ABSTRACT
Understanding adipose tissue heterogeneity is hindered by the paucity of methods to analyze mature adipocytes at the single cell level. Here, we report a system for analyzing live adipocytes from different adipose depots in the adult mouse. Single cell suspensions of buoyant adipocytes were separated from the stromal vascular fraction and analyzed by flow cytometry. Compared to other lipophilic dyes, Nile Red uptake effectively distinguished adipocyte populations. Nile Red fluorescence increased with adipocyte size and granularity and could be combined with MitoTracker® Deep Red or fluorescent antibody labeling to further dissect adipose populations. Epicardial adipocytes exhibited the least mitochondrial membrane depolarization and highest fatty-acid translocase CD36 surface expression. In contrast, brown adipocytes showed low surface CD36 expression. Pregnancy resulted in reduced mitochondrial membrane depolarisation and increased CD36 surface expression in brown and epicardial adipocyte populations respectively. Our protocol revealed unreported heterogeneity between adipose depots and highlights the utility of flow cytometry for screening adipocytes at the single cell level.
Introduction
The growing rates of obesity, diabetes and other metabolic diseases have led to an increased interest in the cell biology and physiology of adipocytes. Adipocytes play central roles in energy storage, body temperature control, blood glucose levels and insulin sensitivity.Citation1 The primary function of white adipose tissue (WAT) is energy storage in the form of the neutral lipid triglyceride in unilocular cytoplasmic lipid droplets.Citation2,Citation3 Brown adipocytes regulate non-shivering thermogenesis and energy expenditure via mitochondrial uncoupling-protein 1.Citation3 Brown adipose tissue (BAT) is primarily restricted to the intrascapular region in adult mice. WAT is found in a range of distinct anatomic locations including; subcutaneous depots such as the inguinal adipose tissue; and visceral depots located in the thorax and abdominal cavity such as the gonadal, peri-renal, mesenteric and epicardial adipose tissues.
The anatomic and functional differences between adipose depots may be due to different developmental pathways.Citation4 Brown adipocytes and skeletal muscle cells arise from Myf5-expressing mesodermal progenitors.Citation5 Wt1-expressing mesothelial progenitors give rise to visceral but not subcutaneous WAT depots.Citation6 Heterogeneity between depots is controlled at the genetic level with distinct genetic loci influencing local adipose behavior.Citation7 Proteomics analysis comparing different white adipose depots in inbred mice revealed significant differences in: the amount of protein expressed; expression profiles of proteins involved in glucose and lipid metabolism; endocrine function and insulin sensitivity.Citation8 Apolipoprotein E expression, as well as responsiveness to metabolic and inflammatory signals, differ across adipose tissue depots.Citation9,Citation10 Adipose depot heterogeneity is also observed in human metabolic disorders and diseases. For example, increased visceral WAT is associated with the development of obesity and type 2 diabetes.Citation11
Adipose tissue consists of developing and mature adipocytes as well as the stromal vascular fraction (SVF) which comprises fibroblasts, immune cells, endothelial cells and pre-adipocytes (adipocyte progenitors).Citation12 Flow cytometry can analyze complex cellular heterogeneity in mixed populations at the single cell level. While flow cytometry has been used extensively to identify pre-adipocytes and to characterize the SVF, flow cytometric analysis of mature adipocytes is not routinely used.Citation6,Citation12 Previous reports of flow cytometric analyses of adipocytes focus on adipocytes derived in vitro from myeloid cells or bone marrow progenitors, or on adipocytes isolated from a single depot.Citation13-17 Recently, Durandt and colleagues identified various subpopulations of adipocytes derived from mesenchymal stromal cells using fatty-acid translocase CD36 and lipophilic dyes Nile Red and BODIPY.Citation18 To improve our understanding of adipose cell biology, a robust flow cytometric protocol was developed to identify and characterize adipocytes according to nuclear content, lipid content, mitochondrial membrane depolarization and adipocyte surface protein phenotype of live cells. In contrast to previous reports, this protocol does not require fixation or permeabilization. We have used this system to assess differences in adipocyte biology during changes in whole-body physiology such as pregnancy. This system allows the robust quantification of live adipocyte phenotype and frequency at the single cell level and could be adapted for use in diagnostic settings in the future.
Results
Flow cytometric analysis of the buoyant adipose fraction
To develop a flow cytometric protocol to analyze adipocyte heterogeneity, adipose tissues were dissected from the regions outlined () in wild type, young adult (6–12 weeks) male outbred mice fed a standard chow diet ad libitum. Depots included; intrascapular BAT; subcutaneous inguinal WAT (lymph nodes removed); and 4 distinct visceral WAT depots namely the gonadal, peri-renal, mesenteric and epicardial. Each tissue was then digested with collagenase type II to obtain single cell suspensions as outlined in Experimental Procedures (G) and pictured beneath their respective depots in . The white arrows indicate multilocular adipocytes dissociated from BAT. Following centrifugation, the buoyant adipose fraction (adipocytes) and pelleted SVF were easily separated and analyzed by flow cytometry.
Figure 1. Single cell suspensions of buoyant adipocytes show distinct flow cytometric profiles to stromal vascular fraction cells. A – F. Adipose tissue was excised from intrascapular brown (BAT), subcutaneous inguinal (WAT:ING), visceral gonadal (WAT:GON), peri-renal (WAT:PR), mesenteric (WAT:MES), and epicardial (WAT:EC). G. The adipose tissue was minced in FACS buffer (PBS + 0.05% BSA) and then digested with 0.1% (w/v) collagenase II for one hour at 37°C. The mixture was pipetted multiple times and passed through a nylon filter. The solution was then centrifuged for 7min at 500 RCF. The buoyant, mature adipocytes were separated from the pelleted SVF using a transfer pipette. Phase contrast images of buoyant adipocytes are placed in the corner of each respective depot in A-F. Arrowheads indicate multi-locular adipocytes. Scale bar represents 50 µm. H and I. Cells from the SVF and buoyant adipocytes were analyzed according to their forward scatter (size) and side scatter (granularity) profile. Cells from both fractions were then stained for propidium iodide (PI) to gate live cells, CD45 for hematopoietic cells, F4/80 for macrophages, and Ter119 for erythroid cells.
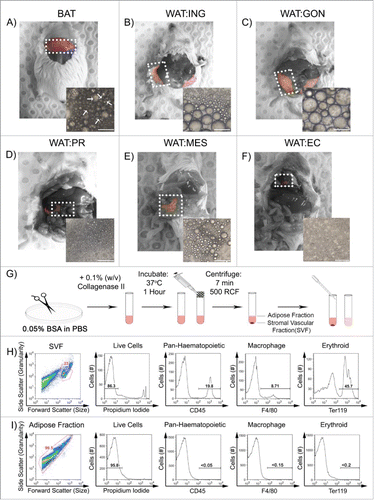
The buoyant fraction consistently showed a forward scatter (size) and side scatter (granularity) profile distinct from the pelleted SVF ( and ). Cell viability was consistently high as shown in ‘live cells’ panel. Propidium iodideneg live cells were gated and analyzed for the presence of contaminating blood cells. The SVF contained CD45+ haematopoietic cells, F4/80+ macrophages and Ter119+ erythrocytes (). The buoyant adipocyte fraction showed no binding to antibodies against blood cell surface proteins ().
Nile Red staining can separate buoyant adipocyte populations by flow cytometry
To confirm the buoyant fraction contained adipocytes, single cell suspensions () were stained with a range of fluorescent lipophilic dyes. These included Nile Red, Oil Red O, LipidTox® Red and LipidTox® Green. Oil Red O is not cell permeable and did not penetrate the live cells (). In contrast, buoyant cells incubated with the neutral lipid dyes LipidTox® Red, LipidTox® Green and Nile Red all emitted a fluorescent signal. Nile Red consistently exhibited a higher fluorescent signal compared with LipidTox® Red and LipidTox® Green. Nile Red is also considerably more cost-effective and hence was chosen as the optimal fluorescent lipophilic dye for this study.
Figure 2. Nile Red is a cell-permeable lipophilic sensor of intracellular lipid droplets useful for flow cytometry. A. Buoyant mature adipocytes were analyzed according to their size and granularity. Buoyant mature adipocytes were stained with: B. Classic lipid dye Oil Red O, C. Neutral lipid dyes; LipidTox® Red; LipidTox® Green and Nile Red. D. Buoyant mature adipocytes were gated according to their size and granularity (R1, R2, and R3) and Nile Red, PI, DRAQ5 and WGA fluorescence measured. Di. Mean fluorescence intensity (MFI ± SEM; n = 6) of Nile RedHigh was 2053 ± 152, Nile RedMid was 234 ± 32 and Nile RedLow was 41 ± 6.1. Dii. Cells from all 3 populations were PI negative (> 97.5% viable). Diii-iv. DRAQ5 and WGA fluorescence was greatest in Nile RedHigh cells (> 99%), while Nile RedMid and Nile RedLow exhibited DRAQ5 (> 87%) and WGA (> 70%) uptake.
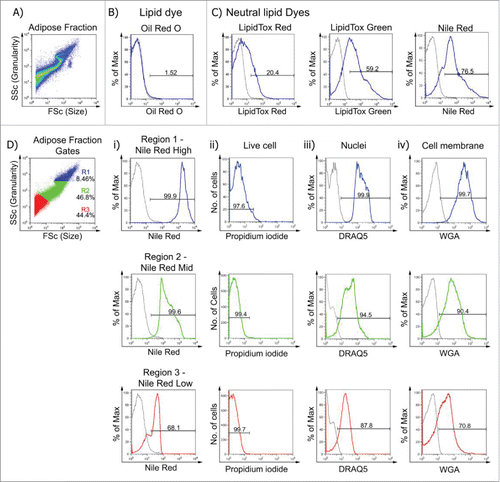
We hypothesized that Nile Red staining would correlate to size and granularity of the buoyant cells. The forward and side scatter profile of the buoyant fraction was subsequently divided into 3 regions. Nile Red uptake for each population revealed highest fluorescence in the largest, most granular cells (, blue). The mean fluorescence intensity (MFI) was significantly different between each population (). Irrespective of the adipose depot assayed, MFI of Nile Red uptake was always greatest in the largest, most granular cells (Nile RedHigh) compared with Nile RedMid and Nile RedLow populations. Dead cells were excluded in all studies using PI. Viability, as determined by lack of PI uptake, was greater than 95% in all Nile Red populations (). DRAQ5, a cell permeable DNA-binding dye, and wheat germ agglutinin (WGA), a probe for cell surface glycosylated structures on the cell membrane, were included to demonstrate the presence of a nucleus and intact cell membranes. More than 90% of Nile RedHigh and Nile RedMid cells were DRAQ5+ and WGA+ while the Nile RedLow population, which may include some cell debris as well as intact cells, showed reduced levels of DRAQ5 uptake and WGA binding (). Nile RedHigh and Nile RedMid cells were not detected in the SVF fraction (data not shown). This demonstrates that the buoyant fraction contains intact, highly viable adipocytes with a high lipid and nuclei content as indicated by the absence of PI and by strong Nile Red, DRAQ5 and WGA staining.
Mitochondrial membrane potential varies among adipose depots
Adipose tissues vary in mitochondrial number and membrane potential.Citation21 As the buoyant fraction can be segregated by Nile Red, it was hypothesized that analysis of combined Nile Red and mitochondrial probe MitoTracker® Deep Red may further differentiate adipose depots. MitoTracker® Deep Red detect changes in mitochondrial membrane potential and measures mitochondrial respiration. To visualize uptake of Nile Red and MitoTracker® Deep Red in live adipocytes, confocal microscopic imaging of buoyant brown, inguinal and gonadal adipocytes was performed ().
Figure 3. Mitochondrial membrane potential as assessed by Mitotracker® Deep Red fluorescence reveals heterogeneity in adipocytes from different depots. A. Confocal images of buoyant brown (left), white inguinal (middle) and gonadal (right) adipocytes stained with Nile Red (red), WGA (green) and MitoTracker® Deep Red (cyan). Scale bar represents 20 μm. B – D. Representative flow cytometric plot of MitoTracker® Deep Red uptake in BAT, WAT:ING, WAT:GON, WAT:PR, WAT:MES and WAT:EC depots according to Nile Red fluorescence (High, Mid and Low). Gating of MitoTracker® Deep Red was defined as MitoTracker−ve, MitoTrackerLow and MitoTrackerHigh. Bi,ii - Di,ii. Adipocytes from the Nile RedHigh, Nile RedMid and Nile RedLow gate of the 6 adipose depots were compared according to MitoTrackerLow and MitoTrackerHigh uptake in male mice (n = 6). Biii-iv – Diii-iv. Frequency of MitoTrackerLow and MitoTrackerHigh positive cells expressed as a proportion of adipose mass in the Nile RedHigh, Nile RedMid and Nile RedLow adipocyte populations. Data presented as mean ± SEM. Differences between adipose depots were determined by 2-tailed, one way ANOVA and pairwise post-hoc comparison by Tukey's HSD test. Groups sharing a numeral are not significantly different from each other.
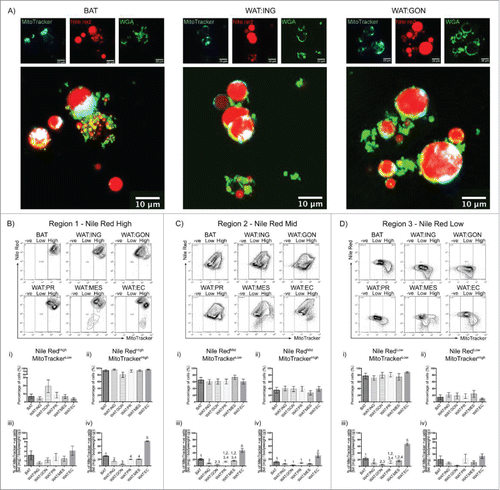
Prior to using Mitotracker Deep Red for flow cytometry of adipocytes, we confirmed uptake in live adipocytes by confocal microscopy (). Adipocytes were dispersed as described previously and the buoyant fraction stained with Nile Red for lipid droplets; FITC-conjugated WGA for cell membrane and Mitotracker® Deep Red for mitochondrial membrane depolarisation. Live cell confocal imaging of adipocytes posed a challenge as the buoyant lipid compartment typically hid the nucleus. Nile Red clearly stained the multilocular lipid droplets of brown adipocytes and the unilocular droplets of white adipocytes. WGA was found to bind to the cell membrane of brown and white adipocytes, however WGA signal rapidly became clustered on the surface of living adipocytes. To our knowledge this has not been reported before as WGA staining is typically performed on fixed or frozen adipose tissue rather than dissociated adipocytes.Citation22 Mitotracker® Deep Red uptake was readily observed by confocal imaging with an intense signal localized eccentrically within the cytoplasm. Together, these data, and those from the previous flow cytometric analyses (, PI and DRAQ5), demonstrate that the cells obtained through the dissociation technique described are indeed live, intact, metabolically active cells.
We were therefore confident to analyze adipocyte populations by flow cytometry according to size, granularity, Nile Red uptake and Mitotracker® Deep Red fluorescence (). No significant differences were observed in mitochondrial membrane potential between adipose depots for the Nile RedHigh, Nile RedMid and Nile RedLow population of cells (). However, when frequency was expressed for each adipose depot as a proportion of body mass (fat (mg)/body weight (g)), epicardial adipocytes exhibited less mitochondrial membrane depolarization compared with all other depots. This was observed in all 3 populations of adipose cells (Nile RedHigh, Nile RedMid and Nile RedLow; and respectively). Mitochondrial membrane depolarization in Nile RedHigh brown adipocytes was less than the similar population found in the inguinal, gonadal, peri-renal and mesenteric depots (). In contrast, gonadal Nile RedHigh adipocytes had the greatest mitochondrial membrane depolarization ().
Multiparametric flow cytometry reveals heterogeneity of CD36 surface protein expression between adipose depots
Initial flow cytometric characterization of adipocytes centered upon surface proteins reported to be present on SVF cells, adipose stem cells or pre-adipocytes such as CD31, CD34, Flk1 and c-Kit.Citation23 No expression of these surface markers was detected on buoyant cells from any depot. The “self-antigen” CD47 and the transferrin receptor CD71 were also not expressed by buoyant cells. A range of adhesion molecules were examined for expression but were not detected including; integrins α2, α4, α5, β3, β4, β7 and CD41; Intercellular Adhesion Molecule 1, CD133 and the epithelial markers E-Cadherin and Epithelial Cell Adhesion Molecule (data not shown).
In contrast, monoclonal antibody against the surface fatty-acid translocase FAT/CD36 bound strongly to buoyant adipocytes. CD36 is a multi-ligand receptor that facilitates the movement of fatty acids into the cell.Citation24 Nile Red staining was combined with anti-CD36 antibody immunoreactivity to determine whether heterogeneity existed between adipose depots (). Buoyant adipocytes showed a broad range of surface CD36 expression from CD36neg through to CD36High levels. In contrast to the other depots, all brown adipocytes exhibited low levels of CD36 surface expression (). Surface CD36 expression was greater in the inguinal, gonadal and epicardial adipocytes compared with brown adipocytes (). As each adipose depot varies significantly in mass (), and the body mass of any individual animal varies, surface expression of CD36 was standardized to ensure that variation of fat mass due to body size is normalized. Epicardial adipocytes showed the highest levels of CD36 expression whereas gonadal adipocytes showed the lowest levels of CD36 among adipose depots examined (). CD36 antibody binding can therefore be used with Nile Red uptake to distinguish adipose populations. We next asked whether there were distinctions between the depots according to physiologic state with particular reference to sex and pregnancy.
Figure 4. CD36 surface expression profiles vary across adipose depots. A – C. Representative flow cytometric plot of CD36 uptake in BAT, WAT:ING, WAT:GON, WAT:PR, WAT:MES and WAT:EC depots according to Nile Red fluorescence (High, Mid and Low). Gating of CD36 was defined as CD36−ve, CD36Low and CD36High. Ai,ii – Ci,ii. Adipocytes from the Nile RedHigh, Nile RedMid and Nile RedLow gate of the 6 adipose depots were compared according to CD36Low and CD36High uptake in male mice (n = 6). Aiii-iv – Ciii-iv. Frequency of CD36Low and CD36High positive cells expressed as a proportion of adipose mass in the Nile RedHigh, Nile RedMid and Nile RedLow adipocyte populations. Data presented as mean ± SEM. Differences between adipose depots were determined by 2-tailed, one way ANOVA and pairwise post-hoc comparison by Tukey's HSD test. Groups sharing a numeral are not significantly different from each other.
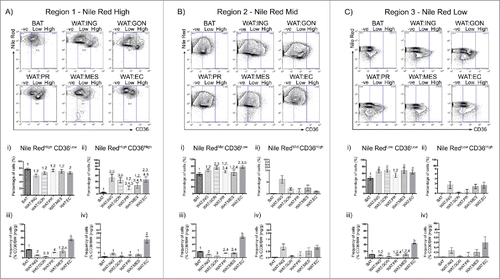
Figure 5. Changes in adipocyte surface phenotype and mitochondrial membrane potential during pregnancy. A. Comparing the mass of BAT, WAT:ING, WAT:GON, WAT:PR, WAT:MES and WAT:EC adipose tissue relative to bodyweight in male (n = 6), virgin (n = 6) and pregnant (n = 6) female mice. B. Expression of CD36 as a proportion of tissue mass in mature adipocytes. BAT, WAT:ING, WAT:GON, WAT:PR, WAT:MES and WAT:EC from male (n = 6), virgin (n = 6) and pregnant (n = 6) female mice were compared. C. Comparing uptake of MitoTracker® Deep Red as a proportion of tissue mass in mature adipocytes. BAT, WAT:ING, WAT:GON, WAT:PR, WAT:MES and WAT:EC from male (n = 6), virgin (n = 6) and pregnant (n = 6) female mice were compared. Data presented as mean ± SEM. Differences between adipose depots were determined by 2-tailed, 2-way ANOVA and pairwise post-hoc comparison by Tukey's multiple comparison test. Groups sharing a numeral are not significantly different from each other.
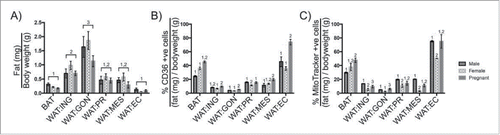
Adipose tissue from pregnant mice have elevated surface CD36 and reduced mitochondrial membrane potential compared with male and virgin female mice
The surface expression of CD36 and mitochondrial membrane potential of adipocytes was compared between different adipose depots from male, virgin female or pregnant female mice as a proportion of body weight (). The adipose mass varied according to sex and pregnancy status of the mice. Interestingly, in healthy adult mice, when adipose mass was expressed as a percentage of body weight, the relative proportions of adipose tissues were not significantly different (). CD36 surface expression was elevated during pregnancy. The frequency of CD36High adipocytes increased in the BAT and epicardial WAT depots of pregnant mice compared with virgin female or male mice (). Brown adipocytes from female mice showed less mitochondrial membrane depolarization compared with equivalent cell types from male mice (). In contrast, virgin female epicardial adipocytes showed greater mitochondrial membrane depolarization than male or pregnant female epicardial adipocytes ().
Discussion
Deeper understanding of adipocyte biology is needed as obesity and related metabolic disorders such as diabetes and metabolic syndrome are rapidly becoming major health burdens throughout the world. It is, therefore, imperative that we develop assay systems to allow for rapid and cost-effective analysis of adipose tissues to monitor human health. Flow cytometry has proven to be very effective in the diagnosis of hematological and oncological diseases.Citation25–28 Flow cytometry has also been used to examine adipocytes derived from myeloid cells in vitro or from single depots.Citation13–17 Recently, Xiao and colleagues used flow cytometry of buoyant gonadal adipocytes to examine adipocyte size in relation to adipose tissue inflammation.Citation29 In contrast, our protocol categorizes adipocyte populations in accordance to lipophilic dye uptake. Irrespective of the depot assayed, all adipocyte populations were strongly fluorescent for DRAQ5 and WGA. DRAQ5 stains nuclei while WGA is a lectin that binds to oligosaccharides containing N-acetyl-D-glucosamine found on the membrane of cells.Citation30 Furthermore all adipocyte suspensions assayed had minimal propidium iodide uptake. Together, these parameters confirmed that the adipocytes are viable and intact with nuclei. These steps were taken to highlight the rigor and robustness of our approach.
In comparing a range of lipophilic dyes to label and quantify adipocytes, we found that Nile Red was the most cost-effective and the most effective at segregating distinct size populations. Nile Red is a lipophilic dye that stains neutral lipids.Citation31,Citation32 Flow cytometric analyses using Nile Red have been conducted on macrophages, smooth muscle cells and Leydig cells.Citation32,Citation33 Adipocytes differentiated from mouse embryonic stem cells in vitro have also been analyzed by flow cytometry using Nile Red.Citation20 More recently, Durandt and colleagues used Nile Red in ex vivo cultures to identify subpopulations of adipocytes derived from mesenchymal stromal cells.Citation18 This is the first study however, that expands upon these protocols by combining Nile Red staining with surface antigen expression and mitochondrial membrane depolarisation to compare primary mature adipocytes from multiple adipose depots at the single cell level. Nile Red staining can be performed rapidly (∼5 minutes) on live cells and does not require fixation or treatment with detergents as used previously.Citation34
Nile Red can be combined with other fluorescent probes to obtain a more complete analysis of adipose cell biology. MitoTracker® Deep Red is a far-red fluorescent probe reported to represent mitochondrial membrane potential, with increasing signal indicating a reduction in mitochondrial membrane depolarization.Citation35 Brown and epicardial adipocytes exhibited less membrane depolarization compared with adipocytes from other adipose depots. The role of mitochondria in BAT is most prevalent in cold temperatures when BAT is activated to stimulate thermogenesis through uncoupling protein-1.Citation36 In humans, epicardial adipose tissue has higher expression of uncoupling protein-1 compared with other WAT depots.Citation37 This is supportive of increasing evidence which suggests epicardial adipocytes may have a similar function to BAT.Citation37 Our findings in the mouse support the similarities between BAT and epicardial adipose tissue in humans.
Fatty acid translocase CD36 facilitates the uptake of fatty acids and lipoproteins by accelerating intracellular esterification into triglycerides.Citation38 Surprisingly, brown adipocytes expressed less surface CD36 than their WAT counterparts. Mice exposed to cold temperatures have enhanced uptake of lipoproteins in BAT.Citation39 Mice that lack both alleles for CD36 have impaired fatty acid, lipoprotein and glucose uptake in BAT.Citation39
As the animals examined here were maintained at thermoneutrality, it is possible that the lower levels of CD36 surface protein expression may change upon exposure to lower temperatures and activation of BAT non-shivering thermogenesis. Epicardial adipocytes exhibited the highest levels of surface protein expression of CD36. This is the first report of CD36 expression in epicardial adipose tissue. Epicardial adipose tissue has been proposed to play roles distinct to those of BAT, subcutaneous WAT and other visceral WAT.Citation37 Reservoirs of adipose tissue surrounding the heart are scarce and largely serve to fuel myocardial contraction through the storage and supply of fatty acids for mitochondrial oxidation.Citation40 Disturbances in this balance results in an accumulation of triglycerides leading to cardiac lipotoxicity.Citation41 However, a specific role of CD36 in cardiac lipotoxicity is yet to be identified.
Evidence is increasing that adipose tissues exhibit sexual dimorphism.Citation42 For example, women have a higher prevalence of BAT compared with men.Citation43 In mice, BAT from pregnant and virgin female mice had greater surface CD36 expression and elevated mitochondrial membrane potential compared with male mice. Pregnancy induced significant changes in adipocyte cell biology. Surface expression of CD36 was elevated in adipose tissue of pregnant mice compared with virgin female mice. This suggests a distinct role for fatty acid translocase during gestation. Ogunyemi and colleagues found a downregulation of CD36 expression in subcutaneous WAT during pregnancy.Citation44 Conversely, populations of inguinal adipocytes from pregnant mice had a minor but significant increase in CD36 surface expression compared with virgin female and male mice. More strikingly however, epicardial adipocytes showed a profound increase in CD36 surface expression. The changes observed may be due to the increased nutrient requirements of the mother during pregnancy, which is supplemented by the mobilization of lipid reserves.Citation45 Further, the changes in circulating hormones during pregnancy may alter the cellular physiology of epicardial adipocytes. For instance, circulating leptin is elevated during pregnancy.Citation46 Enhanced expression of CD36 in epicardial adipose tissue may serve to regulate leptin levels during gestation.Citation47 Additionally, elevated expression of CD36 has been reported in obese pregnant women with gestational diabetes mellitus.Citation48 Gestational diabetes occurs during pregnancy due to the increased load on the mother by the fetus and may lead to type II diabetes postpartum.Citation49 This often causes a larger accumulation of fat in the fetus as well as excess fetal growth.Citation50
Our approach highlights the utility of flow cytometry in assessing adipocyte phenotype and behavior at the single cell level and relating this information to whole body physiology. This tool could be applied for bench-top diagnostics in adipose-related metabolism disorders such as obesity and type 2 diabetes, 2 of the most pressing health concerns for modern society.
Materials and methods
Mice
Young adult, wild type, outbred quackenbush Swiss mice (Animal Resource Center, Perth, Western Australia) were housed in filter top cages. Mice were kept under a 12-hour day-night cycle at constant temperature (21–22°C) and provided food and water ad libitum. The welfare of the animals in the housing area and experiments conducted were in accordance with the Australian Code of Practice for the use of animals in research.
Adipose tissue excision
Mice were killed by cervical dislocation according to University of Sydney Animal Ethics Committee approval. BAT was dissected from the intrascapular region. WAT was dissected from the subcutaneous inguinal (WAT:ING), visceral gonadal (WAT:GON), peri-renal (WAT:PR), mesenteric (WAT:MES) and epicardial (WAT:EC) regions. Tissue was minced thoroughly (∼1–3mm3 in diameter) in fluorescence-activated cell sorting (FACS) buffer (PBS + 0.05% bovine serum albumin) and digested in 0.1% (w/v) Collagenase II (Worthington) for 1 hour at 37oC with occasional shaking. Bovine serum albumin was used to maintain cell integrity of the adipocytes.Citation17,Citation19
Isolation of mature adipocytes
Digested adipose tissue was dispersed further with repeated pipetting before filtering through a ∼350 μM polystyrene mesh to remove cell clusters. The filtered single cell suspension was washed with 3 mL of FACS buffer to inactivate collagenase and centrifuged at 500 RCF for 7 minutes to pellet the SVF. The buoyant adipocytes were separated from the SVF using a plastic transfer pipette into individual tubes. The SVF was resuspended in FACS buffer and ready for antibody/dye labeling.
Reconstitution of Nile Red
Nile Red (Sigma) was reconstituted as described previously in DMSO to a concentration of 10 mg/mL.Citation20 This was diluted to a working solution 100 µg/mL in DMSO. The final concentration used to stain the buoyant adipocytes and SVF was 100 ng/mL in FACS buffer.
Flow cytometry
Buoyant adipocytes and cells from the SVF were incubated with fluorescently-conjugated antibodies () for 30 minutes on ice in the dark. Lipophilic dyes () were subsequently added and incubated for 10 minutes on ice. Cells were then washed with FACS buffer and centrifuged at 500 RCF. Cells from the buoyant fraction were taken and stained with propidium iodide (PI) to exclude dead cells from analysis. Buoyant adipocytes were also stained with the lectin WGA conjugated to FITC (L4895, Sigma) and nuclear probe DRAQ5 (62251, ThermoFisher) to confirm the presence of intact adipocytes with nuclei. For cells from the SVF, the supernatant was removed before being resuspended in FACS buffer containing PI. For single color controls, CD45 (APC and PE, eBioscience, 17–0451–82 and 12–0451–81 respectively) and Ter119 (FITC, eBioscience, 11–5921–81) were used on flushed femoral bone marrow. The single color controls were then used to correct compensation. Isotype matched control was Rat IgG2b (APC, eBioscience, 17–4031–81) and fluorescence minus one controls were Nile Red alone (C, D), CD36 alone and MitoTracker® Deep Red alone (data not shown). Cells were gently shaken before analyses to keep them in suspension and to avoid clumping.
Table 1. Antibodies used in this study.
Table 2. Probes used in this study.
Flow cytometer parameters
A Becton Dickinson FACSCalibur 4 Color Benchtop Analytical Flow Cytometer (Becton Dickinson, San Jose, CA, USA) was used for flow cytometric analysis. The sheath pressure was 4.5 psi and the pressure differential sufficient to establish a sample flow of 60 µL/min on a high flow rate. The flow cell internal dimensions are 180 µM x 430 µM with the flow cell optically coupled to the right angle objective lens for improved sensitivity. The laser used was a 15 mW Argon Ion with laser wavelength of 488 nM. The Sample Injection Port has a diameter of 1 mm. Cells were run at a max sample flow rate of 3000 events/second. Data was acquired using CellQuest Pro® 6.0. Data was analyzed using the FlowJo software package (TreeStar, Ashland, OR, USA).
Phase contrast microscopy
Adipocytes were dissociated into single cells as described above. Buoyant adipocytes were transferred onto a 12-well plate and viewed on an Axiovert35 (Zeiss) microscope under 32x magnification (ACROSTIMA 32x objective, NA: 0.40) and imaged using ZEN 2011 imaging software (Zeiss). Scale bars represent 50 µm.
Confocal microscopy
Adipocytes were prepared and stained as above though PI was not added. Stained adipocytes were pipetted onto a glass slide and a coverslip placed on top. The coverslip was sealed with nail polish and the slide kept in the dark until imaging. Confocal images were taken with a Leica SPEII (Leica, Germany) equipped with the solid-state laser (405 nm, 488 nm, 532 nm and 625 nm). Images were taken using an oil-immersed Leica ACS Apochromat 63x objective coupled to the Leica Application Suite – Advanced Fluorescence software. WGA was excited by the 488 nm laser while Nile Red and MitoTracker® Deep Red were excited by the 532 nm and 625 nm lasers respectively. Scale bars represent 20 µm.
Statistical analysis
All data represented as mean ± SEM. Statistical analyses were performed on GraphPad Prism®. Comparison of One-way ANOVA was used with Tukey's post-hoc analysis to determine differences among lipophilic dye uptake or surface expression of antibodies between adipose depots. Two-way ANOVA with Tukey's multiple comparison test was used to determine significant differences between male, virgin female and pregnant mice. To account for differences in body size between individual mice, raw data are expressed as a proportion of fat mass (as a percentage of body mass) where relevant.
Abbreviations
| BAT | = | brown adipose tissue |
| PI | = | propidium iodide |
| SVF | = | stromal vascular fraction |
| WGA | = | wheat germ agglutinin |
| WAT | = | white adipose tissue |
| WAT:EC | = | epicardial adipose tissue |
| WAT:GON | = | gonadal adipose tissue |
| WAT:ING | = | inguinal adipose tissue |
| WAT:MES | = | mesenteric adipose tissue |
| WAT:PR | = | peri-renal adipose tissue |
Disclosure of potential conflicts of interest
No conflicts of interest relevant for this study.
Acknowledgments
The authors would like to acknowledge the support of the Disciplines of Physiology, Anatomy and Histology at the University of Sydney and the Bosch Institute Live Cell Analysis Facility (Dr Angeles Sanchez-Perez). We also acknowledge the Advanced Microscopy Facility at the University of Sydney. We thank Dr Andrew Hoy (Physiology, University of Sydney) for constructive feedback and suggestions. The authors would also like to acknowledge the laboratory members of the Blood Cell Development laboratory and the Andrology Research Group laboratory.
Funding
No funding sources relevant for this study.
References
- Berry DC, Stenesen D, Zeve D, Graff JM. The developmental origins of adipose tissue. Development 2013; 140(19):3939–49; PMID:24046315; https://doi.org/10.1242/dev.080549</bib>
- Ahmadian M, E Duncan R, Jaworski K, Sarkadi-Nagy E, Sul HS. Triacylglycerol metabolism in adipose tissue. Future Lipidol 2007; 2(2):229–37; PMID:19194515; https://doi.org/10.2217/17460875.2.2.229
- Harms M, Seale P. Brown and beige fat: development, function and therapeutic potential. Nat Med 2013; 19(10):1252–63; PMID:24100998; https://doi.org/10.1038/nm.3361
- Gesta S, Tseng Y-H, Kahn CR. Developmental origin of fat: Tracking obesity to its source. Cell 2007; 131(2):242–56; PMID:17956727; https://doi.org/10.1016/j.cell.2007.10.004
- Lee MJ, Wu Y, Fried SK. Adipose tissue heterogeneity: Implication of depot differences in adipose tissue for obesity complications. Mol Aspects Med 2013; 34(1):1–11; PMID:23068073; https://doi.org/10.1016/j.mam.2012.10.001
- Chau YY, Hastie N. Wt1, the mesothelium and the origins and heterogeneity of visceral fat progenitors. Adipocyte 2014; 4(3):217–21; https://doi.org/10.4161/21623945.2014.985009
- Shungin D, Winkler TW, Croteau-Chonka DC, Ferreira T, Locke AE, Mägi R, Strawbridge J, Pers TH, Fischer K, Justice AE, et al. New genetic loci link adipose and insulin biology to body fat distribution. Nature 2015; 518(7538):187–96; PMID:25673412; https://doi.org/10.1038/nature14132
- Sackmann-Sala L, Berryman DE, Munn RD, Lubbers ER, Kopchick JJ. Heterogeneity among white adipose tissue depots in male C57BL/6J mice. Obesity 2011; 20(1):101–11; PMID:21779095; https://doi.org/10.1038/oby.2011.235
- Huang ZH, Espiritu DJ, Uy A, Holterman AX, Vitello J, Mazzone T. Adipose tissue depot-specific differences in adipocyte apolipoprotein E expression. Metabolism 2011; 60(12):1692–701; PMID:21664633; https://doi.org/10.1016/j.metabol.2011.04.012
- White UA, Tchoukalova YD. Sex dimorphism and depot differences in adipose tissue function. Biochim Biophys Acta 2014; 1842(3):377–92; PMID:23684841; https://doi.org/10.1016/j.bbadis.2013.05.006
- Jeffery E, Church CD, Holtrup B, Colman L, Rodeheffer MS. Rapid depot-specific activation of adipocyte precursor cells at the onset of obesity. Nat Cell Biol 2015; 17(4):376–85; PMID:25730471; https://doi.org/10.1038/ncb3122
- Brake DK, Smith CW. Flow cytometry on the stromal-vascular fraction of white adipose tissue. Methods Mol Biol 2008; 456:221–29; PMID:18516564
- Majka SM, Miller HL, Sullivan T, Erickson PF, Kong R, Weiser-Evans M, Nemenoff R, Moldovan R, Morandi RSA, Davis JA, et al. Adipose lineage specification of bone marrow-derived myeloid cells. Adipocyte 2014; 1(4):215–29; https://doi.org/10.4161/adip.21496
- Majka SM, Fox KE, Psilas JC, Helm KM, Childs CR, Acosta AS, Janssen RC, Friedman JE, Woessner BT, Shade TR, et al. De novo generation of white adipocytes from the myeloid lineage via mesenchymal intermediates is age, adipose depot, and gender specific. Proc Natl Acad Sci U S A 2010; 107(22):14781–86; PMID:20679227; https://doi.org/10.1073/pnas.1003512107
- Crossno JT, Majka SM, Grazia T, Gill RG, Klemm DJ. Rosiglitazone promotes development of a novel adipocyte population from bone marrow–derived circulating progenitor cells. J Clin Invest 2006; 116(12):3220–28; PMID:17143331; https://doi.org/10.1172/JCI28510
- Gavin KM, Gutman JA, Kohrt WM, Wei Q, Shea KL, Miller HL, Sullivan TM, Erickson PD, Helm KM, Acosta AS, et al. De novo generation of adipocytes from circulating progenitor cells in mouse and human adipose tissue. FASEB J 2016; 30(3):1096–1108; PMID:26581599
- Majka SM, Miller HL, Helm KM, Acosta AS, Childs CR, Kong R, Klemm DJ. Analysis and isolation of Adipocytes by flow cytometry. Methods Enzymol 2014; 537:281–96; PMID:24480352
- Durandt C, van Vollenstee FA, Dessels C, Kallmeyer K, de Villiers D, Murdoch C, et al. Novel flow cytometric approach for the detection of adipocyte sub-populations during adipogenesis. J Lipid Red 2016; 57:729–42
- Carswell KA, Lee M-J, Fried SK. Culture of isolated human adipocytes and isolated adipose tissue. Methods Mol Biol 2012; 806:203–214; PMID:22057454
- Schaedlich K, Knelangen JM, Navarrete Santos A, Fischer B, Navarrete Santos A. A simple method to sort ESC-derived adipocytes. Cytometry 2010; 77A(10):990–5
- Bjørndal B, Burri L, Staalesen V, Skorve J, Berge RK. Different Adipose Depots: Their role in the development of metabolic syndrome and mitochondrial response to Hypolipidemic Agents. J Obes 2011; 2011(1):1–15
- Varlamov O, Somwar R, Cornea A, Kievit P, Grove KL, Roberts CT, Jr. Single-cell analysis of insulin-regulated fatty acid uptake in adipocytes. Am J Physiol Endocrinol Metab 2010; 299(3):E486–E496; PMID:20570821
- Cawthorn WP, Scheller EL, MacDougald OA. Adipose tissue stem cells meet preadipocyte commitment: going back to the future. J Lipid Res 2012; 53(2):227–46; PMID:22140268
- Qiao L, Zou C, Shao P, Schaack J, Johnson PF, Shao J. Transcriptional regulation of fatty acid translocase/CD36 expression by CCAAT/enhancer-binding protein alpha. J Biol Chem 2008; 283(14):8788–95; PMID:18263877
- Liu Q, Wang M, Hu Y, Xing H, Chen X, Zhang Y, Zhu P. Significance of CD71 expression by flow cytometry in diagnosis of acute leukemia. Leuk Lymphoma 2015; 55(4):892–8
- Adachi Y, Hino T, Ohsawa M, Ueki K, Murao T, Li M, Cui Y, Okigaki M, Ito M, Ikehara S. A case of CD10-negative angioimmunoblastic T cell lymphoma with leukemic change and increased plasma cells mimicking plasma cell leukemia: A case report. Oncol Lett 2015; 10:1555–60; PMID:26622708
- Rawstron AC, Fazi C, Agathangelidis A, Villamor N, Letestu R, Nomdedeu J, Palacio C, Stehlikova O, Kreuzer KA, Liptrot S, et al. A complementary role of Multiparameter flow cytometry and high-throughput sequencing for minimal residual disease detection in chronic lymphocyte leukemia: an European Research Initiative on CLL study. Leukemia 2015; 30:1–27; PMID:26108693
- Weir EG, Borowitz MJ. Flow cytometry in the diagnosis of acute leukemia. Semin Hematol 2001; 38(2):124–38; PMID:11309694
- Xiao L, Yang X, Lin Y, Li S, Jiang J, Qian S, Tang Q, He R, Li X. Large adipocytes function as antigen-presenting cells to activate CD4(+) T cells via upregulating MHCII in obesity. Int J Obes (Lond) 2016; 40(1):112–20; PMID:26248660
- Yoshida M, Stadler J, Bertholdt G, Gerisch G. Wheat germ agglutinin binds to the contact site A glycoprotein of Dictyostelium discoideum and inhibits EDTA-stable cell adhesion. EMBO J 2984; 3(11):2663–70
- Smith JL. The staining of fat by Nile-blue sulphate. J Pathol Bacteriol 1911; 15:53–5; https://doi.org/10.1002/path.1700150107
- Greenspan P, Mayer EP, Fowler SD. Nile red: a selective fluorescent stain for intracellular lipid droplets. J Cell Biol 1985; 100(3):965–73; PMID:3972906; https://doi.org/10.1083/jcb.100.3.965
- Gocze PM, Freeman DA. Factors underlying the variability of lipid droplet fluorescence in MA-10 Leydig tumor cells. Cytometry 1994; 17(2):151–8; PMID:7835165; https://doi.org/10.1002/cyto.990170207
- Chau YY, Bandiera R, Serrels A, Martínez-Estrada OM, Qing W, Lee M, Slight J, Thornburn A, Berry R, McHaffie S, et al. Visceral and subcutaneous fat have different origins and evidence supports a mesothelial source. Nat Cell Biol 2014; 16(4):367–75; PMID:24609269; https://doi.org/10.1038/ncb2922
- Poot M, Zhang YZ, Krämer JA, Wells KS, Jones LJ, Hanzel DK, Lugade AG, Singer VL, Haugland RP. Analysis of mitochondrial morphology and function with novel fixable fluorescent stains. J Histochem Cytochem 1996; 44(12):1363–72; PMID:8985128; https://doi.org/10.1177/44.12.8985128
- Virtanen KA, Lidell ME, Orava J, Heglind M, Westergren R, Niemi T, Taittonen M, Laine J, Savisto NJ, Enerbäck S, et al. Functional Brown Adipose Tissue in Healthy Adults. N Engl J Med 2009; 360(15):1518–25; PMID:19357407; https://doi.org/10.1056/NEJMoa0808949
- Sacks HS, Fain JN, Bahouth SW, Ojha S, Frontini A, Budge H, Cinti S, Symonds ME. Adult epicardial fat exhibits beige features. J Clin Endocrinol Metab 2013; 98(9):E1448–55; PMID:23824424; https://doi.org/10.1210/jc.2013-1265
- Xu S, Jay A, Brunaldi K, Huang N, Hamilton JA. CD36 enhances fatty acid uptake by increasing the rate of intracellular Esterification but not transport across the plasma membrane. Biochemistry 2013; 52(41):7254–61; PMID:24090054; https://doi.org/10.1021/bi400914c
- Putri M, Syamsunarno MRAA, Iso T, Yamaguchi A, Hanaoka H, Sunaga H, Koitabashi N, Matsui H, Yamazaki C, Kameo S, et al. CD36 is indispensible for thermogenesis under conditions of fasting and cold stress. Biochem Biophys Res Commun 2015; 457(4):520–5; PMID:25596128; https://doi.org/10.1016/j.bbrc.2014.12.124
- Cherian S, Lopaschuk GD, Carvalho E. Cellular cross-talk between epicardial adipose tissue and myocardium in relation to the pathogenesis of cardiovascular disease. AJP: Endocrinol Metab 2012; 303(8):E937–49
- Glatz JFC, Angin Y, Steinbusch LKM, Schwenk RW, Luiken JJFP. CD36 as a target to prevent cardiac lipotoxicity and insulin resistance. Prostaglandins Leukot essent Fatty Acids 2013; 88(1):71–7; PMID:22580174; https://doi.org/10.1016/j.plefa.2012.04.009
- Fuente-Martín E, Argente-Arizón P, Ros P, Argente J, Chowen JA. Sex differences in adipose tissue. Adipocyte 2014; 2(3):128–34; https://doi.org/10.4161/adip.24075
- Cypess AM, Lehman S, Williams G, Tal I, Rodman D, Goldfine AB, Kuo FC, Palmer EL, Tseng YH, Doria A, et al. Identification and importance of brown adipose tissue in adult humans. N Engl J Med 2009; 360(15):1509–17; PMID:19357406; https://doi.org/10.1056/NEJMoa0810780
- Ogunyemi D, Xu J, Mahesan AM, Rad S, Kim E, Yano J, Alexander C, Rotter JI, Chen Y-DI. Differentially expressed genes in adipocytokine signaling pathway of adipose tissue in pregnancy. J Diabetes Mellitus 2013; 03(2):86–95; https://doi.org/10.4236/jdm.2013.32013
- Millican PE, Vernon RG, Pain VM. Protein-Metabolism in the mouse during pregnancy and lactation. Biochem J 1987; 248(1):251–7; PMID:3435442; https://doi.org/10.1042/bj2480251
- Jones HN, Woollett LA, Barbour N, Prasad PD, Powell TL, Jansson T. High-fat diet before and during pregnancy causes marked up-regulation of placental nutrient transport and fetal overgrowth in C57/BL6 mice. FASEB J 2008; 23(1):271–8; PMID:18827021; https://doi.org/10.1096/fj.08-116889
- Hajri T, Hall AM, Jensen DR, Pietka TA, Drover VA, Tao H, Eckel R, Abumrad NA. CD36-facilitated fatty acid uptake inhibits leptin production and signaling in adipose tissue. Diabetes 2007; 56(7):1872–80; PMID:17440173; https://doi.org/10.2337/db06-1699
- Boyle KE, Hwang H, Janssen RC, DeVente JM, Barbour LA, Hernandez TL, Mandarino LJ, Friedman JE. Gestational diabetes is characterized by reduced mitochondrial protein expression and altered calcium signaling proteins in skeletal muscle. PLoS One 2014; 9(9):e106872; PMID:25216282; https://doi.org/10.1371/journal.pone.0106872
- Nar G, Inci S, Aksan G, Unal OK, Nar R, Soylu K. The relationship between epicardial fat thickness and gestational diabetes mellitus. Diabetol Metab Syndr 2014; 6(1):120; PMID:25400702; https://doi.org/10.1186/1758-5996-6-120
- Friedman JE. Obesity and Gestational Diabetes Mellitus pathways for programming in mouse, monkey, and man—Where do we go next? The 2014 Norbert Freinkel award lecture. Diabetes Care 2015; 38(8):1402–11; PMID:26207051; https://doi.org/10.2337/dc15-0628
