The overlooked profiles of non-obese Asian type 2 diabetic patients
pp 71-80
Although most type 2 diabetes studies on Asian populations have focused on obese subjects, the number of Asian type 2 diabetic patients who are non-obese is now 50%. This review by Rattarasarn focuses on the non-obese type 2 diabetic population by looking at insulin resistance, cardiovascular risk, and the role regional fat may play. While non-obese Asian type 2 diabetic patients and non-diabetic patients may not have different body fat content, intra-hepatic and visceral fat proportions appear to be higher which can lead to insulin resistance as well as an increase in cardiovascular risk.
Thermogenesis through fish oil
pp 88-95
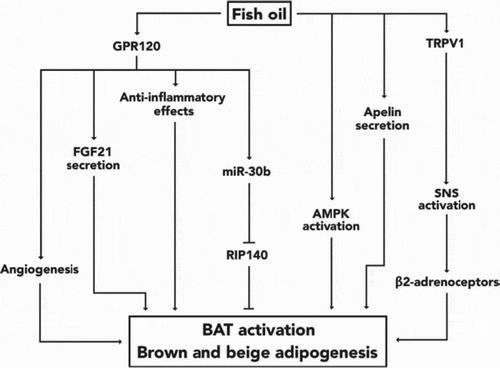
Fish oil, which contains n-3 polyunsaturated fatty acids (n-3 LCPUFAs) has been shown to activate both brown adipose tissue (BAT) as well as white adipose tissue (WAT). Authors Lund, Larsen and Lauritzen review work on n-3 LCPUFAs and its influence on thermogenic fat cells and non-shivering thermogenesis in adipose tissue. The results show that while it is unknown whether human BAT is activated by fish oil, rodent studies show a drop in adiposity. Furthermore, eicosapentaenoic acid, also found in fish oil, has been shown to induce thermogenic gene expression in brown adipocytes as well as the creation of beige adipose via white preadipocyte differentiation ()
Multiple benefits of n-3 Polyunsaturated Fatty acids
pp 81-87
This review by authors Baynes, Mideksa and Ambachew gives readers an overview of prevalent research regarding n-3 Polyunsaturated Fatty acids (PUFAs) and their influence on insulin action and pancreatic β-cells. These fatty acids have been shown to both reverse and inhibit high-fat-diet induced adipose tissue inflammation and insulin resistance and can lead to decreased prostaglandin production. In humans, n-3 PUFAs can also prevent insulin resistance and destruction of beta cells while enhancing insulin secretion.
miRNA regulation during stem cell differentiation
pp 96-105
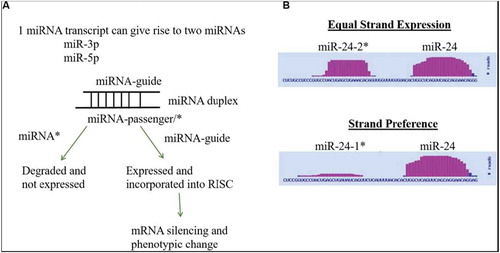
MicroRNAs (miRNAs), via mRNA translation inhibition, are key mediators of stem cell differentiation, and the expression of miRNA during adipogenic and osteogenic differentiation is enhanced. Authors Martin et al. examine adipose-derived stromal stem cell (ASCs) differentiation and how miRNA expression is altered in the process. Here, they show that despite being from the same primary transcript, adipogenic and osteogenic lineage induced ASCs prefer different strands (-3p and -5p) for miRNAs. In addition, there are alterations in the expression of miRNA biogenesis pathway associated genes, as well as alterations of expression of AGOs during adipogenesis and osteogenesis ().
Figure 2. miRNA Strand Preference. (A) Depiction of conventional mechanism for miRNA strand expression where one miRNA strand (guide) is expressed and one miRNA strand (passenger/*) is repressed. (B) Depiction of differences in strand preference of miR-24 as viewed in the miRbase. Martin et al., p. 97.
Gut microbiota manipulation via antibiotics
pp 106-112
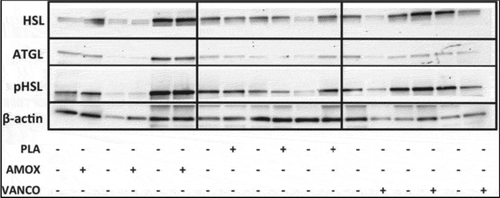
Intestinal microbiota may have an influence on lipolysis and lipid storage in adipose tissue, and alterations of gut microbiota composition could possibly lead to a change in these metabolic processes. Authors Jocken et al. use antibiotics to study the effect of gut microbiota manipulation on lipolysis in human abdominal subcutaneous adipocytes from obese insulin-resistant men. In the short term (7 days), the researchers found that both broad-spectrum antibiotics (amoxicillin) and narrow-spectrum antibiotics (vancomycin) had no significant effect on basal and maximal isoprenaline-mediated glycerol release from adipocytes, nor was there a difference in adipose tissue β-adrenoceptor expression or post-receptor signaling ()
FGF2 signaling and a link to breast epithelial cell transformation
pp 113-120
Although obesity is a known risk factor of post-menopausal breast cancer, the reasons for the link between being overweight and being diagnosed with cancer are not fully known. This brief report by authors Benham, Chakraborty, Bullard and Bernard outlines their recent findings related to the stimulation and transformation of skin epithelial cells via the release of FGF2 from visceral adipose tissue (VAT). With FGF2 and FGFR1 being associated with breast cancer progression, the authors present data suggesting a potential link within VAT-stimulated transformation of breast epithelial cells through FGF2/FGFR1 signaling.
SGLT2 inhibitor’s effect on energy homeostasis
pp 121-128
The low-grade inflammation associated with obesity is related to other ailments such as insulin resistance, type 2 diabetes, and nonalcoholic fatty liver disease, and the activation of adipose tissue macrophages plays an important part in this. Here, authors Xu and Ota look at the sodium-glucose cotransporter 2 (SGLT2) inhibitor empagliflozin and the potential beneficial effects that it may have on insulin resistance and obesity-related inflammation. These findings may lead to more treatment options related to energy metabolism and macrophage polarization in obese subjects.
Adipocyte CD1d as a regulator of iNKT
pp 129-136
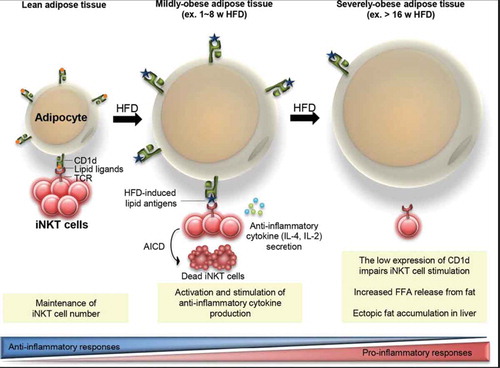
Natural Killer T (iNKT) cells are thought to be involved in anti-inflammatory responses in obesity through the recognition of ‘lipid’ antigens and the modulation of immune response through the secretion of Th1 or Th2 type cytokines. Authors Huh, Park and Kim recently showed that CD1d expressing adipocytes could help stimulate iNKT cells by acting as an antigen presenting cell. This commentary builds on these findings, presenting additional data related to CD1d-dependent regulation of adipose iNKT as well as providing further insights on this interaction as related to obesity ().
The role of ER stress epigenetics
pp 137-142
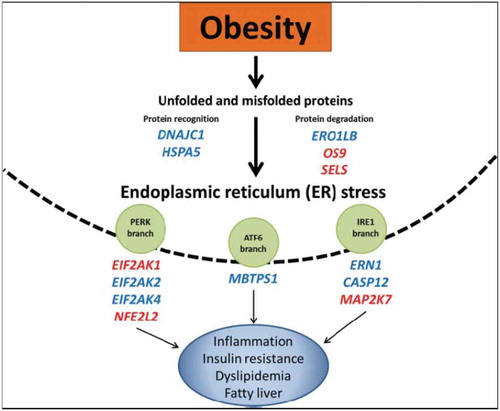
The accumulation of proteins, the result of endoplasmic reticulum folding disruption, is known as endoplasmic reticulum (ER) stress. This condition is linked to many obesity-related metabolic disorders, and authors Ramos-Lopez et al. have shown the association between regulatory genes implicated in ER stress and insulin resistance, dyslipidemia, and abdominal obesity. This commentary builds on previous findings and discusses how epigenetics may play an important role during these processes through the regulation of ER stress response ().
What activates thermogenesis?
pp 143-147
The activation of our sympathetic nervous system in order to warm our bodies when exposed to the cold has been thought to generate heat through the stimulation of cytosolic lipid droplets (LDs) in brown adipocytes. Authors Shin, Shi, Xue, and Yu recently demonstrated that cold-induced nonshivering thermogenesis is not reliant on LD lipolysis in brown adipocytes, and that white adipose tissue (WAT) lipolysis plays a vital role in fasting thermogenesis. In this commentary, the authors discuss these findings as well as potential ways in which thermogenesis may be triggered without the presence of BAT lipolysis.