ABSTRACT
The prevalence of non-obese type 2 diabetes in Asians is up to 50%. This review aims to summarize the role of regional fat in the development of insulin resistance and cardiovascular risk in non-obese Asian type 2 diabetes as well as the role of intra-pancreatic fat and β-cell dysfunction. The body fat content of non-obese Asian type 2 diabetic patients is not different from that of non-diabetic subjects but the proportion of intra-abdominal and intra-hepatic fat are greater. Visceral fat contributes to insulin resistance and cardiovascular risk in non-obese Asian type 2 diabetes. Intra-hepatic fat and the hypertrophic abdominal subcutaneous adipocytes are associated with insulin resistance and cardiovascular risk in non-obese, non-diabetic Asian subjects. It may be true in non-obese Asian type 2 diabetic patients. The role of intra-myocellular lipid and insulin resistance is uncertain. Intra-pancreatic fat may not be involved in β-cell dysfunction in non-obese Asian type 2 diabetes.
It is known that a significant number of type 2 diabetic patients particularly in Asian region are not obese [Citation1–Citation4]. Up to 50% of Asian type 2 diabetic patients have BMI <25 kg/m2 [Citation5]. These non-obese diabetic subjects have lower insulin sensitivity and lower insulin secretion than non-diabetic subjects as similarly observed in obese diabetic counterparts [6−8]. Abdominal obesity, as defined by waist circumference ≥90 cm for men and ≥80 cm for women, has been demonstrated to play a major role in the development of insulin resistance of type 2 diabetes in Asian populations [Citation5,Citation9]. However, most of the studies are from obese subjects, the study of the relationships of total or regional adiposity to insulin resistance and metabolic abnormalities specifically in non-obese patients are relatively few. Furthermore, since structural and biochemical characteristics of subcutaneous and visceral fats are not similar between non-obese and obese subjects [Citation10], it is possible that such relationships may be different between non-obese and obese type 2 diabetes. The purpose of this review is to summarize the latest evidence from English language literature about the role of regional adipose tissues which include subcutaneous, visceral or intra-abdominal, intra-hepatic and intra-myocellular fat in the development of insulin resistance and the increase of cardiovascular risk factors in non-obese Asian patients with type 2 diabetes. The role of intra-pancreatic fat and β cell dysfunction in non-obese Asian patients is also reviewed. The term “diabetes” in this review denotes type 2 diabetes and BMI < 25 kg/m2 is used as a definition of non-obesity in Asian population [Citation9] throughout this review.
Role of differentially distributed adiposity in non-obese Asian type 2 diabetic patients
Visceral or intra-abdominal fat
It is known that despite lower amount of total fat mass, Asian populations tend to accumulate visceral fat greater than other ethnics even in subjects with lower BMI ranges [Citation11]. Non-obese Asian diabetic patients have been shown to have greater visceral fat mass than BMI-matched, non-diabetic subjects with no difference of total body fat. The study by Jang et al [Citation12] in 26 non-obese, Korean diabetic men demonstrated that diabetic men had significantly greater waist-hip ratio and visceral fat areas by CT than age- and BMI-matched non-diabetic men. Two case-control studies in non-obese Indian and Chinese diabetic men and women confirmed those findings from Korean study [Citation13,Citation14]. Jung et al [Citation15] studied 1,603 non-obese Korean subjects and reported visceral fat mass had stronger association with diabetes than other anthropometric measures in both men and women. The longitudinal follow-up study of second generation Japanese-American men and women indicated that the amount of visceral fat was a strong predictor of progression to diabetes [Citation16]. Subjects who started with greater amount of visceral fat are more likely to develop diabetes. There was no difference of BMI or the amount of total body fat between those who did or did not progress to diabetes. Kim et al [Citation17] also demonstrated that increased waist-to-height ratio or waist circumference was better than BMI in prediction of diabetes in the next 5 years in non-obese, non-diabetic Korean subjects.
The direct study of the relationship of visceral fat and insulin resistance in non-obese Asian diabetic population is scarce. Our studies in non-obese, diabetic and non-diabetic Thai women indicated that the amount of visceral fat was higher in diabetic women and visceral fat, not total body fat, was strongly associated with insulin resistance measured by clamp method in diabetic women [Citation18,Citation19]. Visceral fat was also associated with increased blood pressure, fasting insulin and uric acid levels in non-obese diabetic women. However the relationship of visceral fat and insulin resistance could not be observed in non-obese, non-diabetic women [Citation19]. The population-based study from the 4th Thailand National Health Examination Survey in 2008–2009 by Aekplakorn et al (Aekplakorn W, personal communication) confirmed the higher prevalence of abdominal obesity, hypertension, high triglyceride and low HDL cholesterol levels in non-obese diabetic Thai subjects than those of healthy controls. Likewise, the study in 93 non-obese Asian Indian men and women with type 2 diabetes indicated that visceral fat but not abdominal subcutaneous fat was positively associated with insulin resistance, tumor necrosis factor-alpha (TNF-α) and highly sensitive C-reactive protein levels as well as carotid intimal media thickness [Citation20]. Visceral fat, not abdominal subcutaneous fat, was associated with systolic and diastolic blood pressure, glucose and lipid levels in non-obese, non-diabetic and pre-diabetic Chinese adults [Citation21]. The positive dose-response relationships of visceral fat and the prevalence of metabolic syndrome has been reported in non-obese, non-diabetic Japanese subjects [Citation22]. This is possibly true in non-obese diabetic subjects. Fukuda et al [Citation23] reported increased systemic arteriosclerosis measured by ultrasonography and lower adiponectin levels in non-obese Japanese diabetic patients who had visceral fat accumulation compared with those without. Reduction of visceral fat, independent of body weight or abdominal subcutaneous fat, resulted in lowered triglyceride and increased HDL cholesterol levels in 1,106 non-obese, non-diabetic Japanese men [Citation24].
The existing evidence strongly support the role of visceral or intra-abdominal fat in the development of insulin resistance and cardiometabolic risk in non-obese Asian type 2 diabetic patients.
Subcutaneous fat
In general, adipose tissues of healthy subjects have preadipocytes or adipose stem cells residing in stromal vascular fraction which can accommodate positive energy balance in the form of triglyceride. These cells respond to surplus energy through processes of proliferation or expansion so called adipocyte hyperplasia or adipocyte hypertrophy, respectively. Preadipocytes of subcutaneous adipose tissue replicate and differentiate into mature adipocytes (adipocyte hyperplasia) more efficiently than those of visceral depot [Citation25]. The mature adipocyte has its maximum capacity for expansion which is determined by genetic, gender or certain hormones [Citation25–Citation27]. The study by Sato et al [Citation28] in non-diabetic Japanese adults indicates that the maximum storage capacity of abdominal subcutaneous fat occurs at BMI of 23–24 kg/m2 in women and 24–25 kg/m2 in men. Once the adipocyte expansion limit is reached, lipid cannot be stored further and spilled over to other adipose and non-adipose tissues [Citation29–Citation31]. The significant correlation of visceral fat mass and abdominal subcutaneous adipocyte size observed in Tchoukalova et al study in non-diabetic Caucasian population supports this notion [Citation32]. However, the adipocyte capacity to store lipid has inter-depot difference. With positive energy balance, the adipocytes of gluteofemoral depot shows a proliferative responses whereas those of abdominal subcutaneous depot has adipocyte hypertrophy [Citation33,Citation34]. Hence the gluteofemoral depot can accommodate lipid more effectively than the abdominal subcutaneous depot [Citation32,Citation35]. The hypertrophic adipocyte of abdominal subcutaneous depot by itself can activate macrophages, produces pro-inflammatory cytokines and insulin resistance [Citation36]. The abdominal subcutaneous adipocyte size has been shown to be positively correlated with cardiovascular risk factors which include plasma glucose, lipid and blood pressure in non-diabetic and diabetic subjects independent of gender, age and body weight [Citation37]. Thiazolidinedione, a peroxisome proliferator activated receptor-γ agonist, reduces insulin resistance and improve metabolic dysfunction by stimulating preadipocytes to differentiate into mature adipocytes [Citation38]. It makes more lipids stored in mature adipocytes and reduces ectopic lipid deposition in other tissues. Therefore, the high capability of subcutaneous adipose tissue to accommodate lipid has a protective effect against lipotoxicity and metabolic disorders [Citation39]. The more in-depth review of this topic can be read elsewhere [Citation40,Citation41]. Although several genes has been demonstrated to be involved in body fat distribution in human obesity but their functions remain elusive [Citation27,Citation42–Citation45]. Whether there is a defect of adipocyte-regulated gene expression or function in subcutaneous adipose tissue of non-obese Asian diabetic subjects is unknown.
The study in a large number of non-diabetic Swedish men and women by Andersson et al [Citation27] indicated that abdominal subcutaneous fat cell numbers increased in proportion with increasing abdominal subcutaneous fat mass over the BMI ranges of 18 -62 kg/m2 but the much larger fat cell size (adipocyte hypertrophy) was significantly increased only in men with BMI <30 kg/m2. The increased fat cell size was independently associated with insulin resistance in both men and women but was steeper in men. Alligier et al [Citation46] studied the concurrent changes of abdominal subcutaneous and visceral fat after 56 days of high-fat feeding in 41 non-obese, non-diabetic French men who had no family history of diabetes and observed the large individual variation of the relative distribution of visceral and abdominal subcutaneous fat depots. About 50% of subjects experienced a marked increase of visceral fat deposition whereas others did not. Gene expression analysis from abdominal subcutaneous fat indicated the reduced induction of gene involved in triglyceride synthesis in subcutaneous fat of those men who had visceral fat increased. Anand et al [Citation47] studied adipocyte size from abdominal subcutaneous fat of healthy non-obese South Asian women compared with those of obese Canadian women (BMI ∼29 kg/m2). They found that total abdominal subcutaneous fat areas was not different between South Asians and Canadians but adipocyte size of South Asian women was significantly larger and liver fat content was significantly higher despite smaller BMI. The larger adipocyte size was partially accounted for the higher degree of insulin resistance, the lower HDL cholesterol and adiponectin levels observed in non-obese South Asian women. Chandalia et al [Citation48] studied the relationships of body fat distribution and adipocyte size of abdominal subcutaneous depot with insulin resistance in non-obese, non-diabetic South Asian men compared with those of age- and BMI-matched Caucasian men. They found that South Asian men had greater abdominal subcutaneous fat mass, larger adipocyte size and higher insulin resistance (measured by clamp method) than Caucasian men despite similar amount of visceral fat. Adipocyte size was positively correlated with insulin resistance independent of total body fat, abdominal subcutaneous fat or visceral fat mass. In contrast, Meena et al [Citation49] reported omental adipocyte size, although smaller than abdominal subcutaneous adipocyte size, was stronger correlated with HOMA-IR in non-obese, non-diabetic Asian Indian men. However, the relationship was attenuated after adjusting for measures of adiposity. The differences of study design and methods of measurement may explain the discordant results between these studies. Furthermore since body fat distribution particularly of abdominal subcutaneous depot is different among Asian populations [Citation11,Citation50,Citation51], the contribution of the abdominal subcutaneous adipocyte size to systemic insulin resistance in other Asian populations needs to be investigated.
The studies in non-obese diabetic patients or diabetes-prone subjects also support the role of abdominal subcutaneous adipocyte hypertrophy in the generation of insulin resistance. However none of the study was done in Asian diabetic populations. Acosta et al [Citation52] studied abdominal subcutaneous fat of 14 non-obese Caucasian diabetic men and 13 healthy controls and reported significantly larger fat cell volume in diabetic men. The increased fat cell volume was correlated with insulin resistance, lipolysis and TNF-α secretion independent of diabetes status. The expression of gene involving in adipogenesis and adipocyte morphology was inversely correlated with fat cell size in this study. This may indicate the attenuated differentiation capacity of preadipocytes in non-obese diabetic subjects. The studies from a small number of non-obese 1st-degree relatives of Caucasian parents with type 2 diabetes by Hammarsted et al [Citation53] and Henninger et al [Citation54] also showed adipocyte hypertrophy in these diabetes-prone relatives in both men and women compared with age-, sex- and BMI-matched controls who had no history of diabetes in parents. Abdominal subcutaneous adipocyte hypertrophy, independent of waist-hip ratio was associated with insulin resistance and inflammatory markers. However, there was no difference of gene expression of adipogenesis regulator between the groups in this study. These findings indicate that abdominal subcutaneous adipocyte hypertrophy is present long before diabetes develops and contributes to insulin resistance and inflammatory state in non-obese diabetic and non-diabetic subjects independent of total fat mass. The association of adipocyte hypertrophy and insulin resistance is also observed not only in non-obese but also in obese diabetic subjects [Citation55].
These findings support the hypothesis of defective abdominal subcutaneous adipose tissue storage capacity in the development of insulin resistance and its association with excessive visceral or ectopic lipid deposition in non-obese diabetic patients or predisposed-to-diabetes subjects. It is plausible that the defect of abdominal subcutaneous adipose tissue storage capacity could have been observed in non-obese diabetic patients of Asian populations. The study dedicated for this specific population should be performed.
Role of ectopic lipid deposition in non-obese Asian type 2 diabetic patients
Intra-hepatic fat
The prevalence of non-alcoholic fatty liver disease (NAFLD) in non-diabetic subjects who have BMI <25 kg/m2 in Asia-Pacific region is ∼15-21% [Citation56]. The study in ∼2,000 non-diabetic Indian population demonstrated that 75% of subjects with NAFLD had BMI <25 kg/m2 and 54% of which had no abdominal obesity [Citation57]. Data from non-obese, East Asian (Chinese, Japanese, Korean) men and women, of which 50–60% had type 2 diabetes indicated similarly high prevalence of fatty liver measured by CT imaging. The prevalence of fatty liver disease in these populations was higher than those of Caucasian or Hispanic populations despite much lower BMI [Citation11,Citation58]. Likewise, the prevalence of NAFLD measured by MRI in non-obese Asian Indians with type 2 diabetes was ∼50% whereas it was found in only 2% in BMI-matched non-diabetic controls [Citation13]. There was a strong positive relationship of visceral and intra-hepatic fat content in these non-obese Asians. The high free fatty acid in portal circulation from the increased visceral fat lipolysis increases fatty acid esterification and hepatic fat content [Citation59].
It is well known that the presence of intra-hepatic fat is strongly associated with hepatic insulin resistance and perhaps with muscle insulin resistance in obese, non-diabetic and diabetic subjects [Citation60,Citation61]. Intra-hepatic fat is associated with insulin resistance in both liver and muscle in non-obese, non-diabetic Japanese men [Citation62]. Sharma et al [Citation63] reported higher hepatic gluconeogenesis and insulin resistance in non-obese, non-diabetic Asian Indian men with NAFLD than those of age and BMI-matched men without NAFLD. The studies of the relationships of intra-hepatic fat and insulin resistance in non-obese diabetic patients in Asian population are relatively few. Furukawa et al [Citation64] studied 29 non-obese Japanese diabetic patients and found that 7 had increased liver fat. Patients who had high liver fat had more peripheral insulin resistance than those without although no difference of visceral fat area was observed. The studies in Caucasian populations give similar findings. Hwang et al [Citation65] observed the inverse relationship of intra-hepatic fat content and peripheral insulin sensitivity which was stronger than that of visceral fat in twelve non-obese, non-diabetic Caucasian subjects. Likewise, Gastaldelli et al [Citation66] reported similar amount of intra-hepatic fat content in 24 non-obese Caucasian diabetic patients compared with 19 obese patients despite significantly higher amount of visceral fat in the latter. The inverse correlation with peripheral insulin sensitivity was stronger with the amount of intra-hepatic fat than that of visceral fat. How intra-hepatic fat causes systemic insulin resistance independent of visceral fat is intriguing. Some studies indicated that, after high carbohydrate meal, skeletal muscle insulin resistance can promote hepatic lipogenesis by diverting away glucose from muscle glycogen storage to triacylglycerol synthesis in the liver [Citation67,Citation68]. On the other hand, chronic inflammation of the liver secondary to fat accumulation may increase several inflammatory mediators such as interleukin (IL) and TNF and induces peripheral insulin resistance in the muscle [Citation69]. TNF-α can reduce expression of glucose transporter type 4 (GLUT4) which is important for insulin-dependent glucose uptake at adipocytes and muscle cells [Citation70,Citation71]. It also induces serine phosphorylation of insulin receptor substrate (IRS)-1 and inhibits downstream signaling of phosphatidylinositol-3-kinase [Citation71]. IL-6 induces insulin resistance by impairing the phosphorylation of IRS-1 [Citation70,Citation72]. The presence of these inflammatory mediators has been recently shown to predict the development of insulin resistance and type 2 diabetes in non-obese Finnish men [Citation73]. Intra-hepatic fat, independent of visceral fat, has been shown to be associated with numbers of the cardiometabolic risk in diabetic patients [Citation74].
It should be noted that the evidence of the positive relationships of intra-hepatic fat, insulin resistance and cardiometabolic risk in non-obese Asian populations are largely from non-diabetic subjects, it is speculated that such relationships could be observed in diabetic patients as well.
Intra-myocellular lipid (IMCL)
In human physiology, the over-supply of fatty acids from high fat diet or increased adipose tissue lipolysis is transported into muscle cells by fatty acid transporters, after which it can be oxidized in mitochondria (β-oxidation) or accumulated as intramuscular triacylglycerol in the form of lipid droplets or being metabolized to lipid intermediates [Citation75]. The association of IMCL and muscle insulin resistance has previously been demonstrated in obese, non-diabetic and diabetic subjects as well as lean, non-diabetic offspring of type 2 diabetic subjects. Jacob et al [Citation76], Perseghin et al [Citation77] and Lattuada et al [Citation78] independently reported a significantly increased IMCL content measured by magnetic resonance spectroscopy (MRS) in soleus and tibialis anterior muscles of non-obese, insulin-resistant offspring of type 2 diabetic subjects compared with age- and BMI-matched, insulin sensitive controls. However, only Jacob and Perseghin, not Lattuada demonstrated the strong relationship of IMCL content and insulin resistance. The study of IMCL content and insulin resistance in non-obese Asian diabetic patients is rare. Misra et al [Citation79] reported two times higher amount of IMCL in ten non-obese Indian diabetic men compared with sex and BMI-matched healthy control but the relationship of IMCL content and insulin resistance could not be demonstrated. This result in Asian population contradicts those results from Caucasians. It has been known that IMCL can be affected by several factors other than obesity and it is not always associated with insulin resistance. It can be depleted with an acute bout of exercise or can be increased and served as fuel supply in insulin-sensitive endurance exercise training athletes, a phenomenon referred to as “athletes paradox” [Citation80,Citation81]. This may be one of the reasons to explain the inconsistent relationship of IMCL and insulin resistance. Furthermore IMCL can possibly be the result of reduced muscle mitochondrial oxidative capacity of type 2 diabetes although recent evidences prove that this is unlikely to be the case [Citation82–Citation84].
Currently it is believed that the presence of IMCL per se is not the direct cause of muscle insulin resistance. Lipid droplet number and size, the amount of lipid intermediates particularly diacylglycerol and ceramides as well as their subtypes and their cellular location (subsarcolemmal or cytosol) are more important than the amount and may directly involve in muscle insulin resistance of obesity and type 2 diabetes [Citation85]. However, it needs many more studies to understand how they can interfere with skeletal muscle glucose uptake in human physiology. Whether these lipid intermediates or its droplet size and number are altered in non-obese diabetic subjects is unknown. The in-depth review of the role of IMCL and insulin resistance as well as the proposed mechanism of the “athletes paradox” can be read from the references [Citation80,Citation81,Citation86].
Intra-pancreatic fat
In contrast with the extensive studies of other regional fat, intra-pancreatic fat has received least attention in the past due to the limitation of investigation particularly with the non-invasive tools. However, with the recent introduction of MRS, the studies of the association of intra-pancreatic fat and metabolic disturbances in healthy and diseased subjects has rapidly emerging [Citation87]. Intra-pancreatic fat measured by MRS includes peri-pancreatic, inter-lobular and intra-lobular adipose tissue and parenchymal fat. Nevertheless, it cannot distinguish the lipid accumulation within acinar and β-cell [Citation88]. Although the presence of intra-pancreatic fat is commonly observed in both obese non-diabetic and diabetic subjects but it is also present in non-obese diabetic subjects [Citation89,Citation90]. By using 6.2% as a cut-off upper limit of pancreatic fat content, the prevalence of intra-pancreatic fat or “Non-alcoholic fatty pancreas disease” (NAFPD) measured by MRS in 1,209 healthy individuals is 33% [Citation91]. The prevalence of NAFPD assessed by trans-abdominal ultrasonoghraphy in non-obese, non-diabetic Asian populations was 16–35% [92−94]. The prevalence of NAFPD in non-obese Asian diabetic patients is unknown. It was estimated to be 50% by CT in one study from 98 non-obese Korean diabetic patients [Citation90].
Total intra-pancreatic fat content has been shown to be increased in diabetic compared with BMI-matched, non-diabetic subjects in some but not all studies [88,95−97]. The inconsistency may be explained by the difference of age, tools of measurement and degree of obesity. The recent pathological study in non-obese Japanese subjects indicated no difference of intra-pancreatic fat content between diabetic and non-diabetic subjects [Citation98]. The meta-analysis from a large number of subjects by Singh et al [Citation91] indicated that the presence of intra-pancreatic fat was significantly associated with 2 folds higher risk of diabetes mellitus. Nevertheless the long-term follow-up study contradicts this result. Yamazaki et al [Citation99] followed 813 non-obese, non-diabetic Japanese subjects for 5 years and found no independent association of pancreatic fat (measured by CT attenuation) and incidence of diabetes after adjustment with BMI and intra-hepatic fat content. Since intra-pancreatic fat content is increased with increasing BMI and/or intra-hepatic fat content [Citation96,Citation100] and the latters are strongly associated with diabetes, it is possible that the result of Singh et al study may be falsely positive.
The in-vitro and animal studies indicate that prolonged exposure of human β-cell to free fatty acid or high fat diet respectively can induce β-cell apoptosis and results in β-cell dysfunction [Citation101,Citation102]. It is speculated that the accumulated intra-pancreatic fat may play a role in the development of diabetes in this regard. However, the clinical studies in human gave disappointing results. Total intra-pancreatic fat content is not associated with β-cell function at least in obese glucose intolerance or diabetic subjects in several studies [88,92−96,103]. The morphological study in non-obese subjects by Murakami et al [Citation98] indicated that although fractional β-cell areas was significantly decreased in diabetic compared with age- and BMI-matched non-diabetic subjects but there was no difference of intra-pancreatic fat areas between two groups. Furthermore intra-pancreatic fat was not observed within the islets and its content was not associated with the frequency of β-cell replication, β-cell apoptosis or mean islet size.
The evidence so far demonstrate that intra-pancreatic fat may be an innocent bystander and has no or little influence on β-cell function in human.
Conclusions
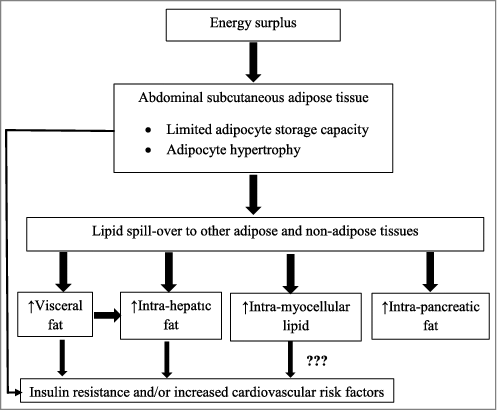
These data indicate that the total body fat content of non-obese Asian type 2 diabetic patients is not different from non-diabetic counterpart but the proportion of visceral fat and intra-hepatic fat is greater. It appears that the increased amount of visceral fat in combination with the increase of liver fat contribute to the development of insulin resistance and the increase of cardiovascular risk in non-obese type 2 diabetes in Asian populations as shown in . The hypertrophic abdominal subcutaneous adipocytes has been shown to contribute to insulin resistance and cardiovascular risk in non-obese, non-diabetic Asian populations as well as in non-obese Caucasian diabetic subjects. This could have been true in non-obese Asian diabetic populations but requires further more study. The role of intra-myocellular lipid and muscle insulin resistance is still uncertain. Intra-pancreatic fat may not be involved in β cell dysfunction in non-obese Asian diabetic population.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed
References
- Kashima S, Inoue K, Matsumoto M, et al. Prevalence and characteristics of non-obese diabetes in Japanese men and women: the Yuport Medical Checkup Center Study. J Diabetes. 2015;7:523–530. doi:10.1111/1753-0407.12213.
- Aekplakorn W. Prevalence, treatment, and control of metabolic risk factors by BMI status in Thai adults: National Health Examination Survey III. Asia Pac J Pub Health. 2011;23:298–306. doi:10.1177/1010539509340690.
- Huxley R, James WPT, Barzi F, et al. Ethnic comparisons of the cross-sectional relationships between measures of body size with diabetes and hypertension. Obes Rev. 2008;9(Suppl 1):53–61. doi:10.1111/j.1467-789X.2007.00439.x.
- Chan WB, Tong PCY, Chow CC, et al. The associations of body mass index, C-peptide and metabolic status in Chinese type 2 diabetic patients. Diabet Med. 2004;21:349–353. doi:10.1111/j.1464-5491.2004.01158.x.
- Chan JCN, Malik V, Jia W, et al. Diabetes in Asia. Epidemiology, risk factors and pathophysiology Lancet. 2009;301:2129–2140.
- Yokoyama H, Emoto M, Fujiwara S, et al. Quantitative insulin sensitivity check index and the reciprocal index of homeostasis model assessment in normal range weight and moderately obese type 2 diabetic patients. Diabetes Care. 2003;26:2426–2432. doi:10.2337/diacare.26.8.2426.
- Kim D-J, Lee M-S, Kim K-W, et al. Insulin secretory dysfunction and insulin resistance in the pathogenesis of Korean type 2 diabetes mellitus. Metabolism. 2001;50:590–593. doi:10.1053/meta.2001.22558.
- Rosalind Marita A, Sarkar JA, Rane S. Type 2 diabetes in non-obese Indian subjects is associated with reduced leptin levels: Study from Mumbai, Western India. Mol Cell Biochem. 2005;275:143–151. doi:10.1007/s11010-005-1204-7.
- WHO, IASO. IOTF. The Asia Pacific perspective: Redefining obesity and its treatment. Melbourne: International Diabetes Institute; 2000.
- Wronska A, Kmiec Z. Structural and biochemical characteristics of various white adipose tissue depots. Acta Physiol. 2012;205:194–208. doi:10.1111/j.1748-1716.2012.02409.x.
- Nazare J-A, Smith JD, Borel J-L, et al. Ethnic influences on the relations between abdominal subcutaneous and visceral adiposity, liver fat and cardiometabolic risk profile: the International Study of Prediction of Intra-abdominal Adiposity and Its Relationships With Cardiometabolic Risk/Intra-abdominal Adiposity. Am J Clin Nutr. 2012;96:714–726. doi:10.3945/ajcn.112.035758.
- Jang Y, Lee JH, Cho EY, et al. Differences in body fat distribution and antioxidant status in Korean men with cardiovascular disease with or without diabetes. Am J Clin Nutr. 2001;73:68–74.
- Misra A, Anoop S, Gulati S, et al. Body fay patterning, hepatic fat and pancreatic volume of non-obese Asian Indians with type 2 diabetes in North India: a case-control study. PLoS ONE. 2015;10:e0140447. doi:10.1371/journal.pone.0140447.
- Bu J, Feng Q, Ran J, et al. Visceral fat mass is always, but adipokines (adiponectin and resistin) are diversely associated with insulin resistance in Chinese type 2 diabetic and normoglycemic subjects. Diabetes Res Clin Pract. 2012;96:163–169. doi:10.1016/j.diabres.2011.12.014.
- Jung SH, Ha KH, Kim DJ. Visceral fat mass has stronger associations with diabetes and prediabetes than other anthropometric obesity indicators among Korean adults. Yonsei Med J. 2016;57:674–680. doi:10.3349/ymj.2016.57.3.674.
- Boyko EJ, Fujimoto WY, Leonetti DL, et al. Visceral adiposity and risk of type 2 diabetes. A prospective study among Japanese Americans Diabetes Care. 2000;23:465–471.
- Kim C-H, Kim H-K, Kim E-H, et al. Impact of body mass index in predictive ability of body fat distribution for type 2 diabetes risk in Koreans. Diabetic Med. 2012;29:1395–8. doi:10.1111/j.1464-5491.2012.03661.x.
- Rattarasarn C, Leelawattana R, Soonthornpun S, et al. Regional abdominal fat distribution in lean and obese Thai type 2 diabetic women: Relationships with insulin sensitivity and cardiovascular risk factors. Metabolism. 2003;52:1444–1447. doi:10.1016/S0026-0495(03)00257-9.
- Rattarasarn C, Leelawattana R, Soonthornpun S, et al. Relationships of body fat distribution, insulin sensitivity and cardiovascular risk factors in lean, healthy non-diabetic Thai men and women. Diabetes Res Clin Pract. 2003;60:87–94. doi:10.1016/S0168-8227(03)00017-2.
- Indulekha K, Anjana RM, Surendar J, et al. Association of visceral and subcutaneous fat with glucose intolerance, insulin resistance, adipocytokines and inflammatory markers in Asian Indians(CURES-113). Clin Biochem. 2011;44:281–7. doi:10.1016/j.clinbiochem.2010.12.015.
- Tang L, Zhang F, Tong N. The association of visceral adipose tissue and subcutaneous adipose tissue with metabolic risk factors in a large population of Chinese adults. Clin Endocrinol. 2016;85:46–53. doi:10.1111/cen.13013.
- Tatsumi Y, Nakao YM, Masuda I, et al. Risk for metabolic diseases in normal weight individuals with visceral fat accumulation: A cross-sectional study in Japan. BMI Open. 2017;7:e013831. doi:10.1136/bmjopen-2016-013831.
- Fukuda S, Hirata A, Nishizawa H, et al. Systemic arteriosclerosis and eating behavior in Japanese patients with visceral fat accumulation. Cardiovasc Diabetol. 2015;14:8. doi:10.1186/s12933-015-0174-7.
- Matsushita Y, Nakagawa T, Yamamoto S, et al. Effect of longitudinal changes in visceral fat area and other anthropometric indices to the changes in metabolic risk factors in Japanese men. The Hitachi Health Study Diabetes Care. 2012;35:1129–43.
- Cleal L, Aldea T, Chau Y-Y. Fifty shades of white: Understanding heterogeneity in white adipose stem cells. Adipocyte. 2017;6:205–216. doi:10.1080/21623945.2017.1372871.
- Andersson DP, Arner E, Hogling DE, et al. Abdominal subcutaneous adipose tissue cellularity in men and women. Int J Obes. 2017;41:1564–1569. doi:10.1038/ijo.2017.148.
- Dahlman I, Ryden M, Brodin D, et al. Numerous genes in loci associated with body fat distribution are linked to adipose function. Diabetes. 2016;65:433–437. doi:10.2337/db15-0828.
- Sato S, Demura S, Nakai M. Storage capacity of subcutaneous fat in Japanese adults. Eur J Clin Nutr. 2015;69:933–938. doi:10.1038/ejcn.2014.292.
- Arner P, Bernard S, Salehpour M, et al. Dynamics of human adipose lipid turnover in health and metabolic disease. Nature. 2011;478:110–113. doi:10.1038/nature10426.
- Lafontan M. Adipose tissue and adipocyte dysregulation. Diabetes Metab. 2014;40:16–28. doi:10.1016/j.diabet.2013.08.002.
- Sniderman AD, Bhopal R, Prabhakaran D, et al. Whu might South Asians be so susceptible to central obesity and its atherogenic consequences? The adipose tissue overflow hypothesis. Int J Eptdemiol. 2007;36:220–225. doi:10.1093/ije/dyl245.
- Tchoukalova YD, Koutsari C, Karpyak MV, et al. Subcutaneous adipocyte size and body fat distribution. Am J Clin Nutr. 2008;87:56–63.
- Monolopoulos KN, Karpe F, Frayn KN. Gluteofemoral body fat as a determinant of metabolic health. Int J Obes. 2010;34:949–959. doi:10.1038/ijo.2009.286.
- Karpe F, Pinnick KE. Biology of upper-body and lower-body adipose tissue- Link to whole-body phenotype. Nature Rev Endocrinol. 2015;11:90–100. doi:10.1038/nrendo.2014.185.
- Todorcevic M, Hilton C, McNeil C, et al. A cellular model for the investigation of depot specific human adipocyte biology. Adipocyte. 2017;6:40–55. doi:10.1080/21623945.2016.1277052.
- Reilly SM, Satiel AR. Adapting to obesity with adipose tissue inflammation. Nature Rev Endocrinol. 2017;13:633–643. doi:10.1038/nrendo.2017.90.
- Ryden M, Arner P. Cardiovascular risk score is linked to subcutaneous adipocyte size and lipid metabolism. J Intern Med. 2017;282:220–228. doi:10.1111/joim.12641.
- Soccio RE, Chen ER, Lazar MA. Thiazolidinediones and the promise of insulin sensitization in type 2 diabetes. Cell Metab. 2014;20:573–591. doi:10.1016/j.cmet.2014.08.005.
- Fryan KN. Adipose tissue as a buffer for daily lipid flux. Diabetologia. 2002;45:1201–1210. doi:10.1007/s00125-002-0873-y.
- Pellegrinelli V, Carobbio S, Vidal-Puig A. Adipose tissue plasticity: how fat depots respond differently to pathophysiological cues. Diabetologia. 2016;59:1075–1088. doi:10.1007/s00125-016-3933-4.
- Thomas D, Apovian C. Macrophage functions in lean and obese adipose tissues. Metabolism. 2017;72:120–143. doi:10.1016/j.metabol.2017.04.005.
- Schleinitz D, Bottcher Y, Bluher M, et al. The genetics of fat distribution. Diabetologia. 2014;57:1276–1286. doi:10.1007/s00125-014-3214-z.
- Gesta S, Bluher M, Yamamoto Y, et al. Evidences for a role of developmental genes in the origin of obesity and body fat distribution. Proc Natl Acad Sci U S A. 2006;103:6676–6681. doi:10.1073/pnas.0601752103.
- Chu AY, Deng X, Fisher VA, et al. Multi-ethnic genome-wide meta-analysis of ectopic fat depots identifies loci associated with adipocyte development and differentiation. Nat Genet. 2017;49:125–130. doi:10.1038/ng.3738.
- Shungin D, Winkler TW, Croteau-Chonka DC, et al. New genetic loci link adipose tissue and insulin biology to body fat distribution. Nature. 2015;518:187–196. doi:10.1038/nature14132.
- Alligier M, Gabert L, Meugnier E, et al. Visceral fat accumulation during lipid overfeeding is related to subcutaneous adipose tissue characteristics in healthy men. J Clin Endocrinol Metab. 2013;98:802–810. doi:10.1210/jc.2012-3289.
- Anand SS, TarnopolskyMA, Rashid S, et al. Adipocyte hypertrophy, fatty liver and metabolic risk factors in South Asians: The Molecular Study of Health and risk in Ethnic Groups (mol-SHARE). PLoS One. 2011;6: e22112. doi:10.1371/journal.pone.0022112.
- Chandalia M, Lin P, Seenivasan T, et al. Insulin resistance and body fat distribution in South Asian men compared with Caucasian men. PLoS ONE. 2007;2:e812. doi:10.1371/journal.pone.0000812.
- Meena VP, Seenu V, Sharma MC, et al. Relationship of adipocyte size and metabolic risk factors in Asian Indians. PLoS ONE. 2014;9:e108421. doi:10.1371/journal.pone.0108421.
- Kohli S, Lear SA. Differences in subcutaneous abdominal adiposity regions in four ethnic groups. Obesity. 2013;21:2288–2295. doi:10.1002/oby.20102.
- Khoo CM, Leow MK, Sadananthan SA, et al. Body fat partitioning does not explain the interethnic variation in insulin sensitivity among Asian ethnicity: the Singapore adults metabolism study. Diabetes. 2014;63:1093–1102. doi:10.2337/db13-1483.
- Acosta JR, Douagi I, Andersson DP, et al. Increased fat cell size: a major phenotype of subcutaneous white adipose tissue in non-obese individuals with type 2 diabetes. Diabetologia. 2016;59:560–570. doi:10.1007/s00125-015-3810-6.
- Hammarstedt A, Graham TE, Kahn BB. Adipose tissue dysregulation and reduced insulin sensitivity in non-obese individuals with enlarged abdominal adipose cells. Diabetology Metab Syndr. 2012;4:42. doi:10.1186/1758-5996-4-42.
- Henninger AMJ, Eliasson B, Jenndahl LE, et al. Adipocyte hypertrophy, inflammation and fibrosis characterize subcutaneous adipose tissue of healthy, non-obese subjects predisposed to type 2 diabetes. PLos One. 2014;9(8): e105260. doi:10.1371/journal.pone.0105262.
- McLaughlin T, Lamendola C, Coghlan N, et al. Subcutaneous adipose cell size and distribution: Relationship to insulin resistance and body fat. Obesity. 2014;22:673–680. doi:10.1002/oby.20209.
- Liu C-J. Prevalence and risk factors for non-alcoholic fatty liver disease in Asian people who are not obese. J Gastroenterol Hepatol. 2012;27:1555–1560. doi:10.1111/j.1440-1746.2012.07222.x.
- Das K, Das K, Mukherjee PS, et al. Nonobese population in a developing country had a high prevalence of nonalcoholic fatty liver and significant liver disease. Hepatology. 2010;51:1593–1602. doi:10.1002/hep.23567.
- Ha Y, Seo N, Shim JH, et al. Intimate association of visceral obesitywith non-alcoholic fatty liver disease in healthy Asians: A case-control study. J Gastroenterol Hepatol. 2015;30:1666–1672. doi:10.1111/jgh.12996.
- Kabir M, Catalano KJ, Ananthnarayan S, et al. Molecular evidence supporting the portal theory: a causative link between visceral adiposity and insulin resistance. Am J Physiol Endocrinol Metab. 2005;288:E454–E461. doi:10.1152/ajpendo.00203.2004.
- Kato K, Takamura T, Takeshita Y, et al. Ectopic fat accumulation and distant organ-specific insulin resistance in Japanese people with nonalcoholic fatty liver disease. PloS ONE. 2014;9:e92170. doi:10.1371/journal.pone.0092170.
- Perry RJ, Samuel VT, Petersen KF, et al. The role of hepatic lipids in hepatic insulin resistance and type 2 diabetes. Nature. 2014;510:84–91. doi:10.1038/nature13478.
- Takeno K, Tamura Y, Kawaguchi M, et al. Relation between insulin sensitivity and metabolic abnormalities in Japanese men with BMI of 23–25 kg/m2. J Clin Endocrinol Metab. 2016;101:3676–3684. doi:10.1210/jc.2016-1650.
- Sharma R, Sinha S, Danishad KA, et al. Investigation of hepatic gluconeogenesis pathway in non-diabetic Asian Indians with non-alcoholic fatty liver disease using in vivo (31P) phosphorus magnetic resonance spectroscopy. Atherosclerosis. 2009;203:291–297. doi:10.1016/j.atherosclerosis.2008.06.016.
- Furukawa Y, Tamura Y, Takeno K, et al. Impaired peripheral insulin sensitivity in non-obese Japanese with type 2 diabetes mellitus and fatty liver. J Diabetes Invest 2017 [Aug 24]; doi:10:1111/jdi12731.
- Hwang J-H, Stein DT, Barzilai N, et al. Increased intrahepatic triglycerides associated with peripheral insulin resistance: in vivo MR imaging and spectroscopy studies. Am J Physiol Endocrinol Metab. 2007;293:E1663–E1669. doi:10.1152/ajpendo.00590.2006.
- Gastaldelli A, Cusi K, Pettiti M, et al. Relationship between hepatic/visceral fat and hepatic insulin resistance in nondiabetic and type 2 diabetic subjects. Gastroenterology. 2007;133:496–506. doi:10.1053/j.gastro.2007.04.068.
- Petersen KF, Dufour S, Savage DB, et al. The role of skeletal muscle insulin resistance in the pathogenesis of the metabolic syndrome. Proc Natl Acad Sci USA. 2007;104:12587–12594. doi:10.1073/pnas.0705408104.
- Rabol R, Petersen KF, Dufour S, et al. Reversal of muscle insulin resistance with exercise reduces postprandial hepatic de novo lipogenesis in insulin resistant individuals. Proc Natl Acad Sci USA. 2011;108:13705–13709. doi:10.1073/pnas.1110105108.
- Cai D, Yuan M, Frantz DF, et al. Local and systemic insulin resistance resulting from hepatic activation of IKK-β and NF-kB. Nat Med. 2005;11:183–190. doi:10.1038/nm1166.
- Jaganathan R, Ravindran R, Dhanasekaran S. Emerging role of adipocytokines in type 2 diabetes as mediators of insulin resistance and cardiovascular disease. Can J Diabetes. 2017; [Dec 8]; doi:10.1016/j.jcjd.2017.10.040.
- Akash MSH, Rehman K, Liaqat A. Tumor necrosis factor-alpha: Role in development of insulin resistance and pathogenesis of type 2 diabetes mellitus. J Cell Biochem. 2018;119:105–110. doi:10.1002/jcb.26174.
- Rehman K, Akash MSH, Liaqat A, et al. Role of interleukin-6 in development of insulin resistance and type 2 diabetes mellitus. Crit Rev Eukaryot Gene Expr. 2017;27:229–236. doi:10.1615/CritRevEukaryotGeneExpr.2017019712.
- Fizelova M, Jauhiainen R, Kangas AJ, et al. Differential associations of inflammatory markers with insulin sensitivity and secretion: The prospective METSIM Study. J Clin Endocrinol Metab. 2017;102:3600–3609. doi:10.1210/jc.2017-01057.
- Targher G, Bertolini L, Rodella S, et al. NASH predicts plasma inflammatory biomarkers independently of visceral fat in men. Obesity. 2008;16:1394–1399. doi:10.1038/oby.2008.64.
- Kitessa SM, Abeywardena MY. Lipid-induced insulin resistance in skeletal muscle: The chase for the culprit goes from total intramuscular fat to lipid intermediates, and finally to species of lipid intermediates. Nutrients. 2016;8:466. doi:10.3390/nu8080466.
- Jacob S, Machann J, Rett K, et al. Association of increased intramyocellular lipid content with insulin resistance in lean nondiabetic offspring of type 2 diabetic subjects. Diabetes. 1999;48:1113–1119. doi:10.2337/diabetes.48.5.1113.
- Perseghin G, Scifo P, De Cobelli F, et al. Intramyocellular triglyceride content is a determinant of in vivo insulin resistance in humans. A 1H-13C nuclear magnetic resonance spectroscopy assessment in offspring of type 2 diabetic parents. Diabetes. 1999;48:1600–1606. doi:10.2337/diabetes.48.8.1600.
- Lattuada G, Costantino F, Caumo A, et al. Reduced whole-body lipid oxidation is associated with insulin resistance, but not with intramyocelllar lipid content in offspring of type 2 diabetic patients. Diabetologia. 2005;48:741–747. doi:10.1007/s00125-005-1686-6.
- Misra A, Sinha S, Kumar M, et al. Proton magnetic resonance spectroscopy study of soleus muscle in non-obese healthy and type 2 diabetic Asian Northern Indian male: high intramyocellular lipid content correlates with excess body fat and abdominal obesity. Diabet Med. 2003;20:361–367. doi:10.1046/j.1464-5491.2003.00932.x.
- Gemmink A, Goodpaster BH, Schrauwen P, et al. Intramyocellular lipid droplets and insulin sensitivity, the human perspective. Mol Cell Biol Lipids. 2017;1862:1242–1249. doi:10.1016/j.bbalip.2017.07.010.
- Bergman BC, Perreault L, Strauss A, et al. Intramuscular triglyceride synthesis- importance in partitioning muscle lipids in humans. Am J Physiol Endocrinol Metab 2017 [Oct 3]; DOI:. 10:1152.
- De Feyter HM, van den Broek NM, Praet SF, et al. Early or advanced stage type 2 diabetes is not accompanied by in vivo skeletal muscle mitochondrial dysfunction. Eur J Endocrinol. 2008;158:643–653. doi:10.1530/EJE-07-0756.
- Lai N, Kummitha C, Hoppel C. Defects in skeletal muscle subsarcolemmal mitochondria in a non-obese model of type 2 diabetes mellitus. PLoS One. 2017;12:e0183978. doi:10.1371/journal.pone.0183978.
- Callahan ZJ, Oxendine MJ, Schaeffer PJ. Intramuscular triglyceride content precedes impaired glucose metabolism without evidences for mitochondrial dysfunction during early development of a diabetic phenotype. Appl Physiol Nutr Metab. 2017;42:963–972.
- Chaurasia B, Summers SA. Ceramides-lipotoxic inducers of metabolic disorders. Trend Endocrinol Metab. 2015;26:538–550. doi:10.1139/apnm-2016-0685. doi:10.1016/j.tem.2015.07.006.
- Brons C, Grunnet LG. Skeletal muscle lipotoxicity in insulin resistance and type 2 diabetes: a causal mechanism or an innocent bystander? Eur J Endocrinol. 2017;176:R67.–R78. doi:10.1530/EJE-16-0488.
- Lingvay I, Esser V, Legendre JL, et al. Noninvasive quantification of pancreatic fat in humans. J Clin Endocrinol Metab. 2009;94:4070–4076. doi:10.1210/jc.2009-0584.
- Begovatz P, Koliaki C, Weber K, et al. Pancreatic adipose tissue infiltration, parenchymal steatosis and beta cell function in humans. Diabetologia. 2015;58:1646–1655. doi:10.1007/s00125-015-3544-5.
- Ma J, Song Z, Yan F. Detection of hepatic and pancreatic fat infiltration in type II diabetes mellitus patients with IDEAL-Quant using 3.0T MR: comparison with single-voxel proton spectroscopy. Chin Med J. 2014;127:3548–3552.
- Kim MK, Chun HJ, Park JH, et al. The association between ectopic fat in the pancreas and subclinical atherosclerosis in type 2 diabetes. Diabetes Res Clin Pract. 2014;106:590–596. doi:10.1016/j.diabres.2014.09.005.
- Singh RG, Yoon HD, Wu LM, et al. Ectopic fat accumulation in the pancreas and its clinical relevance: A systemic review, meta-analysis and meta-regression. Metabolism. 2017;69:1–13. doi:10.1016/j.metabol.2016.12.012.
- Zhou J, Li M-L, Zhang D-D, et al. The correlation between pancreatic steatosis and a metabolic syndrome in a Chinese population. Pancreatology. 2016;16:578–583. doi:10.1016/j.pan.2016.03.008.
- Wang CY, Ou HY, Chen MF, et al. Enigmatic ectopic fat: prevalence of nonalcoholic fatty pancreas disease and its associated factors in a Chinese population. J Am Heart Assoc. 2014;3:e000297. doi:10.1161/JAHA.113.000297.
- Lesmana CRA, Pakasi LS, Inggriani S, et al. Prevalence of non-alcoholic fatty pancreas disease (NAFPD) and its risk factors among adult medical check-up patients in a private hospital: a large cross sectional study. BMC Gastroenterol. 2015;15:174. doi:10.1186/s12876-015-0404-1.
- Tushuizen ME, Bunck MC, Pouwels PJ, et al. Pancreatic fat content and β cell function in men with and without type 2 diabetes. Diabetes Care. 2007;30:2916–2921. doi:10.2337/dc07-0326.
- van der ijl NJ, Goossens GH, Moors CCM, et al. ectopic fat storage in the pancreas, liver, and abdominal fat depots: impact on β-cell function in individuals with impaired glucose metabolism. J Clin Endocrinol Metab. 2011;96:459–467. doi:10.1210/jc.2010-1722.
- Saisho Y, Butler AE, Meier JJ, et al. Pancreas volumes in humans from birth to age one hundred taking into account sex, obesity, and presence of type 2 diabetes. Clin Anat. 2007;20:933–942. doi:10.1002/ca.20543.
- Murakami R, Saisho Y, Watanabe Y, et al. Pancreatic fat and β cell mass in human with and without diabetes: An analysis in the Japanese population. J Clin Endocrinol Metab. 2017;102:3251–3260. doi:10.1210/jc.2017-00828.
- Yamazaki H, Tsuboya T, Katanuma A, et al. Lack of independent association between fatty pancreas and incidence of type 2 diabetes: 5-year Japanese cohort study. Diabetes Care. 2016;39:1677–1683. doi:10.2337/dc16-0074.
- Guglielmi V, Sbraccia P. Type 2 diabetes: does pancreatic fat really matter? Diabetes Metab Res Rev. 2017; [Oct 5]: doi:10.1002/dmrr.2955.
- Dubois M, Kerr-Conte J, Gmyr V, et al. Non-esterified fatty acids are deleterious for human pancreatic islet function at physiologic glucose concentration. Diabetologia. 2004;47:463–469. doi:10.1007/s00125-004-1347-1.
- Goh TT, Mason TM, Gupta N, et al. Lipid-induced beta-cell dysfunction in vivo in models of progressive beta-cell failure. Am J Physiol Endocrinol Metab. 2007;292:E549–E560. doi:10.1152/ajpendo.00255.2006.
- Wicklow BA, Griffith AT, Dumontet JN, et al. Pancreatic lipid content is not associated with beta cell dysfunction in youth-onset type 2 diabetes. Can J Diabetes. 2015;39:398–404. doi:10.1016/j.jcjd.2015.04.001.