ABSTRACT
Brown adipose tissue (BAT) mitochondria are distinct from their counterparts in other tissues in that ATP production is not their primary physiologic role. BAT mitochondria are equipped with a specialized protein known as uncoupling protein 1 (UCP1). UCP1 short–circuits the electron transport chain, allowing mitochondrial membrane potential to be transduced to heat, making BAT a tissue capable of altering energy expenditure and fuel metabolism in mammals without increasing physical activity.
The recent discovery that adult humans have metabolically active BAT has rekindled an interest in this intriguing tissue, with the overarching aim of manipulating BAT function to augment energy expenditure as a countermeasure for obesity and the metabolic abnormalities it incurs. Subsequently, there has been heightened interest in quantifying BAT function and more specifically, determining UCP1-mediated thermogenesis in BAT specimens – including in those obtained from humans.
In this article, BAT mitochondrial bioenergetics will be described and compared with more conventional mitochondria in other tissues. The biochemical methods typically used to quantify BAT mitochondrial function will also be discussed in terms of their specificity for assaying UCP1 mediated thermogenesis. Finally, recent data concerning BAT UCP1 function in humans will be described and discussed.
Introduction
Brown adipose tissue in metabolic physiology
Brown adipose tissue (BAT) occupies an unusual role in mammalian physiology. Although BAT possesses an oxidative capacity comparable to tissues like striated muscle and liver, it is incapable of producing ATP in any great quantity.Citation1 Indeed, much like the extra-ocular superior rectus muscle (heater muscle) of Billfish such as Blue Marlin and Swordfish, BAT functions like a heater organ in mammalian endotherms. Intriguingly, like the Billfish heater muscle, BAT hails from a skeletal muscle lineage.Citation2 However, this is where the similarity ends. Unlike the heater muscle of Billfish, which generates heat through increased ATP hydrolysis on account of an inefficient sarcoplasmic reticulum Ca2+ ATPase,Citation3 BAT mitochondria are equipped with a specialized protein that functions as an energy transducer, short-circuiting the electron transport chain and turning the mitochondrion into a biologic furnace.Citation4
Uncoupling protein 1 (UCP1), originally termed thermogenin owing to its role in non-shivering thermogenesis (NST), resides within the inner mitochondrial membrane.Citation4 When activated, UCP1 permits significant inner membrane proton conductance, uncoupling mitochondrial fuel oxidation and respiration from ATP production.Citation5 In keeping with the laws of thermodynamics, the electro-chemical potential generated by fuel oxidation in BAT mitochondria is largely dissipated as heat as opposed to being used for ADP phosphorylation. Thus, when switched on, UCP1 turns BAT mitochondria into small, internalized radiators - which can assist in maintaining the core temperature of endothermic mammals.Citation1 While this role of BAT in NST has long been appreciated in certain mammals, only very recently has BAT been shown to be present and functional in adult humans.Citation6 This has rekindled interest in a putative role for BAT in human energy metabolism, arising largely from the desire to manipulate BAT as a strategy to increase energy expenditure and substrate metabolism in the context of obesity and its metabolic complications.
BAT mitochondrial bioenergetics
The mitochondrion is the hub of cellular bioenergetics. Acting much like an electrical transducer, mitochondria convert the energy stored in carbon fuels such as glucose and fatty acids, to a potential energy that can be used to produce ATP. Specifically, electron flow coupled to proton excursion in the electron transport chain (ETC) generates electro-chemical potential across the inner mitochondrial membrane. This potential energy, oftentimes referred to as proton-motive force, is harnessed by the penultimate complex of the ETC (ATP synthase), phosphorylating ADP.Citation7 This chemiosmotic mechanism of ATP production represents the quintessential function of the mitochondrion, which has earned it its label as the powerhouse of the cell. Accordingly, when we think of mitochondria, we think of oxidative phosphorylation. BAT mitochondria represent a striking anomaly in this paradigm, owing to their propensity to produce heat instead of ATP. While BAT of both rodents and humans is densely populated with mitochondria, endowing BAT with considerable oxidative capacity, in contrast to tissues such as striated muscle and liver, mitochondrial electron transfer and respiration can be almost completely uncoupled from ATP production in BAT mitochondria.Citation8,9 This ability of BAT mitochondria to respire in the leak or uncoupled state (i.e., respiration not coupled to ATP production) is due to the presence of UCP1 in the inner mitochondrial membrane, which has been shown to be responsible for NST in BAT mitochondria.Citation5,10
UCP1, a 32kDa protein resides within the inner mitochondrial membrane.Citation4 Upon activation, which requires the binding of a fatty acid, UCP1 allows proton transfer from the membrane space to the matrix of the mitochondrion, effectively dissipating the proton gradient that would otherwise be used to drive ATP synthesis. While the specific mechanism by which the binding of a fatty acid allows proton transfer remains contentious, recent evidence suggest that UCP1 functions as a symporter of free fatty acids (FFA) and protons.Citation11 Owning to their hydrophobic nature, FFAs cannot readily disassociate from UCP1, meaning UCP1 effectively acts as a membrane proton carrier that requires a FFA to be active.Citation11 Accordingly, physiologic stimuli that result in an adrenergic response and subsequent lipolysis activate UCP1 by increasing intracellular FFA levels.Citation5,12-19 Further, BAT mitochondria can be recoupled by purine nucleotides such as ADP, ATP, GTP and GDP,Citation12,16 since UCP1 has as a high-affinity nucleotide binding site.Citation20 Interestingly, the knowledge that FFAs and purine nucleotides modulate the coupling of BAT mitochondria pre-dates the identification and characterization of UCP116. Indeed, purine nucleotides, particularly GDP, are often used as a means to directly quantify UCP1 function, which will be discussed in more detail below.
UCP1 is not active in the presence of purine nucleotides.Citation21 However, BAT mitochondria are extremely sensitive to changes in FFA concentrations, where a physiologic increase in intracellular FFA concentrations effectively overcomes purine nucleotide inhibition, activating UCP1.Citation22 This has been demonstrated experimentally by Matthias and colleagues.Citation12 These authors reported that 1mM of GDP could recouple BAT mitochondria, restoring membrane potential to levels comparable to BAT mitochondria from animals lacking UCP1. Further, BAT mitochondria coupled by GDP could be uncoupled again by increasing concentrations of oleic acid (∼20–1000nM).Citation12 Presumably the lowering of FFA concentrations is conducive to purine nucleotide binding and inactivation of UCP1 in vivo.
Assaying mitochondrial function in cells, organelles and permeabilized tissue
Choosing the best parameter
Numerous parameters reflecting different aspects of mitochondrial function can be assayed in cells, isolated organelles and permeabilized tissue; careful consideration should be taken to define the aspect of mitochondrial function that is of interest (i.e., ATP production, Ca2+ uptake, membrane potential, superoxide production, or respiration), to select the most appropriate approach. An comprehensive description and discussion of methods available to biochemically assay mitochondrial function can be found elsewhere.Citation23 In the context of bioenergetics, using bioluminescence to assay ATP production in isolated mitochondria or determining respiratory capacity in cells, isolated mitochondria or permeabilized tissue are approaches frequently used. However, since ATP production is of little interest in the context of BAT bioenergetics, assaying mitochondrial respiratory capacity is the approach most used in studies of BAT mitochondrial function. Importantly, in isolated brown adipoctyes,Citation24 mitochondria isolated from brown adipoctyes,Citation25 and in BAT homogenates,Citation26 respiration has been shown to represent an accurate measure of heat production. Thus, quantification of UCP1 dependent respiration is analogous to quantification of UCP1 dependent thermogenesis.
Choosing an appropriate instrumental platform
Classically, an amperometric approach using a Clarke electrode has been used to determine mitochondrial respiratory rate. Originally developed in the 1950s by Leyland Clarke, a Clarke or oxygen electrode comprises of a platinum (or gold) cathode and a sliver/silver-chloride anode separated by a conduction solution (such as potassium chloride) incased by an oxygen permeable Teflon (polytetrafluoroethylene) membrane. Oxygen in the respiration solution can permeate the Teflon membrane and reduce the cathode, forming hydrogen peroxide. This hydrogen peroxide is then oxidized by the anode, which generates an electrical current that is proportional to the oxygen concentration in the respiration buffer. Thus, the Clarke electrode can be used to quantify the respiratory rate of biologic samples by computing the decline in oxygen concentration over time within the closed chamber.
The advent of high-resolution respirometry systems (i.e., the O2K, Oroboros Instruments, Innsbruck, Austria) combining closed respiration chambers composed of inert glass and plastic that utilize precise electrodes have made the accurate quantification of respiratory rate possible in relatively small biologic specimens (cells, isolated mitochondria or permeabilized tissue). Moreover, the incorporation of software allowing for the real-time visualization and interpretation of experimental data permits the straightforward determination of respiratory steady-states. While the accurate determination of mitochondrial respiratory rates is arguably best achieved with Clarke electrode approaches, the relatively low throughput of this approach may act as a barrier to its wide spread utilization.
A high throughput approach for determining respiration rate became available approximately 10-years ago (the XF Extracellular Flux Analyzer, Seahorse Bioscience, Agilent). A principal benefit of this micro-chamber approach is that respiration rate can be assayed in adherent cells or mitochondria in an 8, 24 or 96 well plate format. The Seahorse XFe utilizes oxygen sensitive fluorophores localized in a sensor probe. These probes are periodically advanced into the wells of the micro-plate forming a transient micro-chamber (7 µl) ∼200 microns above the cell/mitochondria monolayer. Fiber optics incased within the sensor probe excite the sample and the light emission of the fluorophores are recorded and used to calculate the dissolved oxygen concentration within the micro-chamber. While this platform lends itself to high-throughput determination of respiratory rate in cells and mitochondria, it is not without its limitations. The use of components that may be permeable to oxygen such as polystyrene micro-plates, the periodic opening of the micro-chamber, and the small volume of the chamber itself, may introduce artifacts that effect data quality. However, efforts have been made to develop algorithms to correct for some of these limitations.Citation27
The various platforms available that allow mitochondrial respiratory function to be assayed in cells, isolated organelles and tissue all have certain benefits and drawbacks. Careful consideration should be taken to select the most appropriate platform. Broadly speaking, researchers valuing a high-throughput cell based approach will likely find most utility in 24 and 96 well formats offered by the Seahorse XFe. However, researchers with a greater focus on quantitative data that may be generated over several months to years, from samples collected from animals and/or humans, will perhaps find a closed chamber high-resolution respirometer more suitable, such as the O2K (Oroboros Instruments). Irrespective of the instrumental platform used, there are several other important factors that need to be considered when designing assay protocols to quantify BAT mitochondrial function.
Turning on UCP1
UCP1 isn't inherently leaking, and thus must be activated to produce heat. It is therefore important to consider the activation state of UCP1 when attempting to assay its function. Owning to their role in activating UCP1, it may be important to control FFA concentration in respiration buffer when quantifying UCP1 function in isolated BAT adipocytes or mitochondria. This issue has been discussed in detail recently.Citation28,29 In short, for respirometry experiments performed on isolated mitochondria, a buffer containing 0.5–1% fatty acid free albumin appears to effectively scavenge residual FFAs.Citation12 Similarly, others have shown that respiration buffers containing 2–4% albumin are effective in quenching FFAs in experiments using isolated brown adipocytes.Citation5,29 The use of albumin described above allows experiments to be initiated with UCP1 in an inactivated state. Thereafter, addition of fatty acids (for isolated mitochondria) or an adrenergic stressor (for isolated adipocytes) can then be used to activate UCP1.
While it is feasible to quantify the respiratory function of UCP1 in digitonin permeabilized adipose tissue samples from rodentsCitation30 and humansCitation8 in a respiration buffer containing 1% albumin, it is unlikely that extracellular fatty acids were effectively quenched by this albumin concentration. Indeed, several mgs (wet weight) of rodent interscapular or human supraclavicular BAT are required for high-resolution respirometry,Citation8 and as much as 10–20 mg (30) and 50–100 mg (8) (wet weight) of tissue is required for high-resolution respirometry on subcutaneous white fat from rodents and humans, respectively. With relatively large amounts of minced adipose tissue suspended in 2 ml of respiration buffer, not all FFAs are albumin bound in a 1% albumin buffer. Indeed, in permeabolized BAT tissue preparations, leak respiration is responsive to GDP,Citation8 suggesting that fatty acids levels in the respiration buffer are high enough to activate UCP1 when assaying whole tissue. Interestingly, while UCP1 can be inhibited by 1mM GDP when studying isolated adipocytes or isolated mitochondria, a significantly higher concentration of GDP (∼20mM) is required to inhibit UCP1 in BAT tissue samples, likely due to unavoidable high FFA levels in this type of sample preparation.
Assaying UCP1 function
When designing assay protocols to determine BAT mitochondrial function, and in particular UCP1 function, it is critical to acknowledge the fundamental differences between BAT mitochondria and the mitochondria found in almost all other cell types. For example, researchers interested in the respiratory function of mitochondria within striated muscle will likely focus on the ability of mitochondria to make ATP. Accordingly, respiration protocols should focus on the determining respiration coupled to ATP production. The use of mitochondrial uncouplers and inhibitors can also be incorporated to add additional qualitative information on the coupling control and reserve capacity of mitochondria.
Two typical respirometry protocols used to assay mitochondrial respiratory capacity and coupling control are shown in . In the first protocol, the sequential addition of substrates followed by ADP permits the quantification of both leak (state 2) and coupled (state 3) respiration. Thereafter, the addition of the ATP synthase inhibitor oligomycin transition respiration to another leak state; state 4 respiration. While state 2 respiration was classically referred to the respiratory rate when ADP was added but no substrate, and state 4 respiration as the respiratory rate proceeding state 3, once ADP had been used up by the mitochondria,Citation31 here state 2 refers to respiration supported by substrates but no ADP, while state 4 refers to respiration when ATP synthase is pharmacologically blocked by oligomycin. Further, it should be pointed out here that state 2 and state 4 respiration are both referred to as leak respiration and not uncoupled respiration, since they reflect inner membrane proton leaks in mitochondria not chemically treated with an ionophore. This is to differentiate state 2 and state 4 respiration from chemically induced uncoupling of mitochondria by ionophores such as dinitrophenol, Carbonyl cyanide 4-(trifluoromethoxy) phenylhydrazone (FCCP) and Carbonyl cyanide 3-chlorophenylhydrazone (CCCP) (referred to here as state 3U respiration). This may appear trivial, since during state 2, state 4 and state 3U respiration, respiration is indeed not coupled to ATP production. However, the differentiation between leak (state 2 and state 4) and uncoupled (state 3U) respiration is useful since it avoids some important assumptions being made. Specifically, while all mitochondrial respiratory states are influenced by mitochondrial protein levels to a certain degree, state 2 respiration and state 4 respiration offer a measure of the capacity of the mitochondria to respire without making ATP, whereas state 3U respiration is more often used to reflect the overall respiratory capacity of the mitochondrion or pool of mitochondria. By way of example, state 2 and state 4 respiration are comparatively low when compared with state 3U respiration in human skeletal muscle, whereas state 2, state 4 and state 3U respiration are more or less comparable in human BAT when UCP1 is active.Citation8
Figure 1. A typical respiration protocol to determine the oxygen consumption rate (OCR) of mitochondria (isolated organelles or permeabilized tissue) in the leak and coupled state (Panel A). The respiratory states being assayed following the sequential added of substrates, ADP and the ATP synthase inhibitor oligomycin are shown at the top of the oxygraph trace. In Panel B, a similar oxygraph trace is shown, with the exception that this protocol is designed to assay respiration in the leak, coupled and uncoupled states following the sequential added of substrates, ADP and an ionophore, such as FCCP.
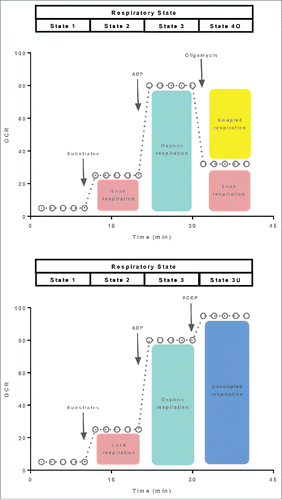
The second protocol depicted in differs from the one described above in that instead of titrating oligomycin once maximal state 3 respiration is achieved, oxidative phosphorylation is uncoupled by adding a ionophore into the respiration chamber. The advantage of this approach in that maximal respiratory capacity can be determined (state 3U respiration). Interestingly, respiratory flux is somewhat curtailed in the coupled state, although the reasons for this are not clear. Addition of an ionophore such as FCCP or CCCP increases respiration to a rate greater than that of state 3. Beyond providing a measure of mitochondrial respiratory capacity, state 3U respiration can be used as another tool to gauge mitochondrial coupling control. Specifically, calculation flux control ratios by normalizing state 2 or state 3 respiration to state 3U respiration provide internal indices of mitochondrial flux control.Citation32 Given the similarity of the 2 assay protocols described in , it may seem cumbersome to perform both assays in parallel. However, it should be noted that state 3U respiration may be curtailed when assayed in the presence of oligomycin. Indeed, experimental evidence in cells supports this conclusion,Citation33 suggesting that state 3U respiration may not be a measure of maximal or total respiratory capacity when assayed in the presence of oligomycin.
The 2 protocols described above provide a robust means to quantify mitochondrial respiratory function. Accordingly, the use of these or similar protocols is widespread. Analogous protocols have been developed to allow similar indices of mitochondrial respiratory capacity and flux control in intact cells (see ), where respiration can be determined in the routine state (in a buffer containing substrates that can cross the cell membrane). Thereafter, ATP production can be blocked by addition of oligomycin before mitochondrial uncoupling with an ionophore such as FCCP.Citation32 While these assays are straightforward to perform and provide useful information on mitochondrial respiratory function, their use as a means of quantifying UCP1 function in adipocytes has several critical limitations that will be discussed below.
Figure 2. A respiration protocol derived from those depicted in to allow mitochondrial respiratory function to be quantified in intact cells. Respiration in the coupled (routine), leak and uncoupled state are quantified following the sequential addition of the ATP synthase inhibitor oligomycin and an inophore. The respiratory states being assayed are shown at the top of the oxygraph trace.
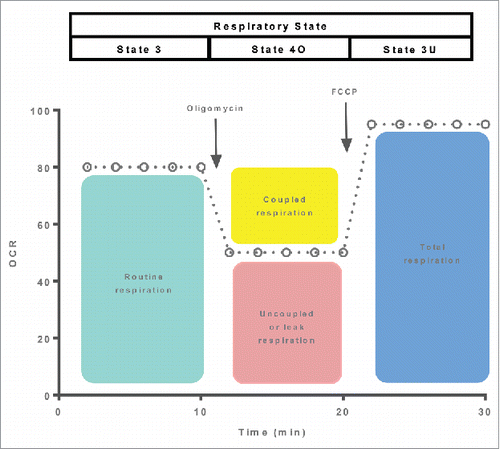
The limitations of assays used to quantify UCP1 function in samples of BAT or BAT like cells/tissue (i.e., ) stem from the fact that these assays were primarily developed to interrogate mitochondrial function in cells where the mitochondrions primary role is ATP production. Therein lies the problem - BAT mitochondria are fundamentally different from their counterparts in other cells in that oxidative phosphorylation is not there main function. Presumably the use of protocols to quantify UCP1 function such as the one depicted in is based on the assumption that BAT mitochondria are more or less analogous to mitochondria found in other cells. Indeed oligomycin insensitive leak respiration and maximal uncoupled respiration derived from the assay described in are frequently reported as measures of BAT UCP1 function. However, since leak respiration and uncoupled respiration are not specific to UCP1, differences in these respiration rates may simply reflect greater mitochondrial respiratory capacity.
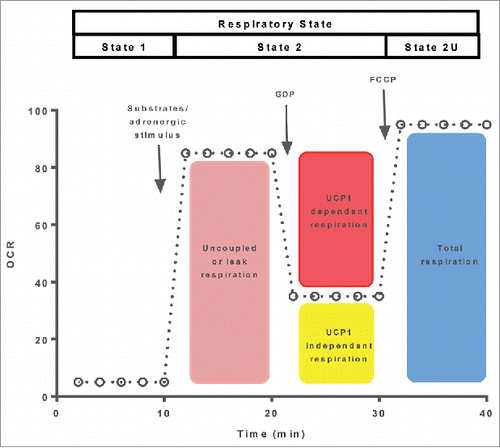
A more appropriate respirometry protocol for the quantification of UCP1 function in mitochondria, adipocytes or adipose tissue is shown in . This approach utilizes the addition of GDP to inhibit UCP1 and thus recouple BAT mitochondria. The reduction in respiration following GDP added represents a direct measure of UCP1s ability to support mitochondrial respiration. It is important to note that samples should first be stimulated to activate UCP1 (i.e., added of fatty acids),Citation5,12,28,29 although this is not necessary when using whole adipose tissue preparations.Citation8,30 Further, when using adipocytes or tissue preparations, permeabilization should be performed since GDP does not readily cross the cell membrane. While approaches similar to the one described above have been used for decades to quantify UCP1in BAT preparations, a Clarke electrode respirometer has typically been used to make such measurements. However, it should be noted that similar protocols have been developed for microplate-based platforms.Citation28
Figure 3. A respiration protocol designed to quantify UCP1 dependent leak respiration and maximal uncoupled respiration is depicted. Following the activation of UCP1, UCP1-dependent leak respiration is differentiated from UCP1-independent leak respiration following the addition of GDP. The respiratory states being assayed following the sequential added of GDP and an ionophore (FCCP) are shown at the top of the oxygraph trace.
Do humans have functional UCP1 in BAT mitochondria?
While radio-labeled glucose uptake data in individuals exposed to acute mild cold indicated that humans do indeed have functional BAT, information regarding the function of the mitochondria within these BAT depots was lacking. Recent data has shed light on this issue, suggesting that gram for gram, UCP1 function in human BAT is more or less comparable to UCP1 function in rodent BAT.Citation8 These data were generated by assaying the respiratory function and in particular GDP dependent respiration in WAT and BAT collected from humans and mice, where BAT was biopsied from the supraclavicular depot of humans and the intrascapular pad of mice.Citation8 Interestingly, while there were species differences in the absolute respiratory capacities of human and murine BAT, their responses to GDP were near identical,Citation8 providing evidence that per mitochondrion, UCP1 function is comparable in human and murine BAT. Specifically, UCP1 dependent respiration was approximately 3-fold greater in mice compared to humans. There are likely several factors contributing to this species difference in BAT oxidative capacity. One possibility is that a life time of being housed at temperatures which constitute mild cold exposure for a mouse (22–24°C), leads to increased mitochondrial volume density and UCP1 abundance in rodent BAT. With that said, despite greater mass specific UCP1 dependent respiration in rodent compared with human BAT, BAT from both species exhibited a comparable response to GDP. Specifically, respiration declined by approximately 50% in rodent and human BAT following addition of GDP.Citation8 In other words, the respiratory control ratio for GDP was comparable in human and rodent BAT, which suggests that per mitochondrion UCP1 function is similar in humans and rodents.
It is important to note that while a significant portion of respiratory capacity in human BAT can be attributed to UCP1, in WAT from the same individuals – UCP1 dependent respiration was essentially undetectable.Citation8 This underlines the critical importance of using GDP to assay UCP1 function in BAT. Indeed, when comparing the respiratory capacity of human BAT to skeletal muscle, it was clear that oligomycin insensitive leak respiration and uncoupled respiration following the addition of an ionophore were more or less the same in these 2 tissues.Citation8 This underscores the point that oligomycin insensitive leak respiration or uncoupled respiration are not particularly useful measures of BAT mitochondrial function – since they do not specifically reflect UCP1 function.
Summary
Brown adipose tissue research has seen a huge resurgence in the last decade. However, in comparison to other fields of human biochemistry and physiology, human BAT research remains in its infancy. The goal of the current article was to discuss BAT bioenergetics and the tools available to assay BAT and in particular UCP1 function. BAT mitochondria are not conventional, in that they do not produce ATP in any great amount. Therefore, assays developed to determine bioenergetic function in coupled mitochondria may not be particularly useful when assaying the function of mitochondria in BAT. In short, determining the respiratory response of brown fat mitochondria to GDP provides a direct measure of UCP1 function. Utilizing this approach, recent data has demonstrated that human supraclavicular BAT mitochondria have functional UCP1, and that UCP1 function in human and rodent BAT are comparable. Future studies should strive to directly quantify UCP1 function to enhance the translation of preclinical data and discern the role of BAT or beige fat cells in human metabolic physiology.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- Cannon B, Nedergaard J. Brown adipose tissue: function and physiological significance. Physiol Rev 2004; 84:277-359; PMID:14715917; https://doi.org/10.1152/physrev.00015.2003
- Timmons J, Wennmalm K, Larsson O, Walden T, Lassmann T, Petrovic N, Hamilton DL, Gimeno RE, Wahlestedt C, Baar K, et al. Myogenic gene expression signature establishes that brown and white adipocytes originate from distinct cell lineages. Proc Natl Acad Sci U S A 2007; 104:4401-6; PMID:17360536; https://doi.org/10.1073/pnas.0610615104
- Block B. Thermogenesis in muscle. Annu Rev Physiol. 1994; 56:535-77; PMID:8010751; https://doi.org/10.1146/annurev.ph.56.030194.002535
- Nicholls D, Bernson V, Heaton G. The identification of the component in the inner membrane of brown adipose tissue mitochondria responsible for regulating energy dissipation. Experientia Suppl 1978; 32:89-93; PMID:348493
- Matthias A, Ohlson K, Fredriksson J, Jacobsson A, Nedergaard J, Cannon B. Thermogenic Responses in brown fat cells are fully UCP1-dependent. J Biol Chem 2000; 275:25073-81; PMID:10825155; https://doi.org/10.1074/jbc.M000547200
- Nedergaard, Bengtsson T, Cannon B. Unexpected evidence for active brown adipose tissue in adult humans. Am J Physiol Endocrinol Metab 2007; 293:444-52; https://doi.org/10.1152/ajpendo.00691.2006
- Mitchell P. Coupling of phosphorylation to electron and hydrogen transfer by a chemi-osmotic type of mechanism. Nature 1961; 191:144-8; PMID:13771349; https://doi.org/10.1038/191144a0
- Porter C, Herndon D, Chondronikola M, Chao T, Annamalai P, Bhattarai N, Saraf MK, Capek KD, Reidy PT, Daquinag AC, et al. Human and Mouse Brown Adipose Tissue Mitochondria Have Comparable UCP1 Function. Cell Metab 2016; 24:246-55; PMID:27508873; https://doi.org/10.1016/j.cmet.2016.07.004
- Shabalina I, Petrovic N, de Jong J, Kalinovich A, Cannon B, Nedergaard J. UCP1 in Brite/Beige adipose tissue mitochondria is functionally thermogenic. Cell Rep 2013; 5:1196-203; PMID:24290753; https://doi.org/10.1016/j.celrep.2013.10.044
- Nedergaard J, Matthias A, Golozoubova V, Jacobsson A, Cannon B. UCP1: The original uncoupling protein - and perhaps the only one? J Bioenerget Biomembranes 1999; 31:475-91
- Fedorenko A, Lishko P, Kirichok Y. Mechanism of fatty-acid-dependent UCP1 uncoupling in brown fat mitochondria. Cell 2012; 151:400-13; PMID:23063128; https://doi.org/10.1016/j.cell.2012.09.010
- Matthias A, Jacobsson A, Cannon B, Nedergaard J. The bioenergetics of brown fat mitochondria from UCP1-ablated mice. Ucp1 is not involved in fatty acid-induced de-energization (“uncoupling”). J Biol Chem. 1999; 274:28150-60; PMID:10497167; https://doi.org/10.1074/jbc.274.40.28150
- Bulychev A, Kramar R, Drahota Z, Lindberg O. Role of a specific endogenous fatty acid fraction in the coupling-uncoupling mechanism of oxidative phosphorylation of brown adipose tissue. Exp Cell Res 1972; 72:169-87; PMID:4260232; https://doi.org/10.1016/0014-4827(72)90579-4
- Hohorst H, Rafael J. Oxidative phosphorylation by mitochondria from brown adipose tissue. Hoppe Seylers Z Physiol Chem 1968; 349:268-70; PMID:5677015
- Rafael J, Klaas D, Hohorst H. Mitochondria from brown fat: enzymes and respiratory chain phosphorylation during the pre- and postnatal development of the interscapular fat body of the guinea pig. Hoppe Seylers Z Physiol Chem 1968; 349:1711-24; PMID:5707038; https://doi.org/10.1515/bchm2.1968.349.2.1711
- Rafael J, Ludolph H, Hohorst H. Mitochondria from brown adipose tissue: uncoupling of respiratory chain phosphorylation by long fatty acids and recoupling by guanosine triphosphate. Hoppe Seylers Z Physiol Chem 1969; 350:1121-31; PMID:5388738; https://doi.org/10.1515/bchm2.1969.350.2.1121
- Fain J, Reed N. A mechanism for hormonal activation of lipolysis and respiration in free brown fat cells. Lipids 1970; 5:210-9; PMID:4314249; https://doi.org/10.1007/BF02532471
- Williams J, Matthews E. Membrane depolarization, cyclic AMP, and glycerol release by brown adipose tissue. Am J Physiol 1974; 227:987-92; PMID:4372898
- Drahota Z, Honová E, Hahn P. The effect of ATP and carnitine on the endogenous respiration of mitochondria from brown adipose tissue. Experientia 1968; 24:431-2; PMID:5674966; https://doi.org/10.1007/BF02144369
- Nicholls D. Hamster brown-adipose-tissue mitochondria. Purine nucleotide control of the ion conductance of the inner membrane, the nature of the nucleotide binding site. Eur J Biochem 1976; 62(2):223-8; https://doi.org/10.1111/j.1432-1033.1976.tb10151.x
- Shabalina I, Ost M, Petrovic N, Vrbacky M, Nedergaard J, Cannon B. Uncoupling protein-1 is not leaky. Biochim Biophys Acta 2010; 1797:773-84; PMID:20399195; https://doi.org/10.1016/j.bbabio.2010.04.007
- Nicholls D, Locke R. Thermogenic mechanisms in brown fat. Physiol Rev 1984; 64:1-64; PMID:6320232
- Perry C, Kane D, Lanza I, Neufer P. Methods for assessing mitochondrial function in diabetes. Diabetes 2013; 62:1041-53; PMID:23520284; https://doi.org/10.2337/db12-1219
- Nedergaard J, Cannon B, Lindberg O. Microcalorimetry of isolated mammalian cells. Nature 1977; 267:518-20; PMID:559946; https://doi.org/10.1038/267518a0
- Ricquier D, Gaillard J, Turc J. Microcalorimetry of isolated mitochondria from brown adipose tissue. Effect of guanosine-di-phosphate. FEBS Lett 1979; 99:203-6; PMID:437127; https://doi.org/10.1016/0014-5793(79)80279-3
- Girardier L, JP S, Giacobino, Chinet A. Catecholamine binding and the modulation of the thermogenic effect in brown adipose tissue. In: Regulation of depressed metabolism and thermogenesis. CC Thomas, Springfield ILL. 1976:196-212
- Gerencser A, Neilson A, Choi S, Edman U, Yadava N, Oh R, Ferrick DA, Nicholls DG, Brand MD. Quantitative microplate-based respirometry with correction for oxygen diffusion. Anal Chem 2009; 81:6868-78; PMID:19555051; https://doi.org/10.1021/ac900881z
- Li Y, Fromme T, Klingenspor M. Meaningful respirometric measurements of UCP1-mediated thermogenesis. Biochimie 2016; 134:56-61: Epub ahead of print
- Li Y, Fromme T, Schweizer S, Schöttl T, Klingenspor M. Taking control over intracellular fatty acid levels is essential for the analysis of thermogenic function in cultured primary brown and brite/beige adipocytes. EMBO Rep 2014; 15(10):1069-76; PMID:25135951; https://doi.org/10.15252/embr.201438775
- Porter C, Herndon D, Bhattarai N, Ogunbileje J, Szczesny B, Szabo C, Toliver-Kinsky T, Sidossis LS. Severe burn injury induces thermogenically functional mitochondria in murine white adipose tissue. Shock 2015; 44:258-64; PMID:26009824; https://doi.org/10.1097/SHK.0000000000000410
- Chance B, Williams G. The respiratory chain and oxidative phosphorylation. Adv Enzymol Relat Subj Biochem 1956; 17:65-134; PMID:13313307
- Gnaiger E. Mitochondrial pathways and respiratory control. An introduction to OXPHOS analysis. OROBOROS MiPNet Publications 2014;4th ed( Mitochondr Physiol Network 19.12):ISBN 978-3-9502399-8-0
- Ruas J, Siqueira-Santos E, Amigo I, Rodrigues-Silva E, Kowaltowski A, Castilho R. Underestimation of the maximal capacity of the mitochondrial electron transport system in oligomycin-treated cells. PLoS One 2016; 11:e0150967; PMID:26950698; https://doi.org/10.1371/journal.pone.0150967
