ABSTRACT
Accumulating evidence highlights the importance of interactions between tumour cells and stromal cells for tumour initiation, progression, and metastasis. In tumours that contain adipocyte in their stroma, adipocytes contribute to modification of tumour microenvironment and affect metabolism of tumour and tumour progression by production of cytokines and adipokines from the lipids. The omentum and bone marrow (BM) are highly adipocyte-rich and are also common metastatic and primary tumour developmental sites. Omental adipocytes exhibit metabolic cross-talk, immune modulation, and angiogenesis. BM adipocytes secrete adipokines, and participate in solid tumour metastasis through regulation of the CCL2/CCR2 axis and metabolic interactions. BM adipocytes also contribute to the progression of hematopoietic neoplasms. Here, we here provide an overview of research progress on the cross-talks between omental/BM adipocytes and tumour cells, which may be pivotal modulators of tumour biology, thus highlighting novel therapeutic targets.
Abbreviations: MCP-1, monocyte chemoattractant protein 1IL, interleukinSTAT3, signal transducer and activator of transcription 3FABP4, fatty acid binding protein 4PI3K/AKT, phosphoinositide 3-kinase/protein kinase BPPAR, peroxisome proliferator-activated receptorPUFA, polyunsaturated fatty acidTAM, tumour-associated macrophagesVEGF, vascular endothelial growth factorVEGFR, vascular endothelial growth factor receptorBM, bone marrowBMA, bone marrow adipocytesrBMA, regulated BMAcBMA, constitutive BMAUCP-1, uncoupling protein-1TNF-α, tumour necrosis factor-alphaRANKL, receptor activator of nuclear factor kappa-Β ligandVCAM-1, vascular cell adhesion molecule 1JAK2, Janus kinase 2CXCL (C–X–C motif) ligandPGE2, prostaglandin E2COX-2, cyclooxygenase-2CCL2, C-C motif chemokine ligand 2NF-κB, nuclear factor-kappa BMM, multiple myelomaALL, acute lymphoblastic leukemiaAML, acute myeloid leukemiaGDF15, growth differentiation factor 15AMPK, AMP-activated protein kinaseMAPK, mitogen-activated protein kinaseAPL, acute promyelocytic leukemiaCCR2, C-C motif chemokine receptor 2SDF-1α, stromal cell-derived factor-1 alphaFFA, free fatty acidsLPrA, leptin peptide receptor antagonistMCD, malonyl-CoA decarboxylase.
Introduction
The tumour microenvironment (TME) affects tumour biology through various biological processes. Adipocytes are a particularly important component of the TME exerting both systemic and local effects on tumour growth and progression when tumours contain adipocyte in their stroma [Citation1,Citation2]. Among the human organs containing adipocytes, the omentum and bone marrow (BM) show particularly high enrichment of adipocytes. Importantly, the omentum and BM are also frequent metastatic sites of tumours as well as common sites of primary tumour development. Adipocytes of the omentum and BM have different origins compared to those derived from other sites, and thus exhibit specialized functions that affect tumour biology. Therefore, it is essential to identify the characteristics of omental and BM adipocytes, and their impacts on tumour biology. We here review the research progress on these adipocyte types with a focus on their roles in metastasis through metabolic interactions, and cross-talk with immune and tumour cells. Gaining a greater understanding of the underlying molecular and cellular mechanisms can highlight novel potential therapeutic targets for tumour treatment.
Basic characteristics of the omentum
The omentum is a visceral adipose tissue, mostly composed white adipose tissue that consisted of vascularized connective tissue, and doubled mesothelial layered membranous and translucent tissue[Citation3]. The omentum, a main lipid storage and a source of bioactive factors, involves in the immune response and fluid exchange [Citation4–Citation6]. Milky spots are the primary functional units of the omentum [Citation7,Citation8], which are distributed along with the blood vessel networksCitation[9]; detailed information of milky spots has been reviewed previously[Citation10]. The structural components of milky spots are fibroblasts, adipocytes, mesothelial cells, endothelial cells, macrophages, stromal cells, and high endothelium of the vein, whereas the migratory components include lymphocytes, granulocytes, and monocytes[Citation11]. Milky spots have recently come into the research spotlight as the main implantation sites of omental cancer cell metastasis. Since adipocytes composing the milky spots are also distributed around the milky spots, various interactions could occur between adipocytes and milky spots that might mediate metastatic processes.
Omental adipocytes have distinct characteristics from adipocytes derived from other sites. In particular, the lipolysis action of catecholamine is increased in omental adipocytes compared with subcutaneous adipocytes, whereas the anti-lipolytic action of insulin and prostaglandin is more prominent in subcutaneous adipocytes than in omental adipocytes[Citation12]. Expression of prostaglandin synthesis- or signalling-related genes is also higher in omental adipocytes than in subcutaneous adipocytes[Citation13]. Similarly, transcriptomics studies revealed that the expression of adipogenesis and lipid metabolism-related genes differs between omental and subcutaneous adipose tissues [Citation14–Citation16], and proteomics analysis revealed differential expression of proteins related to lipid metabolism, oxidation-reduction, and lipid transport between the tissues[Citation17]. Lipidomic analysis of obese individuals showed that compared to subcutaneous adipose tissue, omental adipose tissue contained 54% and 34% more cholesterol and cholesterol epoxide[Citation18]. Moreover, omental and subcutaneous adipocytes have different origins, and only the former express Wilms’ tumour gene (WT-1)Citation[19]; indeed, the presence of a WT-1-positive mesothelial cell layer in the omentum is considered to be the possible origin of these adipocytes. Omental adipocytes differ from other visceral adipocytes in variable circumstances. Omental adipocytes of obesity patients are larger in size, and have lower capillary density compared with periaortic adipocytes[Citation20]. Expression of IL-18, HGF, and MIF is higher in omental adipocytes than in periaortic adipocytes[Citation20]. Omental preadipocytes are also different from mesenteric preadipocytes in having lower replicative potential, lower differentiation, lower adipogenic transcription factor expression and higher TNF-α induced apoptosis[Citation21].
Roles of omental adipocytes in tumour development and metastasis
Most of the tumours found in the omentum are metastatic tumours, and primary omental tumours are extremely rare. Malignant mesothelioma is a representative example of an omental primary tumour. It is suggested that adipocytes can play a key role in tumour promotion during asbestos-induced mesothelial carcinogenesis. Upon exposure to asbestos, the levels of proinflammatory cytokines such as monocyte chemoattractant protein 1 (MCP-1) increase, while anti-inflammatory cytokines such as adiponectin decrease in the adipocytes, resulting in an inflammatory environment. Since MCP-1 promotes mesothelial cell proliferation, these inflammatory stimuli could trigger a carcinogenesis process under a proliferative state[Citation22] ().
Figure 1. The role of omental adipocytes in tumour development and metastasis.
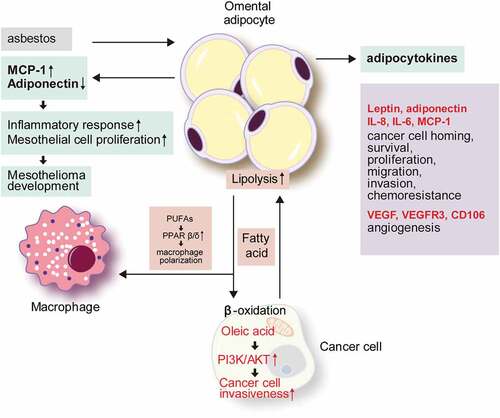
The most common carcinomas that metastasize to the omentum include ovarian cancer, colorectal cancer, gastric cancer, and pancreatic cancer[Citation23]. In particular, 80% of ovarian serous carcinomas exhibit omental metastasis[Citation24]. The main route of omental metastasis is via direct intraperitoneal seeding rather than hematogenous dissemination. Hence, interactions between metastatic tumour cells and the environment of the implantation site are key factors in promoting omental metastasis. To establish omental metastasis, cancer cells seeding in the omentum must pass through several checkpoints, including survival in the peritoneal cavity, evasion of the immune system, reattachment at the secondary site, and angiogenesis[Citation23]. Accumulating evidence has demonstrated that omental adipocytes influence every step of the omental metastasis process.
Adipokines
Adipocyte-derived metabolites and bioactive peptides are collectively referred to as adipokines, with more than 600 identified to date[Citation25]. In general, adipokines play roles in the regulation of appetite, fat distribution, insulin secretion, energy expenditure, inflammation, and blood pressure[Citation26]. In the adipose tissue, adipokines participate in adipogenesis, immune cell migration, and adipocyte metabolism [Citation27,Citation28]. In tumours, adipokines act via adipokine receptors on tumour cells[Citation29]. In an ovarian cancer mouse model, omental adipocytes promote tumour cell homing to omentum after intraperitoneal tumour cell injection through the actions of interleukin (IL)-8, IL-6, MCP-1, and adiponectin secreted by omental adipocytes[Citation30]. The adipokines secreted from omental adipocytes activate pro-survival pathway, p38, and signal transducer and activator of transcription 3 (STAT3) in ovarian cancer cells. Omental adipocytes show increased IL-8 secretion, which promotes the invasiveness of ovarian cancer cells [Citation30,Citation31]. Upon establishment of the omental metastasis of ovarian cancer, secreted IL-8 and TP53 upregulate the expression of fatty acid binding protein 4 (FABP4), which enhances the fatty acid uptake of tumour cells to promote cancer cell growth[Citation31]. Moreover, the peritumoral adipokine profile changes of omental metastases of pancreatic cancer have been described; specifically, leptin expression increases to enhance metastasis[Citation32]. Secretory factors from omental adipocytes promote the reprogramming of pancreatic cancer cells, such as an increase of extracellular matrix and adhesion molecules, resulting in the promotion of cancer cell growth, migration, invasion, and chemo-resistance[Citation33].
Lipid supply and metabolic interactions
Omental adipocytes supply lipids to tumour cells and also support their survival and proliferation in the omentum. An in vitro study with ovarian cancer cells demonstrated the lipid transfer from adipocytes to tumour cells, which was enhanced for omental adipocytes compared with subcutaneous and mesenteric adipocytes[Citation30]. Lipids entering tumour cells supply the energy required for tumour cell proliferation by β-oxidation[Citation30]. To meet the high energy demand, tumour cells upregulate the lipolysis of adipocytes, and their secretion of free fatty acids and glycerol[Citation30]. FABP4 [Citation31] and CD36 [Citation34] are the main lipid transporters during the interaction of tumour cells and adipocytes. After lipid transfer, the size of the adipocytes decreases by consuming lipid droplets. Thus, omental adipocytes become smaller during omental metastasis, and ultimately disappear to become replaced by the metastatic tumour cells, referred to as the ‘omental cake.’[Citation35] In gastric cancer, oleic acids are transferred to tumour cells from omental adipocytes. Intracellular oleic acid activates the phosphoinositide 3-kinase/protein kinase B (PI3K/AKT) pathway of tumour cells, which promotes their invasiveness[Citation36]. The metabolites produced as by-products during lipid metabolism also affect tumour cell metabolism, including glycerol, a by-product of lipolysis, which can act as a substrate for the glycolytic pathway to promote metastatic tumour cell growth and adaptation [Citation37,Citation38].
Cross-talk with immune cells
As mentioned above, a milky spot, the functional unit of the omentum, is composed of diverse cell types, including immune cells such as macrophages, mast cells, and B- and T-lymphocytes, that are important in the immune response of the omentum [Citation39,Citation40]. The milky spot is the initial tumour cell attachment site for metastasis[Citation9], and is thus critical for tumour growth and survival. Milky spots are only found in the omentum and splenoportal adipose tissue among the peritoneal adipose tissues [Citation35,Citation41]. Interactions between adipocytes and peritoneal macrophages have been shown to contribute to ovarian cancer cell metastasis. Peroxisome proliferator-activated receptor (PPAR)β/δ is involved in cancer-associated processes and activated by various lipid ligands[Citation42]. Analyzing of tumour-associated macrophages driven from ovarian cancer ascites showed constantly upregulated PPARβ/δ with impaired ligand response[Citation43]. Ovarian cancer ascites was rich in polyunsaturated fatty acids (PUFAs), particularly linoleic acid[Citation43], which could be derived from omental adipocytes. PUFAs could serve as ligand of PPARs, and PUFA/PPARδ structure promoted their FA sensing ability[Citation44]. PPARδ promotes lipid accumulation in macrophages[Citation45], and this may explain the high concentration of PUFAs and constant upregulation of PPARβ/δ in tumour-associated macrophages (TAMs) in ovarian cancer ascites[Citation43], which play a pro-tumorigenic role in tumour microenvironment. This kind of fatty acid accumulated in TAM is now recognized as tumour promotor [Citation46,Citation47].
Angiogenesis
Milky spots exist along with the vascular network of the omentum and at sites of active angiogenesis. In general, angiogenesis in milky spots occurs by vascular endothelial growth factor A (VEGFA) secreted from omental mesothelial cells and macrophages. In a hypoxic condition, the omental adipocytes secrete VEGF, vascular endothelial growth factor receptor (VEGFR) 3, and CD105 [Citation9,Citation48,Citation49] to induce angiogenesis and thereby promote cancer survival and chemoresistance [Citation50,Citation51]. Microarray analysis with the Oncomine assay revealed higher expression levels of VEGFR1, VEGFR2, CD31, and CD34 in omental metastatic cancer tissues than in the primary ovarian cancer[Citation52].
BM adipocytes (BMA)
BMA are an important component of the BM with diverse roles, and the proportion of BMA in the BM fluctuates under various conditions. The number of adipocytes tends to increase with age, obesity, malnutrition, and stimulation of drugs or radiation [Citation53–Citation56]. Approximately 70% of the adult BM volume is occupied by BMA[Citation57], which can be classified into inducible or regulated BMA (rBMA) and constitutive BMA (cBMA) that differ in terms of development, lipid saturation, gene expression, and vascular density [Citation58,Citation59]. rBMA are characterized by their proximal location and red marrow, whereas cBMA are characterized by their more distal location and yellow marrow. BMA originate from BM mesenchymal stem cells that are bi-potent progenitors, with the ability to differentiate into adipocytes and/or osteoblasts[Citation60]. BMA show several typical adipocyte phenotype characteristics. The brown-like phenotype BMA provide energy to hematopoietic and mesenchymal components [Citation61] and express uncoupling protein-1 (UCP-1)[Citation62], whereas white-like phenotype BMA play roles in the storage and process of triglycerides, and regulate fatty acid metabolism [Citation61,Citation63,Citation64]. This phenotypic difference has been suggested to be based on the location, with BMA in the long bone and vertebrae corresponding to the white and brown phenotype, respectively[Citation65]. BMA secrete various adipokines, including hormones, cytokines, and fatty acids, that affect bone remodelling, energy regulation, and insulin metabolism [Citation66,Citation67]. The secretory profile of BMA also differs from that of adipocytes from other sites: BMA show lower expression of adiponectin mRNA compared with that of extramedullary adipocytes [Citation68,Citation69] but have higher expression levels of tumour necrosis factor-alpha (TNF-α) and IL-6 than those in visceral adipocytes[Citation68], accompanied by higher pro-angiogenic and pro-apoptotic profiles[Citation70]. In bone remodelling, BMA secrete leptin, adiponectin, and chemerin; leptin and adiponectin induce the osteoblastic differentiation and proliferation of mesenchymal stem cells, while chemerin suppresses osteogenesis[Citation65]. Moreover, the abundant saturated fatty acids in BMA induce osteoblast dysfunction and apoptosis, and osteoclastogenesis is facilitated by TNF-α and receptor activator of nuclear factor kappa-Β ligand (RANKL) secreted from BMA[Citation65].
Roles of BMA in solid tumour metastasis
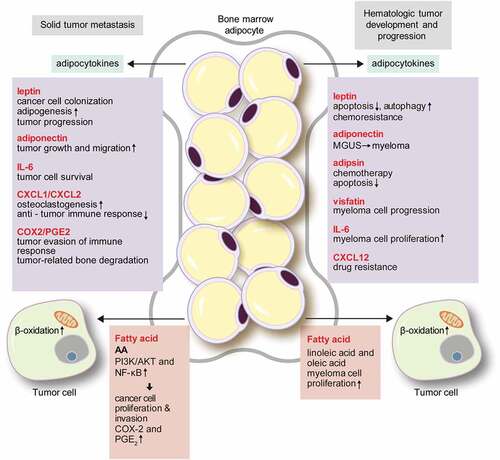
The bone is one of the most common metastatic sites of cancers, especially for prostate, breast, and lung cancers[Citation71]. The incidence of bone metastasis at autopsy is 75–80% for prostate cancer [Citation71,Citation72], and 65–75% for breast cancer[Citation73]. In prostate cancer, old age and obesity are the primary risk factors of metastasis [Citation74–Citation76], which are factors that increase the numbers of BMA. The main metastatic sites of prostate cancer are the axial skeleton and long bone metaphysis, which is a site of active bone remodelling with high marrow cellularity[Citation77]. Thus, a metabolically active BM with abundant adipocytes may be the preferred site of metastasis. Previous studies indicated that metastatic cells forming colonies in the bone were attracted by an adipocyte-rich and metabolically active red BM [Citation78,Citation79]. Thus, substantial research has focused on the contribution of the BMA in the bone metastasis of solid tumours, revealing various mechanisms. Here, we focus on the secretion of adipocytokines and lipid transfer as the representative mechanisms ().
Figure 2. The role of bone marrow adipocytes in solid tumour metastasis and hematologic tumour development.
Adipocytokines secreted by BMA
BMA secrete various adipocytokines such as leptin, adiponectin, IL-1β, IL-6, vascular cell adhesion molecule 1 (VCAM-1), TNF-α, and VEGF, [Citation80] which influence cancer cell biology. Leptin indirectly affects prostate cancer cell growth via promoting bone resorption, [Citation81,Citation82] and increased expression of IL-1β drives cancer cell colonization of BMA in breast cancer[Citation83]. Leptin is also important for BMA generation. Leptin binds to the leptin receptor on BM stem cells, and activates the Janus kinase 2 (JAK2)/STAT3 pathway to trigger adipogenesis [Citation84,Citation85]. Leptin secreted into BMA binds leptin receptor on the tumour cells, which induces tumour progression [Citation86–Citation88]. Indeed, tumour patients show greater amounts of adiponectin secreted by BMA[Citation55]. Although adiponectin generally acts as a tumour suppressor[Citation89], some studies showed that adiponectin enhanced tumour growth and migration [Citation90,Citation91]. This dual effect may be derived from the difference of adiponectin receptor isoforms[Citation90]. BMA secrete abundant IL-6[Citation92], which induces the epithelial-mesenchymal transition in tumour cells via the JAK2/STAT3 pathway[Citation93], and strengthens the metastatic potential of tumour cells via PI3K/AKT[Citation94]. Peculiarly, IL-6 can be activated by an extracellular soluble form of IL-6 receptor (IL-6R), without a receptor of tumour cells[Citation95]. Therefore, if the soluble form IL-6R along with IL-6 are secreted by BMA, they would have a strong effect on the metastatic process. (C–X–C motif) ligand (CXCL)1 and CXCL2, chemokines produced by BMA, enhance osteoclastogenesis in metastatic prostate cancer and prolong tumour cell survival[Citation96]. Upregulated expression of IL-6 in malignant melanoma cells increased osteoclastogenesis, which could induce the proliferation of tumour cells[Citation97]. CXCL1 and CXCL2 also participate in immune modulation, acting as chemoattractants for macrophages, neutrophils, and CD11b+Gr1+ cells [Citation98,Citation99]. These immune cells have CXCL2 receptors and suppress the anti-tumour immune response[Citation100]. Furthermore, the cyclooxygenase-2 (COX-2)/prostaglandin E2 (PGE2) signalling axis induces inflammation and immune suppression, facilitating tumour evasion of the host immune response[Citation101]. Overexpression of COX-2 and PGE2 is the main cause of tumour-related bone degradation in bone metastasis [Citation102,Citation103]. In a breast cancer mouse model, an increase of the COX-2 level increased tumour colonization and osteoclastogenesis, and induced lytic bone metastasis[Citation104]. Adipokines also participate in angiogenesis. When prostate cancer cells were exposed to BMA, VEGF expression increased[Citation105], and CCL2 secreted by adipocytes was found to promote breast cancer progression by inducing angiogenesis[Citation106].
Lipid transfer and characteristic lipid components
BMA provide the lipid source required for the proliferation, migration, and invasion of solid cancer cells [Citation83,Citation105]. In a cell line study, prostate cancer cells co-cultured with BMA were found to be surrounded by lipid droplets and showed increased expression levels of the lipid transfer-associated molecules FABP4, CD36, and perilipin 2[Citation105]. Microarray analysis with Oncomine data also revealed increased expression levels of FABP4 and CD36 in metastatic prostate cancer compared to those of the primary cancer[Citation52]. In addition, CD36 expression was increased in breast cancer and prostate cancer cells co-cultured with BMA[Citation105]. An in vivo study also supported the lipid transfer from BMA to tumour cells: in the early phase of bone metastasis, the number of BMA increased by adipogenesis, but the number of BMA with abundant lipid droplets decreased during tumour progression[Citation107]. Bone metastasis is more frequent in the rBMA-enriched region than in the cBMA-enriched region. rBMA can respond flexibly during metabolic interactions with tumour cells, as they readily adapt to their environment. BMA also influence the metabolic phenotype of metastatic prostate cancer cells. Previous study on prostate cancer showed that BMA induced Warburg-type metabolism in cancer cells in paracrine manner, along with decreased mitochondrial oxidative phosphorylation[Citation108]. Glycolytic enzymes, ENO2, LDHa, PDK1, HK2, and GLUT1 were upregulated in prostate cancer cells that were co-cultured with adipocytes[Citation108]. Exposure of prostate cancer cells to BMA induced hypoxia-inducible factor 1-alpha signalling and persisted Warburg-type metabolism[Citation108].
The lipid droplets of BMA are composed of large-sized saturated and unsaturated fatty acids, particularly oleic, palmitic, and omega-6 PUFAs, and AA[Citation109]. Some of the fatty acids derived from BMA could impact bone metastasis. For example, AA transferred from BMA to prostate cancer cells activated the PI3K/AKT and nuclear factor-kappa B (NF-κB) signalling pathways, and promoted cancer cell proliferation and infiltration [Citation110,Citation111]. Moreover, AA is also related to the expression of COX-2 and PGE2, thus playing a role in the COX-2/PGE2 signalling axis [Citation110,Citation112].
Roles of BMA in hematologic neoplasms
The studies reviewed above clearly demonstrate the roles of interactions between solid tumours and BMA in bone metastasis. However, hematologic neoplasms such as multiple myeloma (MM) and leukaemia are primarily derived from the BM, which could be the primary niche of these neoplasms. In solid tumours, elevated leptin level is associated with cancer risk[Citation113]. Also, in hematologic neoplasms, such as MM, leptin was revealed to have pro-tumour effect[Citation80]. Moreover, previous study showed that adipocytes protected acute lymphoblastic leukaemia (ALL) cells from vincristine, a chemotherapeutic agent, by sequestering lipophilic vincristine, as well as upregulating anti-apoptotic proteins, Pim-2 and Bcl-2[Citation114]. In MM patients, myeloma cells induced adipogenesis from osteoblast progenitor cells, and increased the number of BMA, which contributed to MM progression[Citation115]. In acute myeloid leukaemia (AML) patients, BM mesenchymal stem cells tended to differentiate into adipocytes[Citation116], which implies that tumour microenvironment favours adipocyte-rich state. So far, BMA have been considered as negative regulators in BM microenvironment and hematopoiesis[Citation117]. Preferred differentiation to adipocytes of BM mesenchymal stem cells may lead to the depletion of hematopoietic stem cell niche, and also facilitate tumour growth. Size reduction of BMA surrounding AML cell line is caused by lipolysis of adipocytes by leukemic cells, which leads to increase of free fatty acid utilized by leukemic cells. Growth differentiation factor 15 (GDF15) level, secreted from AML cells, induces morphological remodelling of BMA [Citation118] and lipolytic pathway to generate fatty acid for tumour proliferation[Citation119] ().
Adipokines
An epidemiologic study showed that low adiponectin and high leptin levels are associated with an increased tumour risk in multiple myeloma[Citation113]. In vitro, myeloma cells co-cultured with adipocytes showed enhanced proliferation and migration, and leptin was found to clearly play a role in this process[Citation120]. Leptin activates the AKT/STAT3 pathway, increases Bcl-2 levels, and suppresses caspase-3 and in turn apoptosis, which collectively contribute to the development of chemo-resistance in myeloma[Citation121]. Autophagy, which is also activated by leptin, inhibits chemotherapy-induced apoptosis[Citation122]. Adiponectin activates the AMP-activated protein kinase (AMPK) and mitogen-activated protein kinase (MAPK) pathways in myeloma cells, and reduces the rate of tumour cell proliferation while promoting apoptosis[Citation123]. Decreased adiponectin levels promote the progression to myeloma from the pre-myeloma stage, because a low adiponectin level is not sufficient to properly inhibit acetyl-CoA-carboxylase, a key enzyme of lipid synthesis in tumour cells[Citation124]. Adipsin secreted from adipocytes inhibits chemotherapy-induced apoptosis in myeloma cells by increasing autophagy[Citation108]. Vistafin, a visceral fat-derived protein, has been shown to be related to multiple myeloma progression[Citation125]. IL-6 promotes myeloma cell proliferation both in vitro and in vivo[Citation126], and the IL-6 level is correlated with myeloma progression[Citation127]. TNF-α independently promoted the proliferation of myeloma cells[Citation128], and induced the expression of CCL2 in myeloma cells together with IL-6[Citation129]. CCL2 leads to the macrophage recruitment that supports myeloma cell survival, drug resistance, and angiogenesis[Citation130].
Cytokines and chemokines secreted by BMA induce proliferation of AML cells. Leptin increases survival of leukemic cells [Citation131,Citation132], and induces proliferation of AML cell lines and blasts [Citation133,Citation134]. In acute promyelocytic leukaemia (APL), leptin from adipocytes suppresses APL cell apoptosis via STAT3 and MAPK pathway[Citation135]. In ALL, stromal cell-derived factor-1 alpha (SDF-1α) secreted by adipocytes binds to CXCR receptor, which induces cytoskeletal remodelling and makes leukemic cells migrate to adipose tissue[Citation136]. Finally, CXCL2 secreted from adipocytes was shown to induce drug resistance in a leukaemia mouse model[Citation137]. These above-mentioned adipokines are released by all different deposits of adipose tissue and are found in the circulation.
Lipid metabolism and lipid metabolites
Blast cells of AML induce the phosphorylation of hormone-sensitive lipase in BMA, and activate lipolysis, resulting in the increased production of fatty acids. The fatty acids are transferred to AML blasts via FABP4, which help to promote tumour cell proliferation[Citation138]. BMA also promotes fatty acid β-oxidation as well as the expression of the PPARɣ, FABP4, CD36, and BCL2 genes, which collectively inhibit the apoptosis of acute monocytic leukaemia cells[Citation139]. Thus, adipocytes serve as energy source of AML have reduced size, and small BMA size is known as poor prognostic factor in AML[Citation140]. Furthermore, BMA in AML transfer free fatty acids (FFA) to hematopoietic stem cells that make survival and growth of AML blasts[Citation141]. When ALL cell line was co-cultured with adipocytes, FFA produced from adipocyte lipolysis was used by ALL cell, for tumour cell proliferation[Citation142]. Fatty acids have various effects on myeloma: LA and oleic acid induce the proliferation of myeloma cells [Citation143,Citation144], whereas unsaturated fatty acids such as alpha-LA and eicosapentaenoic acid caused myeloma cell death in vitro[Citation145], and PUFAs induced the apoptosis of human leukemic cells[Citation146].
Therapeutic targets of omental and BM adipocytes for tumour treatment
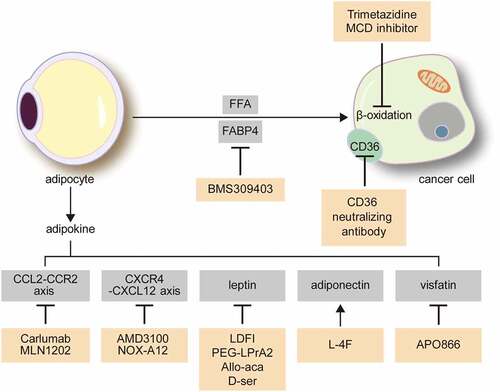
Given the evident roles of omental and BM adipocytes in the various pathways of tumour biology, targeting the interaction between tumour cells and adipocytes could be an effective new cancer treatment strategy ().
Figure 3. Possible treatment targets for the interaction between cancer cells and adipocytes in the omentum and bone marrow.
Adipokine modulators
Adipokine modulators have shown promising tumour-suppressive effects. Leptin has pro-tumorigenic effects as well as induces in chemoresistance via NF-κB and TGF-β signalling pathways [Citation147,Citation148]. Leptin antagonists include leptin mutant proteins[Citation149], leptin peptide antagonist[Citation149], leptin peptide receptor antagonist (LPrA) [Citation150,Citation151], and Allo-aca and D-ser [Citation152,Citation153]. Among these, leptin peptide antagonist, LDFI, inhibited leptin-induced proliferation of breast cancer cells in vivo and in vitro[Citation154]. LPrA2 prevented breast cancer in mouse model, associated with reduction of levels of leptin-induced molecules[Citation155]. Allo-aca and its analogue peptide D-ser inhibited leptin-induced proliferation of cancer cells in in vitro: breast cancer cell line, MDA-MB231[Citation152], leptin-receptor positive breast and colon cancer cells[Citation153]. AMD3100, a CXCR4 inhibitor, was found to increase the sensitivity to therapy in multiple myeloma cells[Citation156]. NOX-A12, a CXCL12 inhibitor, also increased the sensitivity of chronic lymphocytic leukaemia cells to chemotherapy[Citation157]. Carlumab, monoclonal antibody to CCL2 inhibits CCL2 binding to the CCR2 receptor[Citation158]. Also known as CNTO888, carlumab showed promising antitumor effect in pre-clinical study[Citation159]. Although carlumab was well tolerated in solid tumour patients with lesser adverse effect, unlikely to in vitro study, carlumab expected to have lesser binding affinity in human further study and review are required [Citation160,Citation161]. L-4F, an apolipoprotein mimetic, increased the adiponectin level and displayed a chemotherapeutic effect on myeloma[Citation123], breast cancer[Citation162], and ovarian cancer[Citation163]. APO866, a visfatin inhibitor, induced the apoptosis of myeloma cells, and repressed the rate of tumour cell proliferation[Citation125].
Lipid metabolism inhibitors
Given the metabolic interactions between adipocytes and tumour cells, targeting of metabolic pathways has been explored as a treatment target. Fatty acids released during lipolysis are transferred to tumour cells and used for energy production via mitochondrial β-oxidation, which enhances tumour progression. Hence, fatty acid oxidation in cancer cells is a promising therapeutic target. A malonyl-CoA decarboxylase (MCD) inhibitor inhibits fatty acid oxidation by increasing the malonyl-CoA level, which is a key inhibitory enzyme of fatty acid uptake in mitochondria, and in turn could reduce the proliferation of human breast cancer cells[Citation164]. In addition, the transportation of fatty acids from adipocytes to tumour cells has been explored as a potential treatment target. BMS 309403, an inhibitor of FABP4, decreased cancer cell proliferation[Citation30], and a CD36 blocking antibody that blocks the acid uptake of CD36 decreased breast cancer cell metastasis and ovarian cancer cell growth [Citation34,Citation165].
Conclusion and prospects
The omentum and BM are highly enriched in adipocytes and are the main sites of metastasis for various types of solid tumours. The BM is also a primary site of hematologic tumour development. Adipocytes are a component of the TME that dictates tumour development, survival, and progression; thus, targeting adipocytes can be an important strategy for suppressing tumour development, cancer cell survival, and progression. Adipocytes of omentum and BM differ in their origin and location, but both serve as endocrine organs secreting adipokines and are involved in tumour biology. Adipocytes of the omentum and BM secrete various adipokines with pro-tumour effects on growth signalling, angiogenesis, and immune modulation. Furthermore, they transfer lipids to adjacent tumour cells to influence tumour metabolism, and enhance tumour proliferation and survival. Thus, targeting the interaction between tumour cells and adipocytes of the omentum and BM could be an effective tumour treatment. In this regard, IL-6, TNF-α, CXCL12, and CCL2 are therapeutically targetable adipokines, while FABP4 and CD36 are potential targets regarding the metabolic interaction. Further study is required to uncover the detailed relationships between adipocytes of the omentum and BM and their influence on tumour biology, and to identify and validate new potential treatment targets.
Acknowledgments
The authors would like to thank Dong-Su Jang, MFA (Medical Illustrator) for his help with the illustrations.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Choi J, Cha YJ, Koo JS. Adipocyte biology in breast cancer: From silent bystander to active facilitator. Prog Lipid Res. 2018;69:11–20.
- Hoy AJ, Balaban S, Saunders DN. Adipocyte-tumor cell metabolic crosstalk in breast cancer. Trends Mol Med. 2017;23:381–392.
- Wilkosz S, Ireland G, Khwaja N, et al. A comparative study of the structure of human and murine greater omentum. Anat Embryol (Berl). 2005;209:251–261.
- Hall JC, Heel KA, Papadimitriou JM, et al. The pathobiology of peritonitis. Gastroenterology. 1998;114:185–196.
- Goldsmith HS. Role of the omentum in the treatment of Alzheimer’s disease. Neurol Res. 2001;23:555–564.
- Meza-Perez S, Randall TD. Immunological functions of the omentum. Trends Immunol. 2017;38:526–536.
- Beelen RH, Fluitsma DM, Hoefsmit EC. The cellular composition of omentum milky spots and the ultrastructure of milky spot macrophages and reticulum cells. J Reticuloendothel Soc. 1980;28:585–599.
- Cranshaw ML, Leak LV. Milky spots of the omentum: a source of peritoneal cells in the normal and stimulated animal. Arch Histol Cytol. 1990;53(Suppl):165–177.
- Gerber SA, Rybalko VY, Bigelow CE, et al. Preferential attachment of peritoneal tumor metastases to omental immune aggregates and possible role of a unique vascular microenvironment in metastatic survival and growth. Am J Pathol. 2006;169:1739–1752.
- Liu J, Geng X, Li Y. Milky spots: omental functional units and hotbeds for peritoneal cancer metastasis. Tumour Biol. 2016;37:5715–5726.
- Aroeira LS, Aguilera A, Sanchez-Tomero JA, et al. Epithelial to mesenchymal transition and peritoneal membrane failure in peritoneal dialysis patients: pathologic significance and potential therapeutic interventions. J Am Soc Nephrol. 2007;18:2004–2013.
- Arner P. Insulin resistance in type 2 diabetes: role of fatty acids. Diabetes Metab Res Rev. 2002;18(Suppl 2):S5–9.
- Michaud A, Lacroix-Pepin N, Pelletier M, et al. Expression of genes related to prostaglandin synthesis or signaling in human subcutaneous and omental adipose tissue: depot differences and modulation by adipogenesis. Mediators Inflamm. 2014;2014:451620.
- Gesta S, Bluher M, Yamamoto Y, et al. Evidence for a role of developmental genes in the origin of obesity and body fat distribution. Proc Natl Acad Sci U S A. 2006;103:6676–6681.
- MacLaren R, Cui W, Simard S, et al. Influence of obesity and insulin sensitivity on insulin signaling genes in human omental and subcutaneous adipose tissue. J Lipid Res. 2008;49:308–323.
- Perrini S, Laviola L, Cignarelli A, et al. Fat depot-related differences in gene expression, adiponectin secretion, and insulin action and signalling in human adipocytes differentiated in vitro from precursor stromal cells. Diabetologia. 2008;51:155–164.
- Perez-Perez R, Ortega-Delgado FJ, Garcia-Santos E, et al. Differential proteomics of omental and subcutaneous adipose tissue reflects their unalike biochemical and metabolic properties. J Proteome Res. 2009;8:1682–1693.
- Jove M, Moreno-Navarrete JM, Pamplona R, et al. Human omental and subcutaneous adipose tissue exhibit specific lipidomic signatures. Faseb J. 2014;28:1071–1081.
- Chau YY, Bandiera R, Serrels A, et al. Visceral and subcutaneous fat have different origins and evidence supports a mesothelial source. Nat Cell Biol. 2014;16:367–375.
- Kranendonk ME, van Herwaarden JA, Stupkova T, et al. Inflammatory characteristics of distinct abdominal adipose tissue depots relate differently to metabolic risk factors for cardiovascular disease: distinct fat depots and vascular risk factors. Atherosclerosis. 2015;239:419–427.
- Tchkonia T, Tchoukalova YD, Giorgadze N, et al. Abundance of two human preadipocyte subtypes with distinct capacities for replication, adipogenesis, and apoptosis varies among fat depots. Am J Physiol Endocrinol Metab. 2005;288:E267–277.
- Chew SH, Okazaki Y, Nagai H, et al. Cancer-promoting role of adipocytes in asbestos-induced mesothelial carcinogenesis through dysregulated adipocytokine production. Carcinogenesis. 2014;35:164–172.
- Koppe MJ, Nagtegaal ID, de Wilt JH, et al. Recent insights into the pathophysiology of omental metastases. J Surg Oncol. 2014;110:670–675.
- Doig T, Monaghan H. Sampling the omentum in ovarian neoplasia: when one block is enough. Int J Gynecol Cancer. 2006;16:36–40.
- Lehr S, Hartwig S, Sell H. Adipokines: a treasure trove for the discovery of biomarkers for metabolic disorders. Proteomics Clin Appl. 2012;6:91–101.
- Bluher M, Mantzoros CS. From leptin to other adipokines in health and disease: facts and expectations at the beginning of the 21st century. Metabolism. 2015;64:131–145.
- Bluher M. Adipokines - removing road blocks to obesity and diabetes therapy. Mol Metab. 2014;3:230–240.
- Bluher M. Clinical relevance of adipokines. Diabetes Metab J. 2012;36:317–327.
- Jarde T, Caldefie-Chezet F, Damez M, et al. Leptin and leptin receptor involvement in cancer development: a study on human primary breast carcinoma. Oncol Rep. 2008;19:905–911.
- Nieman KM, Kenny HA, Penicka CV, et al. Adipocytes promote ovarian cancer metastasis and provide energy for rapid tumor growth. Nat Med. 2011;17:1498–1503.
- Hu J, Liu Z, Wang X. Does TP53 mutation promote ovarian cancer metastasis to omentum by regulating lipid metabolism? Med Hypotheses. 2013;81:515–520.
- Fan Y, Gan Y, Shen Y, et al. Leptin signaling enhances cell invasion and promotes the metastasis of human pancreatic cancer via increasing MMP-13 production. Oncotarget. 2015;6:16120–16134.
- Feygenzon V, Loewenstein S, Lubezky N, et al. Unique cellular interactions between pancreatic cancer cells and the omentum. PLoS One. 2017;12:e0179862.
- Ladanyi A, Mukherjee A, Kenny HA, et al. Adipocyte-induced CD36 expression drives ovarian cancer progression and metastasis. Oncogene. 2018;37:2285–2301.
- Clark R, Krishnan V, Schoof M, et al. Milky spots promote ovarian cancer metastatic colonization of peritoneal adipose in experimental models. Am J Pathol. 2013;183:576–591.
- Xiang F, Wu K, Liu Y, et al. Omental adipocytes enhance the invasiveness of gastric cancer cells by oleic acid-induced activation of the PI3K-Akt signaling pathway. Int J Biochem Cell Biol. 2017;84:14–21.
- Vaughan M. The production and release of glycerol by adipose tissue incubated in vitro. J Biol Chem. 1962;237:3354–3358.
- Maeda N, Funahashi T, Shimomura I. Metabolic impact of adipose and hepatic glycerol channels aquaporin 7 and aquaporin 9. Nat Clin Pract Endocrinol Metab. 2008;4:627–634.
- Mandache E, Moldoveanu E, Savi G. The involvement of omentum and its milky spots in the dynamics of peritoneal macrophages. Morphol Embryol (Bucur). 1985;31:137–142.
- Rangel-Moreno J, Moyron-Quiroz JE, Carragher DM, et al. Omental milky spots develop in the absence of lymphoid tissue-inducer cells and support B and T cell responses to peritoneal antigens. Immunity. 2009;30:731–743.
- Takemori N, Hirai K, Onodera R, et al. Light and electron microscope study of splenoportal milky spots in New Zealand black mice: comparison between splenoportal milky spots and aberrant spleens. J Anat. 1995;186(Pt 2):287–299.
- Muller R. PPARbeta/delta in human cancer. Biochimie. 2017;136:90–99.
- Schumann T, Adhikary T, Wortmann A, et al. Deregulation of PPARbeta/delta target genes in tumor-associated macrophages by fatty acid ligands in the ovarian cancer microenvironment. Oncotarget. 2015;6:13416–13433.
- Xu HE, Lambert MH, Montana VG, et al. Molecular recognition of fatty acids by peroxisome proliferator-activated receptors. Mol Cell. 1999;3:397–403.
- Vosper H, Patel L, Graham TL, et al. The peroxisome proliferator-activated receptor delta promotes lipid accumulation in human macrophages. J Biol Chem. 2001;276:44258–44265.
- Gueraud F, Tache S, Steghens JP, et al. Dietary polyunsaturated fatty acids and heme iron induce oxidative stress biomarkers and a cancer promoting environment in the colon of rats. Free Radic Biol Med. 2015;83:192–200.
- Khadge S, Sharp JG, McGuire TR, et al. Lipid inflammatory mediators in cancer progression and therapy. Adv Exp Med Biol. 2017;1036:145–156.
- Sorensen EW, Gerber SA, Sedlacek AL, et al. Omental immune aggregates and tumor metastasis within the peritoneal cavity. Immunol Res. 2009;45:185–194.
- Zhang QX, Magovern CJ, Mack CA, et al. Vascular endothelial growth factor is the major angiogenic factor in omentum: mechanism of the omentum-mediated angiogenesis. J Surg Res. 1997;67:147–154.
- Choi HJ, Armaiz Pena GN, Pradeep S, et al. Anti-vascular therapies in ovarian cancer: moving beyond anti-VEGF approaches. Cancer Metastasis Rev. 2015;34:19–40.
- Krock BL, Skuli N, Simon MC. Hypoxia-induced angiogenesis: good and evil. Genes Cancer. 2011;2:1117–1133.
- Chkourko Gusky H, Diedrich J, MacDougald OA, et al. Omentum and bone marrow: how adipocyte-rich organs create tumour microenvironments conducive for metastatic progression. Obes Rev. 2016;17:1015–1029.
- Berry R, Rodeheffer MS, Rosen CJ, et al. Adipose tissue residing progenitors (adipocyte lineage progenitors and adipose derived stem cells (ADSC). Curr Mol Biol Rep. 2015;1:101–109.
- Georgiou KR, Hui SK, Xian CJ. Regulatory pathways associated with bone loss and bone marrow adiposity caused by aging, chemotherapy, glucocorticoid therapy and radiotherapy. Am J Stem Cells. 2012;1:205–224.
- Cawthorn WP, Scheller EL, Learman BS, et al. Bone marrow adipose tissue is an endocrine organ that contributes to increased circulating adiponectin during caloric restriction. Cell Metab. 2014;20:368–375.
- Berendsen AD, Olsen BR. Osteoblast-adipocyte lineage plasticity in tissue development, maintenance and pathology. Cell Mol Life Sci. 2014;71:493–497.
- Fazeli PK, Horowitz MC, MacDougald OA, et al. Marrow fat and bone–new perspectives. J Clin Endocrinol Metab. 2013;98:935–945.
- Scheller EL, Doucette CR, Learman BS, et al. Region-specific variation in the properties of skeletal adipocytes reveals regulated and constitutive marrow adipose tissues. Nat Commun. 2015;6:7808.
- Roche B, David V, Vanden-Bossche A, et al. Structure and quantification of microvascularisation within mouse long bones: what and how should we measure? Bone. 2012;50:390–399.
- Langin D. Control of fatty acid and glycerol release in adipose tissue lipolysis. C R Biol. 2006;329:598–607. discussion 653–595.
- Lecka-Czernik B. Marrow fat metabolism is linked to the systemic energy metabolism. Bone. 2012;50:534–539.
- Nishio M, Yoneshiro T, Nakahara M, et al. Production of functional classical brown adipocytes from human pluripotent stem cells using specific hemopoietin cocktail without gene transfer. Cell Metab. 2012;16:394–406.
- Lecka-Czernik B, Gubrij I, Moerman EJ, et al. Inhibition of Osf2/Cbfa1 expression and terminal osteoblast differentiation by PPARgamma2. J Cell Biochem. 1999;74:357–371.
- Shockley KR, Lazarenko OP, Czernik PJ, et al. PPARgamma2 nuclear receptor controls multiple regulatory pathways of osteoblast differentiation from marrow mesenchymal stem cells. J Cell Biochem. 2009;106:232–246.
- Hardouin P, Rharass T, Lucas S. Bone marrow adipose tissue: to be or not to be a typical adipose tissue? Front Endocrinol (Lausanne). 2016;7:85.
- Roodman GD. Genes associate with abnormal bone cell activity in bone metastasis. Cancer Metastasis Rev. 2012;31:569–578.
- Paula FJ, Rosen CJ. Obesity, diabetes mellitus and last but not least, osteoporosis. Arq Bras Endocrinol Metabol. 2010;54:150–157.
- Liu LF, Shen WJ, Ueno M, et al. Characterization of age-related gene expression profiling in bone marrow and epididymal adipocytes. BMC Genomics. 2011;12:212.
- Poloni A, Maurizi G, Serrani F, et al. Molecular and functional characterization of human bone marrow adipocytes. Exp Hematol. 2013;41:558–566.e552.
- Gasparrini M, Rivas D, Elbaz A, et al. Differential expression of cytokines in subcutaneous and marrow fat of aging C57BL/6J mice. Exp Gerontol. 2009;44:613–618.
- Coleman RE. Metastatic bone disease: clinical features, pathophysiology and treatment strategies. Cancer Treat Rev. 2001;27:165–176.
- Roodman GD. Mechanisms of bone metastasis. N Engl J Med. 2004;350:1655–1664.
- Langley RR, Fidler IJ. The seed and soil hypothesis revisited–the role of tumor-stroma interactions in metastasis to different organs. Int J Cancer. 2011;128:2527–2535.
- Kurahashi N, Iwasaki M, Sasazuki S, et al. Association of body mass index and height with risk of prostate cancer among middle-aged Japanese men. Br J Cancer. 2006;94:740–742.
- Scosyrev E, Messing EM, Mohile S, et al. Prostate cancer in the elderly: frequency of advanced disease at presentation and disease-specific mortality. Cancer. 2012;118:3062–3070.
- Scosyrev E, Wu G, Mohile S, et al. Prostate-specific antigen screening for prostate cancer and the risk of overt metastatic disease at presentation: analysis of trends over time. Cancer. 2012;118:5768–5776.
- Imbriaco M, Larson SM, Yeung HW, et al. A new parameter for measuring metastatic bone involvement by prostate cancer: the Bone Scan Index. Clin Cancer Res. 1998;4:1765–1772.
- Brown MD, Hart CA, Gazi E, et al. Promotion of prostatic metastatic migration towards human bone marrow stoma by omega 6 and its inhibition by omega 3 PUFAs. Br J Cancer. 2006;94:842–853.
- Gazi E, Gardner P, Lockyer NP, et al. Direct evidence of lipid translocation between adipocytes and prostate cancer cells with imaging FTIR microspectroscopy. J Lipid Res. 2007;48:1846–1856.
- Caers J, Deleu S, Belaid Z, et al. Neighboring adipocytes participate in the bone marrow microenvironment of multiple myeloma cells. Leukemia. 2007;21:1580–1584.
- Bussard KM, Gay CV, Mastro AM. The bone microenvironment in metastasis; what is special about bone? Cancer Metastasis Rev. 2008;27:41–55.
- Thobe MN, Clark RJ, Bainer RO, et al. From prostate to bone: key players in prostate cancer bone metastasis. Cancers (Basel). 2011;3:478–493.
- Templeton ZS, Lie WR, Wang W, et al. Breast cancer cell colonization of the human bone marrow adipose tissue niche. Neoplasia. 2015;17:849–861.
- Yue R, Zhou BO, Shimada IS, et al. Leptin receptor promotes adipogenesis and reduces osteogenesis by regulating mesenchymal stromal cells in adult bone marrow. Cell Stem Cell. 2016;18:782–796.
- Zhou BO, Yu H, Yue R, et al. Bone marrow adipocytes promote the regeneration of stem cells and haematopoiesis by secreting SCF. Nat Cell Biol. 2017;19:891–903.
- Snoussi K, Strosberg AD, Bouaouina N, et al. Leptin and leptin receptor polymorphisms are associated with increased risk and poor prognosis of breast carcinoma. BMC Cancer. 2006;6:38.
- Jarde T, Perrier S, Vasson MP, et al. Molecular mechanisms of leptin and adiponectin in breast cancer. Eur J Cancer. 2011;47:33–43.
- Kumar J, Fang H, McCulloch DR, et al. Leptin receptor signaling via janus kinase 2/signal transducer and activator of transcription 3 impacts on ovarian cancer cell phenotypes. Oncotarget. 2017;8:93530–93540.
- Dalamaga M, Diakopoulos KN, Mantzoros CS. The role of adiponectin in cancer: a review of current evidence. Endocr Rev. 2012;33:547–594.
- Libby EF, Frost AR, Demark-Wahnefried W, et al. Linking adiponectin and autophagy in the regulation of breast cancer metastasis. J Mol Med (Berl). 2014;92:1015–1023.
- Jia Z, Liu Y, Cui S. Adiponectin induces breast cancer cell migration and growth factor expression. Cell Biochem Biophys. 2014;70:1239–1245.
- Laharrague P, Fontanilles AM, Tkaczuk J, et al. Inflammatory/haematopoietic cytokine production by human bone marrow adipocytes. Eur Cytokine Netw. 2000;11:634–639.
- Wang L, Cao L, Wang H, et al. Cancer-associated fibroblasts enhance metastatic potential of lung cancer cells through IL-6/STAT3 signaling pathway. Oncotarget. 2017;8:76116–76128.
- Tu Y, Gardner A, Lichtenstein A. The phosphatidylinositol 3-kinase/AKT kinase pathway in multiple myeloma plasma cells: roles in cytokine-dependent survival and proliferative responses. Cancer Res. 2000;60:6763–6770.
- Knupfer H, Preiss R. sIL-6R: more than an agonist? Immunol Cell Biol. 2008;86:87–91.
- Hardaway AL, Herroon MK, Rajagurubandara E, et al. Marrow adipocyte-derived CXCL1 and CXCL2 contribute to osteolysis in metastatic prostate cancer. Clin Exp Metastasis. 2015;32:353–368.
- Chen GL, Luo Y, Eriksson D, et al. High fat diet increases melanoma cell growth in the bone marrow by inducing osteopontin and interleukin 6. Oncotarget. 2016;7:26653–26669.
- De Filippo K, Dudeck A, Hasenberg M, et al. Mast cell and macrophage chemokines CXCL1/CXCL2 control the early stage of neutrophil recruitment during tissue inflammation. Blood. 2013;121:4930–4937.
- Acharyya S, Oskarsson T, Vanharanta S, et al. A CXCL1 paracrine network links cancer chemoresistance and metastasis. Cell. 2012;150:165–178.
- Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol. 2009;9:162–174.
- Kalinski P. Regulation of immune responses by prostaglandin E2. J Immunol. 2012;188:21–28.
- Safina A, Sotomayor P, Limoge M, et al. TAK1-TAB2 signaling contributes to bone destruction by breast carcinoma cells. Mol Cancer Res. 2011;9:1042–1053.
- Singh B, Berry JA, Shoher A, et al. COX-2 involvement in breast cancer metastasis to bone. Oncogene. 2007;26:3789–3796.
- Li Z, Schem C, Shi YH, et al. Increased COX2 expression enhances tumor-induced osteoclastic lesions in breast cancer bone metastasis. Clin Exp Metastasis. 2008;25:389–400.
- Herroon MK, Rajagurubandara E, Hardaway AL, et al. Bone marrow adipocytes promote tumor growth in bone via FABP4-dependent mechanisms. Oncotarget. 2013;4:2108–2123.
- Arendt LM, McCready J, Keller PJ, et al. Obesity promotes breast cancer by CCL2-mediated macrophage recruitment and angiogenesis. Cancer Res. 2013;73:6080–6093.
- Wang J, Chen GL, Cao S, et al. Adipogenic niches for melanoma cell colonization and growth in bone marrow. Lab Invest. 2017;97:737–745.
- Diedrich JD, Rajagurubandara E, Herroon MK, et al. Bone marrow adipocytes promote the Warburg phenotype in metastatic prostate tumors via HIF-1alpha activation. Oncotarget. 2016;7:64854–64877.
- Sumida T. Clinical and experimental study on fatty acid composition of bone marrow lipid in hematologic disorders. Acta Med Nagasaki. 1965;9:222–241.
- Hughes-Fulford M, Li CF, Boonyaratanakornkit J, et al. Arachidonic acid activates phosphatidylinositol 3-kinase signaling and induces gene expression in prostate cancer. Cancer Res. 2006;66:1427–1433.
- Rose DP. Effects of dietary fatty acids on breast and prostate cancers: evidence from in vitro experiments and animal studies. Am J Clin Nutr. 1997;66:1513s–1522s.
- Tjandrawinata RR, Dahiya R, Hughes-Fulford M. Induction of cyclo-oxygenase-2 mRNA by prostaglandin E2 in human prostatic carcinoma cells. Br J Cancer. 1997;75:1111–1118.
- Kelesidis I, Kelesidis T, Mantzoros CS. Adiponectin and cancer: a systematic review. Br J Cancer. 2006;94:1221–1225.
- Behan JW, Yun JP, Proektor MP, et al. Adipocytes impair leukemia treatment in mice. Cancer Res. 2009;69:7867–7874.
- Trotter TN, Gibson JT, Sherpa TL, et al. Adipocyte-lineage cells support growth and dissemination of multiple myeloma in bone. Am J Pathol. 2016;186:3054–3063.
- Chen Q, Yuan Y, Chen T. Morphology, differentiation and adhesion molecule expression changes of bone marrow mesenchymal stem cells from acute myeloid leukemia patients. Mol Med Rep. 2014;9:293–298.
- Naveiras O, Nardi V, Wenzel PL, et al. Bone-marrow adipocytes as negative regulators of the haematopoietic microenvironment. Nature. 2009;460:259–263.
- Lu W, Wan Y, Li Z, et al. Growth differentiation factor 15 contributes to marrow adipocyte remodeling in response to the growth of leukemic cells. J Exp Clin Cancer Res. 2018;37:66.
- Zaidi N, Lupien L, Kuemmerle NB, et al. Lipogenesis and lipolysis: the pathways exploited by the cancer cells to acquire fatty acids. Prog Lipid Res. 2013;52:585–589.
- Harte AL, Tripathi G, Piya MK, et al. NFkappaB as a potent regulator of inflammation in human adipose tissue, influenced by depot, adiposity, T2DM status, and TNFalpha. Obesity (Silver Spring). 2013;21:2322–2330.
- Yu W, Cao DD, Li QB, et al. Adipocytes secreted leptin is a pro-tumor factor for survival of multiple myeloma under chemotherapy. Oncotarget. 2016;7:86075–86086.
- Liu Z, Xu J, He J, et al. Mature adipocytes in bone marrow protect myeloma cells against chemotherapy through autophagy activation. Oncotarget. 2015;6:34329–34341.
- Fowler JA, Lwin ST, Drake MT, et al. Host-derived adiponectin is tumor-suppressive and a novel therapeutic target for multiple myeloma and the associated bone disease. Blood. 2011;118:5872–5882.
- Medina EA, Oberheu K, Polusani SR, et al. PKA/AMPK signaling in relation to adiponectin’s antiproliferative effect on multiple myeloma cells. Leukemia. 2014;28:2080–2089.
- Venkateshaiah SU, Khan S, Ling W, et al. NAMPT/PBEF1 enzymatic activity is indispensable for myeloma cell growth and osteoclast activity. Exp Hematol. 2013;41:547–557.e542.
- Gado K, Domjan G, Hegyesi H, et al. Role of INTERLEUKIN-6 in the pathogenesis of multiple myeloma. Cell Biol Int. 2000;24:195–209.
- Birmann BM, Neuhouser ML, Rosner B, et al. Prediagnosis biomarkers of insulin-like growth factor-1, insulin, and interleukin-6 dysregulation and multiple myeloma risk in the multiple myeloma cohort consortium. Blood. 2012;120:4929–4937.
- Jourdan M, Tarte K, Legouffe E, et al. Tumor necrosis factor is a survival and proliferation factor for human myeloma cells. Eur Cytokine Netw. 1999;10:65–70.
- Arendt BK, Velazquez-Dones A, Tschumper RC, et al. Interleukin 6 induces monocyte chemoattractant protein-1 expression in myeloma cells. Leukemia. 2002;16:2142–2147.
- Zheng Y, Yang J, Qian J, et al. PSGL-1/selectin and ICAM-1/CD18 interactions are involved in macrophage-induced drug resistance in myeloma. Leukemia. 2013;27:702–710.
- Uddin S, Mohammad RM. Role of leptin and leptin receptors in hematological malignancies. Leuk Lymphoma. 2016;57:10–16.
- Kohler JA, Moon RJ, Wright S, et al. Increased adiposity and altered adipocyte function in female survivors of childhood acute lymphoblastic leukaemia treated without cranial radiation. Horm Res Paediatr. 2011;75:433–440.
- Foss B, Mentzoni L, Bruserud O. Effects of vascular endothelial growth factor on acute myelogenous leukemia blasts. J Hematother Stem Cell Res. 2001;10:81–93.
- Gorska E, Popko K, Wasik M. Leptin receptor in childhood acute leukemias. Adv Exp Med Biol. 2013;756:155–161.
- Tabe Y, Konopleva M, Munsell MF, et al. PML-RARalpha is associated with leptin-receptor induction: the role of mesenchymal stem cell-derived adipocytes in APL cell survival. Blood. 2004;103:1815–1822.
- Juarez J, Bradstock KF, Gottlieb DJ, et al. Effects of inhibitors of the chemokine receptor CXCR4 on acute lymphoblastic leukemia cells in vitro. Leukemia. 2003;17:1294–1300.
- Pramanik R, Sheng X, Ichihara B, et al. Adipose tissue attracts and protects acute lymphoblastic leukemia cells from chemotherapy. Leuk Res. 2013;37:503–509.
- Shafat MS, Oellerich T, Mohr S, et al. Leukemic blasts program bone marrow adipocytes to generate a protumoral microenvironment. Blood. 2017;129:1320–1332.
- Tabe Y, Yamamoto S, Saitoh K, et al. Bone marrow adipocytes facilitate fatty acid oxidation activating AMPK and a transcriptional network supporting survival of acute monocytic leukemia cells. Cancer Res. 2017;77:1453–1464.
- Lu W, Weng W, Zhu Q, et al. Small bone marrow adipocytes predict poor prognosis in acute myeloid leukemia. Haematologica. 2018;103:e21–e24.
- Veldhuis-Vlug AG, Rosen CJ. Clinical implications of bone marrow adiposity. J Intern Med. 2018;283:121–139.
- Sheng X, Mittelman SD. The role of adipose tissue and obesity in causing treatment resistance of acute lymphoblastic leukemia. Front Pediatr. 2014;2:53.
- Holley RW, Baldwin JH, Kiernan JA. Control of growth of a tumor cell by linoleic acid. Proc Natl Acad Sci U S A. 1974;71:3976–3978.
- Butler M, Huzel N, Barnabe N. Unsaturated fatty acids enhance cell yields and perturb the energy metabolism of an antibody-secreting hybridoma. Biochem J. 1997;322(Pt 2):615–623.
- Sravan Kumar G, Das UN. Cytotoxic action of alpha-linolenic and eicosapentaenoic acids on myeloma cells in vitro. Prostaglandins Leukot Essent Fatty Acids. 1997;56:285–293.
- Finstad HS, Myhrstad MC, Heimli H, et al. Multiplication and death-type of leukemia cell lines exposed to very long-chain polyunsaturated fatty acids. Leukemia. 1998;12:921–929.
- Sarraf P, Frederich RC, Turner EM, et al. Multiple cytokines and acute inflammation raise mouse leptin levels: potential role in inflammatory anorexia. J Exp Med. 1997;185:171–175.
- Gonzalez-Perez RR, Xu Y, Guo S, et al. Leptin upregulates VEGF in breast cancer via canonic and non-canonical signalling pathways and NFkappaB/HIF-1alpha activation. Cell Signal. 2010;22:1350–1362.
- Shpilman M, Niv-Spector L, Katz M, et al. Development and characterization of high affinity leptins and leptin antagonists. J Biol Chem. 2011;286:4429–4442.
- Rene Gonzalez R, Watters A, Xu Y, et al. Leptin-signaling inhibition results in efficient anti-tumor activity in estrogen receptor positive or negative breast cancer. Breast Cancer Res. 2009;11:R36.
- Harmon T, Harbuzariu A, Lanier V, et al. Nanoparticle-linked antagonist for leptin signaling inhibition in breast cancer. World J Clin Oncol. 2017;8:54–66.
- Otvos L Jr., Kovalszky I, Riolfi M, et al. Efficacy of a leptin receptor antagonist peptide in a mouse model of triple-negative breast cancer. Eur J Cancer. 2011;47:1578–1584.
- Beccari S, Kovalszky I, Wade JD, et al. Designer peptide antagonist of the leptin receptor with peripheral antineoplastic activity. Peptides. 2013;44:127–134.
- Catalano S, Leggio A, Barone I, et al. A novel leptin antagonist peptide inhibits breast cancer growth in vitro and in vivo. J Cell Mol Med. 2015;19:1122–1132.
- Gillespie C, Guo S, Zhou W, et al. Abstract 825: leptin signaling disruption prevents DMBA-induced mammary tumors in lean and diet-induced-obesity (DIO) mice. Cancer Res. 2011;71:825.
- Azab AK, Runnels JM, Pitsillides C, et al. CXCR4 inhibitor AMD3100 disrupts the interaction of multiple myeloma cells with the bone marrow microenvironment and enhances their sensitivity to therapy. Blood. 2009;113:4341–4351.
- Hoellenriegel J, Zboralski D, Maasch C, et al. The Spiegelmer NOX-A12, a novel CXCL12 inhibitor, interferes with chronic lymphocytic leukemia cell motility and causes chemosensitization. Blood. 2014;123:1032–1039.
- Obmolova G, Teplyakov A, Malia TJ, et al. Structural basis for high selectivity of anti-CCL2 neutralizing antibody CNTO 888. Mol Immunol. 2012;51:227–233.
- Loberg RD, Ying C, Craig M, et al. CCL2 as an important mediator of prostate cancer growth in vivo through the regulation of macrophage infiltration. Neoplasia. 2007;9:556–562.
- Lim SY, Yuzhalin AE, Gordon-Weeks AN, et al. Targeting the CCL2-CCR2 signaling axis in cancer metastasis. Oncotarget. 2016;7:28697–28710.
- Brana I, Calles A, LoRusso PM, et al. Carlumab, an anti-C-C chemokine ligand 2 monoclonal antibody, in combination with four chemotherapy regimens for the treatment of patients with solid tumors: an open-label, multicenter phase 1b study. Target Oncol. 2015;10:111–123.
- Korner A, Pazaitou-Panayiotou K, Kelesidis T, et al. Total and high-molecular-weight adiponectin in breast cancer: in vitro and in vivo studies. J Clin Endocrinol Metab. 2007;92:1041–1048.
- Gao F, Chattopadhyay A, Navab M, et al. Apolipoprotein A-I mimetic peptides inhibit expression and activity of hypoxia-inducible factor-1alpha in human ovarian cancer cell lines and a mouse ovarian cancer model. J Pharmacol Exp Ther. 2012;342:255–262.
- Zhou W, Tu Y, Simpson PJ, et al. Malonyl-CoA decarboxylase inhibition is selectively cytotoxic to human breast cancer cells. Oncogene. 2009;28:2979–2987.
- Pascual G, Avgustinova A, Mejetta S, et al. Targeting metastasis-initiating cells through the fatty acid receptor CD36. Nature. 2017;541:41–45.
