ABSTRACT
Adipose-derived stem cells represent a reliable adult stem cell source thanks to their abundance, straightforward isolation, and broad differentiation abilities. Consequently, human adipose-derived stem cells (hASCs) have been used in vitro for several innovative cellular therapy and regenerative medicine applications. However, the translation of a novel technology from the laboratory to the clinic requires first to evaluate its safety, feasibility, and potential efficacy through preclinical studies in animals. The anatomy and physiology of pigs and humans are very similar, establishing pigs as an attractive and popular large animal model for preclinical studies. Knowledge of the properties of porcine adipose-derived stem cells (pASCs) used in preclinical studies is critical for their success. While hASCs have been extensively studied this past decade, only a handful of reports relate to pASCs. The aim of this concise review is to summarize the current findings about the isolation of pASCs, their culture, proliferation, and immunophenotype. The differentiation abilities of pASCs and their applications in porcine preclinical models will also be reported.
Isolation and Culture of pASCs
Adult stem cells have been identified in several tissues and organs including peripheral blood, bone marrow, adipose tissue, skin, and skeletal muscle.Citation1,2 Bone marrow mesenchymal stem cells have been the established standard for adult stem cells, but their harvest from bone marrow is a highly invasive procedure involving pain, morbidity and low cell yield.Citation2 Adipose tissue has proven to be an attractive alternative cell source to bone marrow.Citation3,4 It has the advantage of being an abundant and easily attainable cell source, with a straightforward and significant less invasive isolation procedure.
The standard process highlighted in to isolate pASCs from adipose tissue is similar to protocols previously reported for hASCs.Citation5–7 The goal of this procedure is to isolate the stromal vascular fraction (SVF) containing the pASCs from the adipocytes by using simple physical treatments. The first step consists of obtaining adipose tissue from a pig biopsy. The most common locations for subcutaneous adipose tissue are the dorsal and abdominal areas. Other white adipose tissue sources can also be used to obtain pASCs. Niada et al. determined that the buccal fat pad, which is an encapsulated fat mass in the cheek, contains pASCs with comparable properties to cells harvested from the subcutaneous interscapular region.Citation8 After procurement, the adipose tissue is finely minced then digested (typically via collagenase type I treatment). Centrifugation and filtration with cell strainers separate adipocytes from the SVF containing the pASCs. After separation, adipocytes remain in the supernatant while the SVF pelletizes. The adipocytes are then discarded and the SVF pellet is resuspended in culture medium and seeded into culture flasks. Typical cell seeding densities range from 5000 to 7000 cells/cm2.Citation5,9,10 Dulbecco's Modified Eagle Medium (DMEM) mixed 1:1 with Ham's F-12 Nutrient Mixture and supplemented with 10% Fetal Bovine Serum has been reported as ideal for the culture of pASCs.Citation5,10 After 48/72hrs in culture the non-adherent haematopoietic cells are removed. The remaining adherent cells are pASCs who display an elongated morphology, similar to fibroblasts. These primary pASCs complete a cell cycle in 60 to 80 hours.Citation5,11,12 Reports suggest that pASCs can reach up to 30–40 population doublings without reaching replicative senescence.Citation5,11
Figure 1. Illustrations of the standard protocol used to isolate pASCs. Subcutaneous porcine adipose tissue is finely minced before being digested in a collagenase type I solution at 37°C. Centrifugation separates the supernatant containing adipocytes from the SVF pellet. Cells are then plated in a tissue culture flask. pASCs are adherent and adopt a fibroblast-like morphology in culture.

On average, 0.5 to 1×106 viable and adherent pASCs are obtained per mL of adipose tissue.Citation5,12 One parameter that has been shown to affect the recovery yield of pASCs is the age of the source animal from whom the cells are extracted.Citation13,14 However, the abundance and accessibility of subcutaneous adipose tissue in pigs results in the ability to isolate several million cells from a single biopsy. Long term cryopreservation can also be used to store pASCs indefinitely. Cryopreserved pASCs have been shown to display similar proliferative characteristics, expression of cell surface markers, and differentiation abilities to fresh pASCs.Citation15
Immunophenotype of pASCs
hASCs have been thoroughly characterized with an extensive literature available detailing their isolation, proliferation, immunophenotype, and differentiation abilities. The International Federation for Adipose Therapeutics and Science (IFATS) and the International Society for Cellular Therapy (ISCT) have defined phenotypic and functional criteria to identify hASCs.Citation16 Currently, no criteria have been established to facilitate the identification of porcine stem cells, either bone marrow or adipose derived. Characterization of these animal cells is largely based on morphologic, phenotypic and functional properties, and can still appear rather ambiguous.
Flow cytometry is a convenient and fast method to analyze the immunophenotype of a cell population. It is a powerful tool routinely used to assess the characteristics of a freshly isolated population of cells and verify that they have not been contaminated with endothelial or haematopoietic cells. Indeed, the SVF can include cells other than adipose stem cells such as blood cells, smooth muscle cells, fibroblasts, and endothelial cells.
Fluorescence-activated cell sorting (FACS) can be used to purify a cell population by removing undesired subpopulations. Protocols have also been established with hASCs to isolate specific subpopulations of progenitor cells among the hASCs population.Citation1,3 Similar strategies haven't been implemented on pASCs yet but could prove beneficial for future studies.
Flow cytometry analysis of some pASCs surface markers can prove challenging. Many porcine surface antigens are not cross-reactive with antibodies designed for other species and require porcine-specific antibodies. For instance, among the 7 porcine surface antigens reported in , only CD44, CD90, and CD105 are cross-reacting with anti-human antibodies. A limited number of porcine-specific antibodies are currently commercially available. Consequently, published studies were consistent with one another and reported the use of similar antibodies. The expression of several cell surface markers for pASCs and hASCs are summarized in .
Table 1. Expression of cell surface markers for pASCs and hASCs determined by flow cytometry analysis for cells cultured at low passage numbers and in regular FBS supplemented culture medium. “+” corresponds to a positive expression of the cell surface marker, “−” for a low or non-expressed cell surface antigen.
CD29, CD44, CD90 and CD105 are part of the typical panel of surface markers characteristic of mesenchymal stem cells. These markers are positively expressed by pASCs, demonstrating their stemness. Analysis of stem cell transcription factors (Oct-4, Sox-2, and Nanog) by RT-PCR also reveals that pASCs express these markers of primitive stem cells.Citation20
pASCs do not express haematopoietic stem cell markers CD14 and CD45, nor do they express CD31 which is a marker characteristic of endothelial cells. Measuring the expression of CD14, CD45, and CD31 after isolating a new population of pASCs is a useful technique to verify that the stem cell population is not contaminated with endothelial or haematopoietic cells.
Overall, the cell surface marker expression profile for these typical markers appears similar between pASCs and hASCs. However, the few antibodies available for pASCs only provide a limited representation of the expression of surface antigens by pASCs.
Multilineage differentiation abilities
Besides self-renewal, a high proliferative capacity, and the expression of specific cell surface markers, another defining characteristic of a stem cell is its ability to differentiate into multiple lineages.Citation1,16 hASCs have demonstrated the ability to differentiate into multiple cell types such as osteoblasts, chondrocytes, adipocytes, epithelial cells, endothelial cells, smooth muscle cells, neural cells, and hepatocytes.Citation1,2,21 While the differentiation abilities of hASCs have been extensively studied this past decade, only a handful of reports have been related to pASCs. illustrates the differentiation pathways previously reported with pASCs along with the major reagents typically used to promote each cell lineage.
Figure 2. Multilineage differentiation abilities of pASCs. Major typical reagents are specified for each pathway. pASCs have been shown to be able to differentiate into adipocytes, osteoblasts, chondrocytes, hepatocytes, and neurons, as well as being able to be reprogrammed into induced pluripotent stem cells.
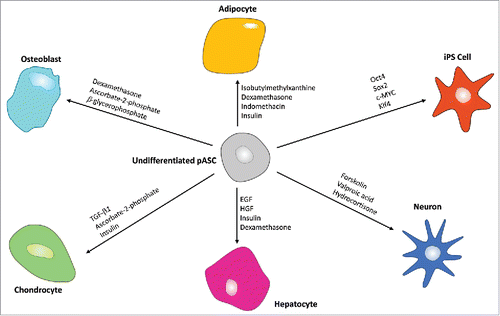
The adipogenic, osteogenic and chondrogenic differentiation are classic and easily obtainable mesodermal lineages differentiation pathways. Differentiation protocols for these 3 lineages are well established, and have been reported with pASCs.Citation9,22,23 However, studies related to ectodermal or endodermal lineages are scarce. Currently, it has been reported that pASCs can transdifferentiate into hepatocytes,Citation17,24 neurons,Citation11,25 and pancreatic islet-like clusters.Citation26 Using lentivirals carrying reprogramming factors, pASCs have also been reprogrammed into induced pluripotent stem cells (iPSCs).Citation19,27 Song et al. also described that pASCs could also differentiate into oocyte-like cells.Citation20
pASCs have been shown to demonstrate similar characteristics to other porcine-derived adult stem cells such as those derived from bone marrow, peripheral blood, adipose tissue, synovial membrane, and skin.Citation7,28,29 These include comparable morphology, proliferative capacity, alkaline phosphatase activity, cell surface marker expression, metabolic pathways, biologic functions, and transcription factors.Citation10,23,30 pASCs have often been compared side-by-side with porcine stem cells from these other tissue sources, revealing characteristics and comparable multilineage differentiation abilities as well.Citation9,23,28
While knowledge of the differentiation abilities of pASCs is currently limited to a few reports, porcine bone marrow-derived stem cells (pBMSCs) have been differentiated into myocytes,Citation31 endothelial cells,Citation32 and epithelial cells.Citation33 Since pBMSCs and pASCs characteristics are very similar,Citation34 it can be assumed that these differentiation pathways reported for pBMSCs should also apply to pASCs.
Applications in preclinical models
Adult stem cells have proven to be effective for the treatment of several diseases and the repair and regeneration of damaged tissues in vitro.Citation1,3,29 Preclinical animal studies represent a critical step in the translation of a cell transplantation or tissue engineering technology from the laboratory to the clinic. They are required to evaluate the safety, feasibility and potential efficacy of novel therapies.Citation2,9 Two criteria need to be addressed in order for an animal study assessing a novel cell-based approach to be beneficial. The animal chosen for the study has to mimic human physiology as closely as possible, and the animal cells that are being used need to be precisely identified and characterized. Some previous clinical trials did not adhere to these guidelines and concluded with unsatisfactory results. One shortcoming often comes from the use of rodents whose physiology and organ size does not properly match that of humans.Citation35 Results obtained with small animals, whose anatomy is different to humans, do not typically extrapolate properly to human clinical trials.Citation9 Large animals such as pigs represent a preferable model. Their organ size along with cell number and distribution more closely mimic human characteristics. Pigs are some of the most attractive and relevant large animal models for preclinical studies since their size, anatomy, genomic organization, and physiology are very similar to humans.Citation7,9,36 In a porcine preclinical model, the autologous transplantation of pASCs avoids triggering an adverse immune response.
Pigs have been used to investigate innovative pASCs bone regeneration strategies.Citation36 The osteogenic differentiation of pASCs is a well-known process.Citation23,37,38 Several studies combined pASCs differentiated into osteocytes with various types of scaffold such as hydroxyapatite,Citation22 polycaprolactone,Citation39 or oligo (polyethylene glycol) fumarate (OPF) hydrogelCitation40 to repair osteochondral defects. pASCs have also been implanted in pigs to evaluate their therapeutic effect in the treatment of osteonecrosis of the femoral headCitation41 and for the regeneration of osteochondral defects.Citation42,43 Pigs have also proven a suitable model for oral and maxillofacial studies.Citation8,44 Wilson et al. demonstrated that injections of pASCs enhanced healing of mandibular defects in pigs.Citation45
The vascular anatomy and physiology of pigs are quite similar to humans. Pigs have consequently been broadly used to evaluate novel vascular therapies.Citation46 Recent studies include the intracoronary administration of pASCs after an acute myocardial infarction modelCitation47–49 and the transplantation of pASCs cell sheets in a porcine model of chronic heart failure.Citation50
The structures of porcine and human skin are similar, making pigs a suitable model for dermatologic preclinical studies. Hanson et al. harvested pASCs and pBMSCs and injected them in a dermal wound model to study the feasibility of stem cells injections to promote wound healing.Citation18 pASCs performed similarly to pBMSCs, safely promoting tissue regeneration.
Future perspectives
pASCs express cell surface markers characteristic of mesenchymal stem cells and are able to differentiate into several lineages. Knowledge about the possible transdifferentiation abilities of pASCs is currently limited but will expand in the foreseeable future. The development of standard protocols for the isolation, culture and differentiation of pASCs would further improve pASC-based preclinical studies.Citation51
Pigs represent an excellent animal model for preclinical studies. Besides their similar morphology and physiology to humans, they also have the advantage of providing large quantities of easily obtainable subcutaneous tissue, resulting in a generous supply of pASCs.
Miniature pigs are being developed with the goal to increase the efficiency and translation of preclinical studies in pigs. Minipigs have the advantage of slower growth curves and a similar weight to an average human male, between 150 to 200lbs.Citation40,52,53 Such a new advantageous animal model has the potential to become increasingly popular and supplant rodents for relevant preclinical animal studies.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Funding
This work was supported by the Vice President for Research of the University of Oklahoma.
References
- De Francesco F, Ricci G, D'Andrea F, Nicoletti GF, Ferraro GA. Human adipose stem cells: From bench to bed-side. Tissue Eng Part B Rev 2015; 21(6):572-84; PMID:25953464; http://dx.doi.org/10.1089/ten.teb.2014.0608
- Kokai LE, Marra K, Rubin JP. Adipose stem cells: biology and clinical applications for tissue repair and regeneration. Translational Res 2014; 163(4):399-408; PMID:24361334; http://dx.doi.org/10.1016/j.trsl.2013.11.009
- Feisst V, Meidinger S, Locke MB. From bench to bedside: use of human adipose-derived stem cells. Stem Cells Cloning 2015; 8:149-62; PMID:26586955
- Lindroos B, Suuronen R, Miettinen S. The potential of adipose stem cells in regenerative medicine. Stem Cell Reviews Reports 2011; 7(2):269-91; PMID:20853072; http://dx.doi.org/10.1007/s12015-010-9193-7
- Williams KJ, Picou AA, Kish SL, Giraldo AM, Godke RA, Bondioli KR. Isolation and characterization of Porcine Adipose tissue-derived adult stem cells. Cells Tissues Organs 2008; 188(3):251-8; PMID:18349524
- Chen YJ, Liu HY, Chang YT, Cheng YH, Mersmann HJ, Kuo WH, Ding ST. Isolation and differentiation of adipose-derived stem cells from porcine subcutaneous adipose tissues. J Vis Exp 2016; (109):e53886; PMID:27077225; http://dx.doi.org/10.3791/53886
- Rho GJ, Kumar BM, Balasubramanian SS. Porcine mesenchymal stem cells–current technological status and future perspective. Front Biosci (Landmark Ed) 2009; 14:3942-61; PMID:19273325; http://dx.doi.org/10.2741/3503
- Niada S, Ferreira LM, Arrigoni E, Addis A, Campagnol M, Broccaioli E, Brini AT. Porcine adipose-derived stem cells from buccal fat pad and subcutaneous adipose tissue for future preclinical studies in oral surgery. Stem Cell Res Ther 2013; 4(6):1-11; PMID:23290259; http://dx.doi.org/10.1186/scrt359
- Casado JG, Gomez-Mauricio G, Alvarez V, Mijares J, Tarazona R, Bernad A, Sanchez-Margallo FM. Comparative phenotypic and molecular characterization of porcine mesenchymal stem cells from different sources for translational studies in a large animal model. Veterinary Immunol Immunopathol 2012; 147(1-2):104-12; PMID:22521281; http://dx.doi.org/10.1016/j.vetimm.2012.03.015
- Lee AY, Lee J, Kim CL, Lee KS, Lee SH, Gu NY, Kim JM, Lee BC, Koo OJ, Song JY, Cha SH. Comparative studies on proliferation, molecular markers and differentiation potential of mesenchymal stem cells from various tissues (adipose, bone marrow, ear skin, abdominal skin, and lung) and maintenance of multipotency during serial passages in miniature pig. Res Veterinary Sci 2015; 100:115-24; PMID:25823860; http://dx.doi.org/10.1016/j.rvsc.2015.03.010
- Wang KH, Kao AP, Wangchen H, Wang FY, Chang CH, Chang CC, Lin SD. Optimizing proliferation and characterization of multipotent stem cells from porcine adipose tissue. Biotechnol Applied Biochem 2008; 51(4):159-66; PMID:18279148; http://dx.doi.org/10.1042/BA20070201
- Schwarz C, Leicht U, Rothe C, Drosse I, Luibl V, Röcken M, Schieker M. Effects of different media on proliferation and differentiation capacity of canine, equine and porcine adipose derived stem cells. Res Veterinary Sci 2012; 93(1):457-62; PMID:21940026; http://dx.doi.org/10.1016/j.rvsc.2011.08.010
- Perruchot MH, Lefaucheur L, Barreau C, Casteilla L, Louveau I. Age-related changes in the features of porcine adult stem cells isolated from adipose tissue and skeletal muscle. AJP: Cell Physiol 2013; 305(7):C728-38
- Ock SA, Lee YM, Park JS, Shivakumar SB, Moon SW, Sung NJ, Lee WJ, Jang SJ, Park JM, Lee SC, et al. Evaluation of phenotypic, functional and molecular characteristics of porcine mesenchymal stromal/stem cells depending on donor age, gender and tissue source. J Vet Med Sci 2016; 78(6):987-95; PMID:26922917; http://dx.doi.org/10.1292/jvms.15-0596
- Dariolli R, Bassaneze V, Nakamuta JS, Omae SV, Campos LCG, Krieger JE. Porcine adipose tissue-derived Mesenchymal stem cells retain their proliferative characteristics, senescence, karyotype and plasticity after long-term cryopreservation. Covas DT, editor. PLoS One 2013; 8(7):e67939; PMID:23874472; http://dx.doi.org/10.1371/journal.pone.0067939
- Bourin P, Bunnell BA, Casteilla L, Dominici M, Katz AJ, March KL, Redl H, Rubin JP, Yoshimura K, Gimble JM. Stromal cells from the adipose tissue-derived stromal vascular fraction and culture expanded adipose tissue-derived stromal/stem cells: a joint statement of the International Federation for Adipose Therapeutics and Science (IFATS) and the International Society for Cellular Therapy (ISCT). Cytotherapy 2013; 15(6):641-8; PMID:23570660; http://dx.doi.org/10.1016/j.jcyt.2013.02.006
- Brückner S, Tautenhahn H-M, Winkler S, Stock P, Dollinger M, Christ B. A fat option for the pig: Hepatocytic differentiated mesenchymal stem cells for translational research. Exp Cell Res 2014; 321(2):267-75; PMID:24200501; http://dx.doi.org/10.1016/j.yexcr.2013.10.018
- Hanson SE, Kleinbeck KR, Cantu D, Kim J, Bentz ML, Faucher LD, Kao WJ, Hematti P. Local delivery of allogeneic bone marrow and adipose tissue-derived mesenchymal stromal cells for cutaneous wound healing in a porcine model. J Tissue Eng Regen Med 2016; 10(2):E90-E100; PMID:23418160; http://dx.doi.org/10.1002/term.1700
- Zhang Y, Wei C, Zhang P, Li X, Liu T, Pu Y, Li Y, Cao Z, Cao H, Liu Y, et al. Efficient reprogramming of Naïve-Like induced Pluripotent stem cells from porcine adipose-derived stem cells with a Feeder-independent and Serum-free system. PLoS One 2014; 9(1):e85089; PMID:24465482; http://dx.doi.org/10.1371/journal.pone.0085089
- Song SH, Kumar BM, Kang E-J, Lee Y-M, Kim T-H, Ock S-A, Lee S-L, Jeon B-G, Rho GJ. Characterization of porcine multipotent stem/stromal cells derived from skin, adipose, and ovarian tissues and their differentiation in vitro into putative oocyte-like cells. Stem Cells Dev 2011; 20(8):1359-70; PMID:21299414; http://dx.doi.org/10.1089/scd.2010.0203
- Gimble JM, Katz AJ, Bunnell BA. Adipose-derived stem cells for regenerative medicine. Circ Res 2007; 100(9):1249-60; PMID:17495232; http://dx.doi.org/10.1161/01.RES.0000265074.83288.09
- Arrigoni E, Lopa S, de Girolamo L, Stanco D, Brini AT. Isolation, characterization and osteogenic differentiation of adipose-derived stem cells: from small to large animal models. Cell Tissue Res 2009; 338(3):401-11; PMID:19882172; http://dx.doi.org/10.1007/s00441-009-0883-x
- Bionaz M, Monaco E, Wheeler MB. Transcription Adaptation during In Vitro Adipogenesis and Osteogenesis of Porcine Mesenchymal stem cells: Dynamics of Pathways, Biological Processes, Up-Stream Regulators, and gene networks. Plos One 2015; 10(9):e0137644; PMID:26398344; http://dx.doi.org/10.1371/journal.pone.0137644
- Stock P, Brückner S, Ebensing S, Hempel M, Dollinger MM, Christ B. The generation of hepatocytes from mesenchymal stem cells and engraftment into murine liver*. Nature Protocols 2010; 5(4):617-27; PMID:20224562; http://dx.doi.org/10.1038/nprot.2010.7
- Huang T, He D, Kleiner G, Kuluz J. Neuron-like differentiation of adipose-derived stem cells from infant piglets in vitro. J Spinal Cord Med 2007; 30(Suppl 1):S35-40; PMID:17874685; http://dx.doi.org/10.1080/10790268.2007.11753967
- Liu HY, Chen CC, Lin YY, Chen YJ, Liu BH, Wong SC, Wu CY, Chang YT, Chou HYE, Ding ST. Chitosan-assisted differentiation of porcine adipose tissue-derived stem cells into glucose-responsive insulin-secreting clusters. PLoS One 2017; 12(3):e0172922; PMID:28253305; http://dx.doi.org/10.1371/journal.pone.0172922
- Tang L, Yin Y, Zhou H, Song G, Fan A, Tang B, Shi W, Li Z. Proliferative Capacity and Pluripotent characteristics of Porcine adult stem cells derived from adipose tissue and bone marrow. Cell Reprogram 2012; 14(4):342-52; PMID:22775457
- Ock SA, Baregundi Subbarao R, Lee YM, Lee JH, Jeon RH, Lee SL, Park JK, Hwang SC, Rho GJ. Comparison of immunomodulation properties of Porcine Mesenchymal Stromal/stem cells derived from the bone marrow, Adipose Tissue, and Dermal skin tissue. Stem Cells Int 2015; 2016:e9581350
- Bharti D, Belame Shivakumar S, Baregundi Subbarao R, Rho GJ. Research advancements in Porcine derived Mesenchymal stem cells. Curr Stem Cell Res Therapy 2016; 11(1):78-93; http://dx.doi.org/10.2174/1574888X10666150723145911
- Hwang IS, Bae HK, Cheong HT. Comparison of the characteristics and multipotential and in vivo cartilage formation capabilities between porcine adipose-derived stem cells and porcine skin-derived stem cell–like cells. Am J Veterinary Res 2015; 76(9):814-21; PMID:26309110; http://dx.doi.org/10.2460/ajvr.76.9.814
- Du M, Huang Y, Lu NS, Shu G, Zhu X, Wang L, Gao P, Xi Q, Zhang Y, Wang S, Jiang Q. Characterization and differentiation into adipocytes and myocytes of porcine bone marrow mesenchymal stem cells. J Integrative Agriculture 2014; 13(4):837-48; http://dx.doi.org/10.1016/S2095-3119(13)60497-9
- Pankajakshan D, Kansal V, Agrawal DK. In vitro differentiation of bone marrow derived porcine mesenchymal stem cells to endothelial cells. J Tissue Eng Regen Med 2013; 7(11):911-20; PMID:22605545; http://dx.doi.org/10.1002/term.1483
- Kokubun K, Pankajakshan D, Kim M-J, Agrawal DK. Differentiation of porcine mesenchymal stem cells into epithelial cells as a potential therapeutic application to facilitate epithelial regeneration. J Tissue Eng Regen Med 2016; 10(2):E73-83; PMID:23696537; http://dx.doi.org/10.1002/term.1758
- Monaco E, Bionaz M, Rodriguez-Zas S, Hurley WL, Wheeler MB. Transcriptomics comparison between Porcine Adipose and Bone Marrow Mesenchymal stem cells during in vitro osteogenic and adipogenic differentiation. Beltrami AP, editor. PLoS One 2012; 7(3):e32481; PMID:22412878; http://dx.doi.org/10.1371/journal.pone.0032481
- Vatner SF. Why so few new cardiovascular drugs translate to the clinics. Circulation Rese 2016; 119(6):714-7; http://dx.doi.org/10.1161/CIRCRESAHA.116.309512
- Rubessa M, Polkoff K, Bionaz M, Monaco E, Milner DJ, Holllister SJ, Goldwasser MS, Wheeler MB. Use of pig as a model for Mesenchymal stem cell therapies for bone regeneration. Animal Biotechnol 2017; 1-13; [Epub ahead of print]; PMID:28267421; http://dx.doi.org/10.1080/10495398.2017.1279169
- Monaco E, Bionaz M, Hollister SJ, Wheeler MB. Strategies for regeneration of the bone using porcine adult adipose-derived mesenchymal stem cells. Theriogenology 2011; 75(8):1381-99; PMID:21354606; http://dx.doi.org/10.1016/j.theriogenology.2010.11.020
- Qu C, Zhang G, Zhang L, Yang G. Osteogenic and adipogenic potential of porcine adipose mesenchymal stem cells. Vitro Cell Dev Biol Animal 2007; 43(2):95-100; http://dx.doi.org/10.1007/s11626-006-9008-y
- Liao HT, Lee MY, Tsai WW, Wang HC, Lu WC. Osteogenesis of adipose-derived stem cells on polycaprolactone–β-tricalcium phosphate scaffold fabricated via selective laser sintering and surface coating with collagen type I. J Tissue Eng Regen Med 2016; 10(10):E337-53; PMID:23955935; http://dx.doi.org/10.1002/term.1811
- de Girolamo L, Niada S, Arrigoni E, Di Giancamillo A, Domeneghini C, Dadsetan M, Yaszemski MJ, Gastaldi D, Vena P, Taffetani M, et al. Repair of osteochondral defects in the minipig model by OPF hydrogel loaded with adipose-derived mesenchymal stem cells. Regenerative Med 2015; 10(2):135-51; PMID:25835479; http://dx.doi.org/10.2217/rme.14.77
- Jo WL, Kang ML, Kim JE, Kim EA, Kwon SY, Im GI. Co-transplantation of adipose and bone marrow derived stromal cells for treatment of osteonecrosis of femoral head. Tissue Engineering Regenerative Med 2015; 12(6):410-6; http://dx.doi.org/10.1007/s13770-015-0017-3
- Cui L, Wu Y, Cen L, Zhou H, Yin S, Liu G, Liu W, Cao Y. Repair of articular cartilage defect in non-weight bearing areas using adipose derived stem cells loaded polyglycolic acid mesh. Biomaterials 2009; 30(14):2683-93; PMID:19217157; http://dx.doi.org/10.1016/j.biomaterials.2009.01.045
- Murata D, Tokunaga S, Tamura T, Kawaguchi H, Miyoshi N, Fujiki M, Nakayama K, Misumi K. A preliminary study of osteochondral regeneration using a scaffold-free three-dimensional construct of porcine adipose tissue-derived mesenchymal stem cells. J Orthopaedic Surg Res 2015; 10(1):35; PMID:25890366; http://dx.doi.org/10.1186/s13018-015-0173-0
- Liu N, Lyu X, Fan H, Shi J, Hu J, Luo E. Animal models for craniofacial reconstruction by stem/stromal cells. Curr Stem Cell Res Ther 2014; 9(3):174-86; PMID:24524796; http://dx.doi.org/10.2174/1574888X09666140213150811
- Wilson SM, Goldwasser MS, Clark SG, Monaco E, Bionaz M, Hurley WL, Rodriguez-Zas S, Feng L, Dymon Z, Wheeler MB. Adipose-derived mesenchymal stem cells enhance healing of mandibular defects in the Ramus of swine. J Oral Maxillofacial Surg 2012; 70(3):e193-e203; PMID:22374062; http://dx.doi.org/10.1016/j.joms.2011.10.029
- Swartz DD, Andreadis ST. Animal models for vascular tissue-engineering. Curr Opinion Biotechnol 2013; 24(5):916-925; PMID:23769861; http://dx.doi.org/10.1016/j.copbio.2013.05.005
- Valina C, Pinkernell K, Song Y-H, Bai X, Sadat S, Campeau RJ, Jemtel THL, Alt E. Intracoronary administration of autologous adipose tissue-derived stem cells improves left ventricular function, perfusion, and remodelling after acute myocardial infarction. European Heart J 2007; 28(21):2667-2677; PMID:17933755; http://dx.doi.org/10.1093/eurheartj/ehm426
- Gomez-Mauricio RG, Acarregui A, Sánchez-Margallo FM, Crisóstomo V, Gallo I, Hernández RM, Pedraz JL, Orive G, Martín-Cancho MF. A preliminary approach to the repair of myocardial infarction using adipose tissue-derived stem cells encapsulated in magnetic resonance-labelled alginate microspheres in a porcine model. European J Pharmaceutics Biopharmaceutics 2013; 84(1):29-39; PMID:23266493; http://dx.doi.org/10.1016/j.ejpb.2012.11.028
- Vilahur G, Oñate B, Cubedo J, Béjar MT, Arderiu G, Peña E, Casaní L, Gutiérrez M, Capdevila A, Pons-Lladó G, et al. Allogenic adipose-derived stem cell therapy overcomes ischemia-induced microvessel rarefaction in the myocardium: systems biology study. Stem Cell Res Therapy 2017; 8:52; http://dx.doi.org/10.1186/s13287-017-0509-2
- Ishida O, Hagino I, Nagaya N, Shimizu T, Okano T, Sawa Y, Mori H, Yagihara T. Adipose-derived stem cell sheet transplantation therapy in a porcine model of chronic heart failure. Translational Res 2015; 165(5):631-9; PMID:25613060; http://dx.doi.org/10.1016/j.trsl.2014.12.005
- Gimble JM, Bunnell BA, Chiu ES, Guilak F. Concise Review: Adipose-Derived Stromal vascular fraction cells and stem cells: Let's not get lost in translation. Stem Cells 2011; 29(5):749-54; PMID:21433220; http://dx.doi.org/10.1002/stem.629
- Schomberg DT, Tellez A, Meudt JJ, Brady DA, Dillon KN, Arowolo FK, Wicks J, Rousselle SD, Shanmuganayagam D. Miniature swine for preclinical modeling of complexities of human disease for translational scientific discovery and accelerated development of therapies and medical devices. Toxicol Pathol 2016; 44(3):299-314; PMID:26839324; http://dx.doi.org/10.1177/0192623315618292
- Stramandinoli-Zanicotti RT, Carvalho AL, Rebelatto CLK, Sassi LM, Torres MF, Senegaglia AC, Boldrinileite LM, Correa-Dominguez A, Kuligovsky C, Brofman PRS. Brazilian minipig as a large-animal model for basic research and stem cell-based tissue engineering. Characterization and in vitro differentiation of bone marrow-derived mesenchymal stem cells. J Applied Oral Sci 2014; 22(3):218-27; PMID:25025563; http://dx.doi.org/10.1590/1678-775720130526
