new interventions that would take advantage of its potential to ameliorate metabolic diseases.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- Bragdon B, Moseychuk O, Saldanha S, King D, Julian J, Nohe A. Bone morphogenetic proteins: a critical review. Cellular Signalling 2011; 23:609-20; PMID: 20959140; https://doi.org/10.1016/j.cellsig.2010.10.003
- Modica S, Wolfrum C. Bone morphogenic proteins signaling in adipogenesis and energy homeostasis. BBA - Molecular and Cell Biology of Lipids 2013; 1-11.
- Zhang DJ, Wu JH, Husile G, Sun HL, Zhang WG. Sequence variation and molecular evolution of BMP4 genes. Genet Mol Res 2014; 13:9196-201; PMID: 25501141; https://doi.org/10.4238/2014.November.7.6
- Cui Y, Jean F, Thomas G, Christian JL. BMP-4 is proteolytically activated by furin and/or PC6 during vertebrate embryonic development. The EMBO Journal 1998; 17:4735-43; PMID: 9707432; https://doi.org/10.1093/emboj/17.16.4735
- Goldman DC, Hackenmiller R, Nakayama T, Sopory S, Wong C, Kulessa H, Christian JL. Mutation of an upstream cleavage site in the BMP4 prodomain leads to tissue-specific loss of activity. Development 2006; 133:1933-42; PMID: 16624858; https://doi.org/10.1242/dev.02368
- Chen D, Zhao M, Mundy GR. Bone morphogenetic proteins. Growth Factors 2004; 22:233-41; PMID: 15621726; https://doi.org/10.1080/08977190412331279890
- Kawabata M, Imamura T, Miyazono K. Signal transduction by bone morphogenetic proteins. Cytokine and Growth Factor Reviews 1998; 9:49-61; PMID: 9720756; https://doi.org/10.1016/S1359-6101(97)00036-1
- Nohe A. Signal transduction of bone morphogenetic protein receptors. Cellular Signalling 2004; 16:291-9; PMID: 14687659; https://doi.org/10.1016/j.cellsig.2003.08.011
- Miyazono K, Kamiya Y, Morikawa M. Bone morphogenetic protein receptors and signal transduction. Journal of Biochemistry 2010; 147:35-51; PMID: 19762341; https://doi.org/10.1093/jb/mvp148
- Wozney JM, Rosen V, Celeste AJ, Mitsock LM, Whitters MJ, Kriz RW, Hewick RM, Wang EA. Novel regulators of bone formation: molecular clones and activities. Science 1988; 242:1528-34; PMID: 3201241; https://doi.org/10.1126/science.3201241
- Oida S, Iimura T, Maruoka Y, Takeda K, Sasaki S. Cloning and sequence of bone morphogenetic protein 4 (BMP-4) from a human placental cDNA library. DNA Seq 1995; 5:273-5; PMID: 7579580; https://doi.org/10.3109/10425179509030980
- Yanai I, Benjamin H, Shmoish M, Chalifa-Caspi V, Shklar M, Ophir R, Bar-Even A, Horn-Saban S, Safran M, Domany E, et al. Genome-wide midrange transcription profiles reveal expression level relationships in human tissue specification. Bioinformatics 2005; 21:650-9; PMID: 15388519; https://doi.org/10.1093/bioinformatics/bti042
- Urist MR. Bone: formation by autoinduction. 1965. Clin Orthop Relat Res 2002; 395: 4-10; PMID: 11937861
- Bowers RR, Kim JW, Otto TC, Lane MD. Stable stem cell commitment to the adipocyte lineage by inhibition of DNA methylation: role of the BMP-4 gene. Proc Natl Acad Sci USA 2006; 103:13022-7; PMID: 16916928; https://doi.org/10.1073/pnas.0605789103
- Huang H, Song T-J, Li X, Hu L, He Q, Liu M, Lane MD, Tang QQ. BMP signaling pathway is required for commitment of C3H10T1/2 pluripotent stem cells to the adipocyte lineage. Proceedings of the National Academy of Sciences 2009; 106:12670-5; https://doi.org/10.1073/pnas.0906266106
- Tang QQ, Otto TC, Lane MD. Commitment of C3H10T1/2 pluripotent stem cells to the adipocyte lineage. Proc Natl Acad Sci USA 2004; 101:9607-11; PMID: 15210946; https://doi.org/10.1073/pnas.0403100101
- Schulz TJ, Tseng Y-H. Emerging role of bone morphogenetic proteins in adipogenesis and energy metabolism. Cytokine and Growth Factor Reviews 2009; 20:523-31.
- Qian SW, Tang Y, Li X, Liu Y, Zhang YY, Huang HY, Xue RD, Yu HY, Guo L, Gao HD, et al. BMP4-mediated brown fat-like changes in white adipose tissue alter glucose and energy homeostasis. Proc Natl Acad Sci U S A 2013; 110(9):E798-807
- Tang QQ, Lane MD. Adipogenesis: From Stem Cell to Adipocyte. Annu Rev Biochem 2012; 81:715-36; PMID: 22463691; https://doi.org/10.1146/annurev-biochem-052110-115718
- Rosen ED, Spiegelman BM. What We Talk About When We Talk About Fat. Cell 2014; 156:20-44; PMID: 24439368; https://doi.org/10.1016/j.cell.2013.12.012
- Berry DC, Stenesen D, Zeve D, Graff JM. The developmental origins of adipose tissue. Development 2013; 140:3939-49; PMID: 24046315; https://doi.org/10.1242/dev.080549
- Tseng Y-H, Kokkotou E, Schulz TJ, Huang TL, Winnay JN, Taniguchi CM, Tran TT, Suzuki R, Espinoza DO, Yamamoto Y, et al. New role of bone morphogenetic protein 7 in brown adipogenesis and energy expenditure. Nature 2008; 454:1000-4; PMID: 18719589; https://doi.org/10.1038/nature07221
- Qian S-W, Tang Y, Li X, Liu Y, Zhang Y-Y, Huang H-Y, Xue R-D, Yu H-Y, Guo L, Gao H-D, et al. BMP4-mediated brown fat-like changes in white adipose tissue alter glucose and energy homeostasis. Proceedings of the National Academy of Sciences 2013; 110:E798-807; https://doi.org/10.1073/pnas.1215236110
- Elsen M, Raschke S, Tennagels N, Schwahn U, Jelenik T, Roden M, Romacho T, Eckel J. BMP4 and BMP7 induce the white-to-brown transition of primary human adipose stem cells. AJP: Cell Physiology 2014; 306:C431-40.
- Gustafson B, Hammarstedt A, Hedjazifar S, Hoffmann JM, Svensson P-A, Grimsby J, Rondinone C, Smith U. BMP4 and BMP Antagonists Regulate Human White and Beige Adipogenesis. Diabetes 2015; 64:1670-81; PMID: 25605802; https://doi.org/10.2337/db14-1127
- Nedergaard J, Cannon B. UCP1 mRNA does not produce heat. BBA - Molecular and Cell Biology of Lipids 2013; 1831(5):943-9; PMID: 23353596; https://doi.org/10.1016/j.bbalip.2013.01.009
- Modica S, Straub LG, Balaz M, Sun W, Varga L, Stefanicka P, Profant M, Simon E, Neubauer H, Ukropcova B, et al. Bmp4 Promotes a Brown to White-like Adipocyte Shift. CellReports 2016; 16:2243-58.
- Harms M, Seale P. Brown and beige fat: development, function and therapeutic potential. Nature Medicine 2013; 19:1252-63; PMID: 24100998; https://doi.org/10.1038/nm.3361
- Giralt M, Villarroya F. White, brown, beige/brite: different adipose cells for different functions? Endocrinology 2013; 154:2992-3000; PMID: 23782940; https://doi.org/10.1210/en.2013-1403
- Sidossis L, Kajimura S. Brown and beige fat in humans: thermogenic adipocytes that control energy and glucose homeostasis. J Clin Invest 2015; 125:478-86; PMID: 25642708; https://doi.org/10.1172/JCI78362
- Ohno H, Shinoda K, Spiegelman BM, Kajimura S. PPARγ agonists induce a white-to-brown fat conversion through stabilization of PRDM16 protein. Cell Metabolism 2012; 15:395-404; PMID: 22405074; https://doi.org/10.1016/j.cmet.2012.01.019
- Paulik MA, Lenhard JM. Thiazolidinediones inhibit alkaline phosphatase activity while increasing expression of uncoupling protein, deiodinase, and increasing mitochondrial mass in C3H10T1/2 cells. Cell Tissue Res 1997; 290:79-87; PMID: 9377645; https://doi.org/10.1007/s004410050910
- Jones DL, Wagers AJ. No place like home: anatomy and function of the stem cell niche. Nat Rev Mol Cell Biol 2008; 9:11-21; PMID: 18097443; https://doi.org/10.1038/nrm2319
- Qi X, Li T-G, Hao J, Hu J, Wang J, Simmons H, Miura S, Mishina Y, Zhao G-Q. BMP4 supports self-renewal of embryonic stem cells by inhibiting mitogen-activated protein kinase pathways. Proc Natl Acad Sci USA 2004; 101:6027-32; PMID: 15075392; https://doi.org/10.1073/pnas.0401367101
- Suenaga M, Kurosawa N, Asano H, Kanamori Y, Umemoto T, Yoshida H, Murakami M, Kawachi H, Matsui T, Funaba M. Bmp4 expressed in preadipocytes is required for the onset of adipocyte differentiation. CYTOKINE 2013; 64(1):138-45
- Gregorieff A, Clevers H. Wnt signaling in the intestinal epithelium: from endoderm to cancer. Genes & Development 2005; 19:877-90; https://doi.org/10.1101/gad.1295405
- Ashe HL. BMP Signalling: Synergy and Feedback Create a Step Gradient. Current Biology 2005; 15:R375-7; PMID: 15916937; https://doi.org/10.1016/j.cub.2005.05.003
- Ramel M-C, Hill CS. Spatial regulation of BMP activity. FEBS Letters 2012; 586:1929-41; PMID: 22710177; https://doi.org/10.1016/j.febslet.2012.02.035
- Tang W, Zeve D, Suh JM, Bosnakovski D, Kyba M, Hammer RE, Tallquist MD, Graff JM. White fat progenitor cells reside in the adipose vasculature. Science 2008; 322:583-6; PMID: 18801968; https://doi.org/10.1126/science.1156232
- Jiang Y, Berry DC, Tang W, Graff JM. Independent stem cell lineages regulate adipose organogenesis and adipose homeostasis. CellReports 2014; 9:1007-22.
- Jeffery E, Berry R, Church CD, Yu S, Shook BA, Horsley V, Rosen ED, Rodeheffer MS. Characterization of Cre recombinase models for the study of adipose tissue. Adipocyte 2014; 3:206-11; PMID: 25068087; https://doi.org/10.4161/adip.29674
- Bluher M, Michael MD, Peroni OD, Ueki K, Carter N, Kahn BB, Kahn CR. Adipose tissue selective insulin receptor knockout protects against obesity and obesity-related glucose intolerance. DEVCEL 2002; 3:25-38.
- Boucher J, Softic S, Ouaamari El A, Krumpoch MT, Kleinridders A, Kulkarni RN, O'Neill BT, Kahn CR. Differential Roles of Insulin and IGF-1 Receptors in Adipose Tissue Development and Function. Diabetes 2016; 65:2201-13; PMID: 27207537; https://doi.org/10.2337/db16-0212
- Giordano A, Frontini A, Cinti S. Convertible visceral fat as a therapeutic target to curb obesity. Nat Rev Drug Discov 2016; 15:405-24; PMID: 26965204; https://doi.org/10.1038/nrd.2016.31
- Lidell ME, Betz MJ, Enerbäck S. Association of serum bone morphogenetic protein 4 levels with obesity and metabolic syndrome in non diabetic individuals. Adipocyte 2011; 3:1-8.
- Chen Y, Siegel F, Kipschull S, Haas B, hlich HFO, Meister G, Pfeifer A. miR-155 regulates differentiation of brown and beige adipocytes via a bistable circuit. Nature Communications 1AD 4:1769-13; https://doi.org/10.1038/ncomms2742
- Whittle AJ, Jiang M, Peirce V, Relat J, Virtue S, Ebinuma H, Fukamachi I, Yamaguchi T, Takahashi M, Murano T, et al. Soluble LR11/SorLA represses thermogenesis in adipose tissue and correlates with BMI in humans. Nature Communications 2015; 6:8951; PMID: 26584636; https://doi.org/10.1038/ncomms9951
