ABSTRACT
Elastin is a highly elastic protein present in connective tissue. As a result of protease activity, elastin hydrolysis occurs, and during this process, elastin-derived peptides (EDPs) are released. One of the constitutively repeating elastin and EDP building sequences is the hexapeptide VGVAPG. Therefore, the aim of our research was to define the effect of VGVAPG peptide on adipogenesis in a mouse 3T3-L1 cell line. 3T3-L1 cells were differentiated according to a previously described protocol and exposed to increasing concentrations of VGVAPG or VVGPGA peptide. The obtained results showed that VGVAPG peptide does not stimulate reactive oxygen species (ROS) production, caspase-1 activation, and 3T3-L1 cell proliferation. In the second part of the experiments, it was proved that VGVAPG peptide decreased lipid accumulation as measured by oil red O staining, which was confirmed by the profile of increased expression markers of undifferentiated preadipocytes. In our experiments, 10 nM VGVAPG added for differentiating to adipocytes increased the expression of Pref-1, serpin E1, and adiponectin as compared to rosiglitazone (PPARγ agonist)-treated group and simultaneously decreased the expression of VEGF and resistin as compared to the rosiglitazone-treated group. The obtained results show that VGVAPG peptide sustains 3T3 cells in undifferentiated state.
Abbreviations: DMSO: dimethyl sulphoxide; EBP: elastin-binding protein; EDPs: elastin-derived peptides; FBS: foetal bovine serum; Glb1: gene for beta-galactosidase; LDL: low-density-lipoprotein; PAI-1 (Serpin E1): plasminogen activator inhibitor-1; PBS: phosphate-buffered saline; PPARγ: peroxisome proliferator-activated receptor gamma; Pref-1: preadipocyte factor 1; ROS: reactive oxygen species; VEGF-A: vascular endothelial growth factor-A; VGVAPG: Val-Gly-Val-Ala-Pro-Gly; β-Gal: beta-galactosidase; ORO: oil red O; IBMX: 3-isobutyl-1-methylxanthine; H2DCFDA: 2ʹ,7ʹ-dichlorodihydrofluorescein diacetate; DMEM: Dulbecco’s Modified Eagle’s Medium; VVGPGA: Val-Val-Gly-Pro-Gly-Ala.
1. Introduction
Elastin is a highly elastic protein that occurs in connective tissue. Together with microfibrils, they are the main building blocks of elastin fibres. Skin, lung tissue, tendons, ligaments, and walls of blood vessels are rich in elastin [Citation1]. This protein is synthesized in fibroblasts, chondroblasts, endothelial cells, and smooth muscle cells [Citation2–5]. In physiological conditions, elastin-rich extracellular matrix (ECM) is slowly degraded; however, this process is accelerated during ageing or pathological conditions such as inflammation, atherosclerosis, and carcinogenesis in the breast, skin, and lung [Citation6–9]. Elastin is degraded by proteolytic enzymes produced by monocytes, thrombocytes, neutrophils, lymphocytes, smooth muscle cells, skin fibroblasts, certain malignant tumour cells, and cells of adipose tissue [Citation10–16]. As a result of protease activity, elastin hydrolysis occurs, and during this process, elastin-derived peptides (EDPs) are released [Citation17]. One of the constitutively repeating elastin and EDP building sequences is the hexapeptide valine-glycine-valine-alanine-proline-glycine (VGVAPG) [Citation18]. This peptide interacts with cells through a 67-kDa elastin-binding protein (EBP) located on the cell surface [Citation18]. EBP is a catalytically inactive form of the alternatively spliced gene for β-galactosidase (GLB1 gene) [Citation19,Citation20]. The second receptor for the VGVAPG peptide is galectin-3, which also has an important role in cell–ECM interactions [Citation21]. Galectin-3 is mostly expressed in inflammatory cells [Citation22,Citation23]; however, its expression has been linked with tumour progression, and cancer aggressiveness [Citation24–26]. There are several intracellular signalling pathways elicited by the EDP. The elastin receptor complex is linked to the G proteins which activation, opening of L-type calcium channels, and sequential activation of tyrosine kinases: FAK, c-Src, platelet-derived growth factor receptor kinase and then Ras-Raf- MEK1/2-ERK1/2 phosphorylation cascade [Citation27].
It has been shown that EDPs and/or VGVAPG induce some biological effects such as cell proliferation, migration, differentiation, and inflammation through EBP activation [Citation2,Citation28–30]. Recently, it has been reported that VGVAPG peptide affects peroxisome proliferator-activated receptor gamma (PPARγ) mRNA and protein expression [Citation31]. Furthermore, similar to EDPs, PPARγ is involved in cell proliferation, migration, and differentiation and most importantly in adipocyte differentiation [Citation32]. Moreover, PPARγ is directly related to obesity in rodents, resulting in the overexpression of PPARγ in adipose tissue [Citation33]. Adipocytes are characterized by secretion of many proteins such as adipocyte-derived vascular endothelial growth factor-A (VEGF-A) whose level is increased in obesity [Citation34]; adiponectin related to insulin resistance [Citation35]; preadipocyte factor 1 (Pref-1) that prevents fibroblasts from differentiation (adipose tissue generation) [Citation36]; adipose-tissue secretory factor (ADSF/resistin) involved in low-density lipoprotein (LDL) level regulation [Citation37]; and plasminogen activator inhibitor-1 (PAI-1/serpin E1) that plays a major role in insulin resistance [Citation38]. All these proteins are also closely related to obesity, diabetes, atherosclerosis, or other conditions such as heart diseases. Hence, research studies are necessary to clarify the expression of these proteins in adipocytes. In addition to their role in glucose and lipid metabolism, adipocytes respond differentially to physiological indications or metabolic stress by releasing endocrine factors that regulate diverse processes, such as energy expenditure, appetite control, glucose homoeostasis, insulin sensitivity, inflammation and tissue repair [Citation39]. Furthermore, reactive oxygen species (ROS) have been implicated as a contributor to both the onset and progression of insulin resistance and obesity, which are also associated with chronic low-grade inflammation [Citation40]. To date, studies on the significance of VGVAPG peptide in the formation and metabolism of adipose tissue are limited. Blaise and team (2013) described that EDPs are involved in the development of insulin resistance in mice [Citation41]. Moreover, Robert and colleagues, demonstrated that the concentration of anti-EDP antibodies of IgG is increased threefold in type 2 diabetic patients compared with the control population [Citation42–44].
Therefore, the aim of our research was to determine the effect of VGVAPG peptide on adipogenesis in 3T3-L1 cell line that can function as an in vitro model for lipid accumulation.
2. Materials and methods
2.1. Reagents
Dulbecco’s Modified Eagle’s Medium (DMEM) without phenol red (10–013-CVR) and phosphate-buffered saline without calcium and magnesium (PBS) were purchased from Corning (Manassas, VA, USA). Trypsin, rosiglitazone, oil red O (ORO), Ac-YVAD-pNA (caspase-1 substrate), resazurin, penicillin, streptomycin, amphotericin B, 3-isobutyl-1-methylxanthine (IBMX), dexamethasone, insulin, RIPA buffer (product number R0278 – 50 mM Tris-HCl, pH 8.0, with 150 mM sodium chloride, 1.0% Igepal CA-630 (NP-40), 0.5% sodium deoxycholate, and 0.1% sodium dodecyl sulphate), 2ʹ,7ʹ-dichlorodihydrofluorescein diacetate (H2DCFDA), and dimethyl sulphoxide (DMSO) were purchased from Sigma-Aldrich (St. Louis, MO, USA). VGVAPG and VVGPGA peptides were synthesized by LipoPharm.pl (Gdańsk, Poland). Foetal bovine serum (FBS) was purchased from EURx (Gdańsk, Poland). Ki67 (EM1473) was purchased from Fine Biotech (Wuhan, China). Proteome profiler mouse adipokine array (ARY013) was purchased from R&D Systems, Inc. (Minneapolis, MN, USA). Stock solutions of VGVAPG and VVGPGA peptides were prepared in DMSO and then added to the DMEM medium. The final concentration of DMSO in the culture medium was always 0.1%.
2.2. 3T3-L1 cell culture, differentiation procedure, and treatment
Mouse embryonic fibroblast cell line 3T3-L1 was obtained from the American Type Culture Collection (ATCC, distributor: LGC Standards, Łomianki, Poland). The 3T3-L1 cell line was maintained in DMEM supplemented with 10% FBS, 100 U/mL penicillin, 0.10 mg/mL streptomycin, and 250 ng/mL amphotericin B. The cells were maintained at 37°C in a humidified atmosphere with 5% CO2. The cells were seeded in 96-well culture plates at a density of 6 × 103 cells/well and in 6-well culture plates at a density of 3 × 105 cells/well, and then initially cultured before the experiment for 24 h. Subsequently, the medium was replaced with a fresh one by increasing the concentrations (1, 10, 50, and 100 nM and 1, 10, 50, and 100 µM) of VGVAPG or VVGPGA, respectively. The levels of ROS, caspase-1 activation, resazurin reduction, and Ki67 protein expression were then measured.
Differentiation procedure was assessed according to Zebisch et al. (2012) with slight modifications [Citation45] Briefly, 3T3-L1 cells were routinely cultured in basal medium (DMEM containing 10% FBS, 100 U/mL penicillin, 0.10 mg/mL streptomycin, and 250 ng/mL amphotericin B). The cells were seeded in 48-well plates at a density of 3 × 104 cells/well (for ORO staining) or in 6-well plates at a density of 3 × 105 cells/well (for protein). Forty-eight hours after seeding (day 2), cell differentiation was induced by changing the medium to basal medium containing 0.5 mM IBMX, 0.25 µM dexamethasone, and 1 µg/mL insulin. The experimental groups were negative control (without rosiglitazone), positive control with 2 µM rosiglitazone, and groups treated with 10 nM VGVAPG or 10 nM VVGPGA. After 48 h, the medium was changed to basal medium containing 1 µg/mL insulin for the next 48 h. After this time, the cell culture medium was again changed to basal medium, and the procedure was repeated after every two days. At 14 day (measured from cell seeding), ORO staining (48-well plates) and cell harvesting (6-well plates) were performed.
2.3. Production of ROS induced by VGVAPG peptide
The method for ROS determination is based on the oxidation of the fluorogenic dye 2ʹ,7ʹ-dichlorodihydrofluorescein diacetate (H2DCFDA), which is added to the cells before treatment with the studied peptide [Citation46]. In the experiment, the cells were seeded in 96-well plates in DMEM medium supplemented with 10% FBS. After 24 h, the medium was replaced with DMEM without FBS containing the fluorogenic dye at 5 μM concentration for 45 min. The dye was then removed, and a series of dilutions of VGVAPG peptide (1 nM–100 µM) in DMEM supplemented with 1% FBS were added to the plate. To assess the ability of VGVAPG or VVGPGA to induce ROS production in 3T3-L1 cells, fluorescence was measured after 3, 6, 24, and 48 h. A microplate reader (FilterMax F5) was used to measure the maximum excitation and emission spectrum at 485 nm and 535 nm wavelengths, respectively.
2.4. Caspase-1 activity
Caspase-1 activity was assessed according to Nicholson et al. [Citation47]. To measure caspase-1 activity, the cells were plated on 96-well plates and exposed to increasing concentrations of VGVAPG or VVGPGA peptides. Controls with or without DMSO vehicle were included in the experimental design to determine the effect of DMSO (results not shown). After thawing (−80ºC), the cells were lysed using lysis buffer (50 mM HEPES, pH 7.4, 100 mM NaCl, 0.1% CHAPS, 1 mM EDTA, 10% glycerol, and 10 mM DTT) at 10°C for 10 min. The lysates were incubated in the caspase-1 substrate Ac-YVAD-pNA at 37°C. After 30 min, the absorbance of the lysates at 405 nm was measured using a microplate reader (FilterMax F5). The amount of the colorimetric product was continuously monitored for 120 min.
2.5. Resazurin reduction assay
To determine changes in the level of cell metabolism/proliferation stimulated by VGVAPG, we used the redox dye resazurin. Depending on the metabolic activity of the cells, the dye shows both colorimetric and fluorometric changes. The metabolically active cells reduce nonfluorescent resazurin to fluorescent resorufin [Citation48]. The cells were seeded on 96-well plates and exposed to increasing concentrations of VGVAPG or VVGPGA peptides at 24 h after seeding. After 48 or 72 h of incubation, the medium was removed and replaced with a new one (DMEM containing 1% FBS and 10% resazurin). The plates were then incubated for 60 min. Following this incubation, fluorescence was measured using a microplate reader (FilterMax F5) at the maximum excitation and emission spectrum of 530 nm and 590 nm wavelengths, respectively.
2.6. Enzyme-linked Immunosorbent Assay (ELISA) for Ki67
The level of Ki67 protein was determined after 48 h of exposure to 1, 10, 50, and 100 nM and 1, 10, 50, and 100 µM of VGVAPG or VVGPGA treatment, respectively, by enzyme-linked immunosorbent assay (ELISA). These proteins were specifically detected by ELISA and subsequently subjected to quantitative sandwich enzyme immunoassay. The assay was performed according to the manufacturer’s instructions. Briefly, a 96-well plate was precoated with monoclonal antibodies specific to Ki67. Standards and the collected cell extracts were added to the wells and incubated for 90 min at 37°C. Next, after removing the liquid, 100 µL of biotinylated detection antibodies were added for 60 min. After 3 times washing to remove any unbound substances, horseradish peroxidase-conjugated avidin was added. Following additional washing, 90 µL of substrate solution was added to the wells for 15 min. Then, 50 µL of the reaction termination solution was added, and the absorbance was measured at 450 nm. The obtained values were proportional to the amount of Ki67. The total protein concentration was determined in triplicate in each sample by using a Thermo Fisher NanoDrop device.
2.7. Oil Red O (ORO) staining and quantification
Oil red O (ORO) staining was performed according to Zebish et al. with slight modifications [Citation45]. To perform the staining of cells, 1 day before the experiment, a stock solution of ORO was prepared by dissolving 1 g of ORO in 100 mL of absolute isopropanol. The prepared solution was left standing overnight on a stirrer at room temperature. The solution was then filtered through a filter paper. The working solution was prepared just before staining by mixing the stock solution with distilled water (3:2). The cells were washed twice with PBS and incubated for 1 h at room temperature in a 10% formalin solution. After incubation, the cells were washed twice with distilled water and once with 60% isopropanol. The cells were then incubated with the working solution of the ORO dye for 15 min. The cells were washed 5 times with distilled water, and after the 5th wash, the cells were photographed. The cells were then washed three times with 60% isopropanol. Finally, the cells were washed with 100% isopropanol. The samples were then transferred to new 96-well plates to measure absorbance at 450 nm. The blank was 100% isopropanol.
2.8. Proteome profiler mouse adipokine array
Briefly, cells were collected in RIPA buffer, and protein concentration was determined using a Thermo Fisher NanoDrop device. A protein profiler membrane was prepared according to the manufacturer’s protocol. Next, equal amounts of protein (500 µg/mL) from the experimental group (Control – undifferentiated; Rosiglitazone – positive control, cell differentiated with 2 µM rosiglitazone; VVGPGA – cell differentiated with 10 nM VVGPGA, VGVAPG – cell differentiated with 10 nM VGVAPG) were mixed with 15 μL of reconstituted mouse adipokine detection antibody cocktail and incubated at room temperature for 1 h. The sample/antibody mixtures were added to 4-well plates and incubated overnight at 2–8°C on a rocking platform shaker. On the next day, the membranes were washed in a wash buffer two times for 10 min. After this step, the membranes were incubated with streptavidin-HRP antibody for 30 min at room temperature on a rocking platform shaker. After washing for three times, the chemiluminescent reagent was added to each membrane and incubated for 1 min. In the last step, the membranes were placed in a C-DiGit Blot Scanner (LI-COR) and scanned for chemiluminescence. Quantification of protein band densitometry was carried out using ImageJ 1.52a software.
2.9. Statistical analysis
Data are presented as mean ± SD of three independent experiments. Each treatment was repeated six times (n = 6) and measured in triplicate. The data were normalized to the vehicle-treated control cells and presented as percentage of control. The data were analysed by one-way analysis of variance (ANOVA) followed by Tukey’s multiple comparison procedure ***p < 0.001, **p < 0.01, and *p < 0.05 vs. the control were considered statistically significant.
3. Results
3.1. Measurement of ROS production
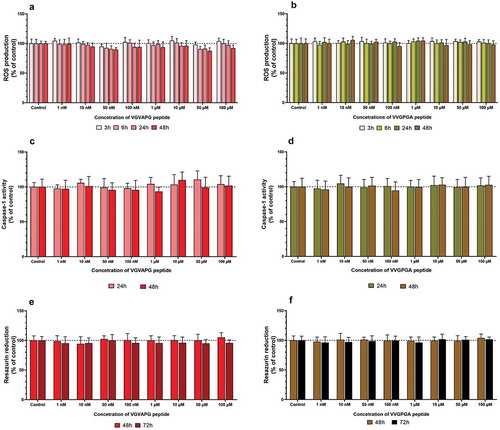
After 3T3-L1 cell treatment with increasing concentrations of VGVAPG or VVGPGA peptides, no changes were found in ROS production in all studied time intervals (3, 6, 24, and 48 h) ().
Figure 1. Effect of the increasing concentrations of VGVAPG or VVGPGA peptides on ROS production (a and b), activity of caspase-1 (c and d), and resazurin reduction (e and f). Measurement were performed after 3, 6, 24, and 48 h for ROS; after 24 and 48 h for caspase-1 activity; and after 48 and 72 h for resazurin reduction assay in mouse 3T3-L1 cell line. Data are expressed as mean ± SD of three independent experiments, each of which comprised six replicates per treatment group
3.2. Measurement of caspase-1 activity
After 3T3-L1 cell treatment with increasing concentrations of VGVAPG or VVGPGA peptides, no significant changes were found in caspase-1 activity in both 24 and 48 h time intervals ().
3.3. Measurement of cell metabolism
After 3T3-L1 cell treatment with increasing concentrations of VGVAPG or VVGPGA peptides, no significant changes were found in the resazurin reduction assay in both 48 and 72 h time intervals ().
3.4. Measurement of Ki67 protein level
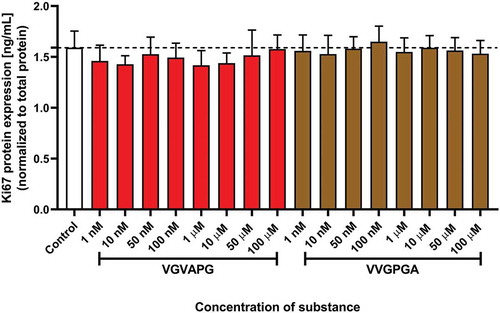
After 48 h of 3T3-L1 cell exposure to increasing concentrations of VGVAPG or VVGPGA peptide, no significant changes were found in Ki67 protein expression level ().
Figure 2. Effect of the increasing concentrations of VGVAPG or VVGPGA peptides on the expression of Ki67 protein. Ki67 level was measured by the ELISA method after 48 h of exposure of 3T3-L1 cell line to the studied peptides. Data are expressed as mean ± SD of three independent experiments, each of which comprised six replicates per treatment group
3.5. Measurement of lipid accumulation by ORO staining
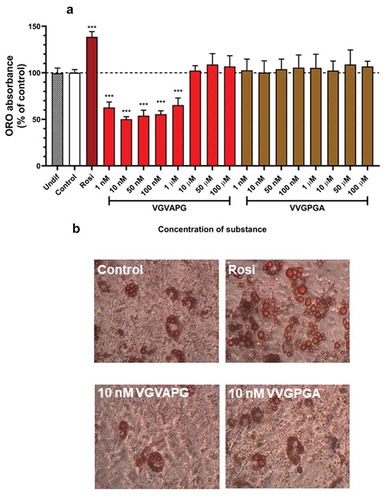
After 14 days of differentiation using IBMX, dexamethasone, insulin, and other appropriate compounds (increasing concentrations of VGVAPG or VVGPGA or 2 µM rosiglitazone), ORO staining and its quantification were performed. Undifferentiated 3T3-L1 cells (Undif); cells differentiated using only IBMX, dexamethasone, and insulin (Control); and cells differentiated using IBMX, dexamethasone, and insulin and increasing concentrations of VVGPGA or VGVAPG (1, 10, 50, and 100 nM and 1, 10, 50, 100 µM, respectively) did not accumulate lipid (). In cells differentiated using IBMX, dexamethasone, insulin, and 2 µM rosiglitazone (Positive Control), lipid accumulation significantly increased as compared to that in Control without rosiglitazone (increase by 38.57%). In differentiated 3T3-L1 cells with VGVAPG concentrations in range of 1–100 nM and 1 µM peptide, lipid accumulation decreased as compared to that in Control (decrease from 50.01% to 34.54%). However, VGVAPG peptide treatment in the range of 10–100 µM did not affect lipid accumulation as compared to that in Control ().
Figure 3. Effect of the increasing concentrations of VGVAPG or VVGPGA (a) peptides on lipid accumulation in 3T3-L1 cell line. Undif – undifferentiated cells; Control – cells differentiated with IBMX, dexamethasone, insulin, without rosiglitazone; Rosi – positive control, differentiated cells with IBMX, dexamethasone, insulin and 2 µM rosiglitazone; groups treated with VGVAPG or VVGPGA were differentiated with IBMX, dexamethasone, insulin and appropriate concentration of VGVAPG or VVGPGA. ORO staining (b) and quantification were performed after 14 days of differentiation
3.6. Proteome profiler mouse adipokine array
After 14 days of differentiation using IBMX, dexamethasone, insulin, and other appropriate compounds (10 nM VGVAPG, 10 nM VVGPGA, or 2 µM rosiglitazone), protein quantification with the proteome profiler array was performed. Two controls (Control: undifferentiated cells and VVGPGA Control: cells differentiated with 10 nM VVGPGA – that peptide that does not activate EBP) were used. Cells differentiated with rosiglitazone were used as a positive control ().
Figure 4. Effect of 10 nM VGVAPG or VVGPGA peptides on the protein expression profile in 3T3-L1 cell line. Control – undifferentiated cells; Rosiglitazone – positive control, cells differentiated with IBMX, dexamethasone, insulin, and 2 µM rosiglitazone; VGVAPG – cells differentiated with IBMX, dexamethasone, insulin, and 10 nM VGVAPG; VVGPGA – negative control VVGPGA peptide that does not activate EBP, cells differentiated with IBMX, dexamethasone, insulin, and 10 nM VVGPGA (a). Protein measurement was performed after 14 days of differentiation. Densitometry was performed by ImageJ 1.52a software (b)
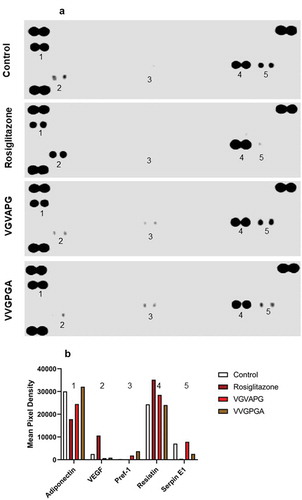
VGVAPG peptide decreased the expression of adiponectin as compared to that in Control and VVGPGA cells. However, rosiglitazone caused a higher reduction of adiponectin expression than VGVAPG peptide.
Further, VGVAPG peptide decreased the expression of VEGF compared to that in Control and VVGPGA cells, but rosiglitazone significantly increased VEGF expression in 3T3-L1 cells.
In cells treated with IBMX, dexamethasone, insulin, and VGVAPG or VVGPGA, the expression of Pref-1 was increased significantly as compared to that in undifferentiated cells. However, Pref-1 expression was lower in cells treated with VGVAPG than in cells treated with VVGPGA. Pref-1 expression was not detected in the group treated with rosiglitazone.
The expression level of resistin did not differ in Control and VVGPGA cells. Cells treated with VGVAPG peptide showed increased level of resistin expression. Resistin expression was much higher in cells treated with rosiglitazone than in the VGVAPG peptide-treated group.
In cells treated with VGVAPG peptide, the expression of serpin E1 was similar to that of undifferentiated cells and much higher than that in cells treated with VVGPGA or rosiglitazone. The lowest expression of serpin E1 was noted in the rosiglitazone-treated group ().
4. Discussion
It is well known that EDPs or VGVAPG increase ROS production in different cells such as murine monocytes and astrocytes, human fibroblasts, and neuroblastoma (SH-SY5Y) cells [Citation49–53]. Moreover, ROS are key signalling molecules that play an important role in the progression of inflammatory disorders. Interleukin-1 beta (IL-1β) is one of the key pro-inflammatory cytokines in an organism [Citation54]. IL-1β is formed when its inactive precursor pro-IL-1β is activated by limited proteolysis through the interleukin-1-beta-converting enzyme (ICE), which is currently known as caspase-1 [Citation55]. ROS activation can initiate or result in an inflammation process in which caspase-1 plays an important role [Citation56]. Caspase-1 is a member of the intracellular cysteine protease family that mediates inflammation and activates IL-1β and IL-18 [Citation57]. Mice lacking IL-18 become obese and insulin resistant, and both IL-1β and IL-18 play a role in overall energy balance [Citation58]. Moreover, it has been reported that in mouse with caspase-1 knocked out, obesity develops similar to mice with IL-18 deficiency [Citation59]. Interestingly, the IL-18 protein level is upregulated in the adipose tissue of obese mice [Citation60]. Furthermore, ROS production and/or inflammation process can initiate cell death and decrease cell metabolism, but in some cases, a contrasting effect might be observed and metabolism and/or cell proliferation could increase [Citation61,Citation62]. Our data show that VGVAPG peptide did not increase ROS production and induce caspase-1 activation and cell proliferation in all studied concentrations and time intervals in 3T3-L1 cell line. To date, previous studies have shown that EDPs and/or VGVAPG peptide increase the production and/or secretion inflammatory markers such as IL-1α, IL-1β, and IL-6 in ligamentum flavum cells, synovial cells, and melanoma cell lines [Citation63–65]. On the other hand, our previous studies show that VGVAPG peptide increases the expression of PPARγ and decreases the expression of nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) and production of IL-1β in mouse astrocytes in a PPARγ-dependent manner, which suggest that the effect of VGVAPG is tissue dependent [Citation31,Citation66]. Interestingly, our latest study revealed that caspase-1 activity increases, which suggests that this caspase does not play an inflammatory role in mouse astrocytes [Citation66].
ORO staining and its quantification are a recognized marker for determining the maturity of adipocytes [Citation67]. This method is based on a correlation between higher values of ORO dye in the fully matured adipocytes than in less differentiated or undifferentiated cells. This method has been successfully validated, indicating that the ORO assay can be used to analyse adipocyte differentiation [Citation68–70]. Our obtained data show that VVGPGA peptide did not induce lipid accumulation in 3T3-L1 cells, while VGVAPG peptide in the range of 1 nM to 1 µM decreased lipid accumulation as compared to that in control. Based on the obtained data from ORO measurement for further research, we chose 10 nM VGVAPG peptide as the concentration to evaluate the level of activated proteins during the differentiation process in adipocytes.
During the differentiation process in preadipocytes, the expression profile of proteins changed in 3T3-L1 cell line. Our data show that VGVAPG peptide altered the expression profile of Pref-1, serpin E1, adiponectin, VEGF, and resistin which are the key proteins involved in developing obesity and/or insulin resistance. In our experimental model, VGVAPG peptide increased the expression of Pref-1, serpin E1, and adiponectin as compared to rosiglitazone, which is a PPARγ agonist used to trigger differentiation of 3T3-L1 cells into mature adipocytes. It is well known that Pref-1 is highly expressed in 3T3-L1 cells but shows reduced expression during adipocyte differentiation [Citation71]. Therefore, Pref-1 serves as an excellent marker for preadipocytes. Pref-1 is also an inhibitor of adipogenesis, and its constitutive expression inhibits 3T3-L1 adipocyte differentiation [Citation36]. Moreover, in our experiments VGVAPG peptide increased PAI-1 (synonym serpin E1) expression, which is linked to obesity and insulin resistance [Citation72]. Liang et al. (2006) reported that the overexpression of PAI-1 by adenovirus-mediated gene transfer in 3T3-L1 adipocytes inhibited differentiation and reduced PPARγ expression [Citation72]. Finally, compared to the rosiglitazone-treated group, VGVAPG peptide increased adiponectin expression in 3T3-L1 cells. Adiponectin produced by adipocytes is an important vascular protective adipocytokine that possesses antidiabetic, antiatherogenic, and anti-inflammatory properties [Citation73]. Furthermore, adiponectin is an important insulin-sensitizing adipocytokine that is downregulated in insulin resistance and obesity, and replenishment of this protein in adiponectin-deficient state improves insulin sensitivity [Citation74]. Additionally, leptin, adiponectin, and glucose transporter type 4 (Glut 4) are the major target genes in adipose tissue for PPARγ, and therefore, they are essential for maintaining homoeostasis of carbohydrate metabolism. It was shown that any disorders related to the regulation of these genes may contribute to the development of obesity due to the important role of these genes in hunger regulation (leptin), glucose transport (Glut 4), and insulin susceptibility (adiponectin) [Citation75–79].
In our experimental model, VGVAPG peptide decreased the expression of VEGF and resistin compared to that observed in the rosiglitazone-treated group. Vascular endothelial growth factor (VEGF) is the family of secreted polypeptides, characterized as cysteine-knot superfamily of hormones, including VEGF-A and others, which are formed by alternative splicing process [Citation80]. VEGF is recognized as a key factor in normal and abnormal angiogenesis and regulates multiple biological responses in endothelial cells, including cell proliferation, migration, survival, and production of vasoactive mediators [Citation81]. For example, VEGF-A released from adipocytes promotes angiogenesis and thereby ameliorates the local hypoxia-induced adipose inflammation and insulin resistance [Citation82]. Our data suggest that reducing VEGF expression by VGVAPG could reduce angiogenesis in adipose tissue.
The last studied protein resistin promotes 3T3-L1 preadipocyte differentiation and is an important mediator of obesity-induced insulin resistance; moreover, this protein could be secreted by adipocytes and is involved in insulin regulation [Citation83]. According to literature data, the level of resistin increases in 3T3-L1 during the differentiation process [Citation84]. On the other hand, in adult/mature adipocytes, resistin levels were shown to be decreased by rosiglitazone, and ligand-induced PPARγ activation strongly downregulated resistin expression; thus, this protein has been proposed as a therapeutic target of PPARγ ligands, which are used clinically to improve insulin sensitivity [Citation85–87]. In our study, the VGVAPG peptide decreased the expression of resistin as compared to that in rosiglitazone-treated cells. Because resistin is strictly controlled by PPARγ activation, our data allow to assume that VGVAPG peptide by interaction with this receptor can control resistin gene expression [Citation88]. Interaction between PPARγ and VGVAPG has been proven in our previous study, in which VGVAPG peptide was shown to affect PPARγ expression and PPARγ-mediated effects in mouse astrocytes and SH-SY5Y cells [Citation31,Citation49]. As mentioned above, PPARγ is involved in resistin expression, which is strictly related to obesity and type 2 diabetes. To date, Blaise and co-workers (2013) described that EDPs are involved in the development of insulin resistance in mice [Citation41]. Moreover, several papers, demonstrated that the concentration of anti-EDP antibodies of IgG is increased three fold in type 2 diabetic patients compared with the control population [Citation42–44]. Based on that we believed that, VGVAPG peptide affecting the PPARγ pathway could first prevent the maturation of preadipocytes and next make it difficult to break insulin resistance. According to available literature data which described mechanisms of action EDPs and PPARγ we hypothesized that in our experimental model VGVAPG could interfere with MAP-kinase [Citation27,Citation89]. MAP-kinase is commonly known as an agent taking part in many cell types differentiation processes, such as: adipocytes [Citation90], osteocytes [Citation91] or neurons [Citation92], therefore we think that VGVAPG can affect differentiation process of 3T3-L1 cells in PPARγ-MAPK-dependent way.
Taking into account all the above data, our obtained results show that VGVAPG peptide sustains 3T3 cells in less undifferentiated state. This could potentially have many repercussions such as preventing maturation of preadipocytes, possibility of carcinogenesis in undifferentiated cells, and making it difficult to break insulin resistance.
5. Conclusions
The present study is the first to describe that VGVAPG peptide does not increase ROS production and induce caspase-1 activation and cell proliferation in all studied time intervals in 3T3-L1 cell line. Moreover, VGVAPG peptide decreased lipid accumulation measured by ORO assay and increased the expression of Pref-1, serpin E1 and adiponectin as compared to that in rosiglitazone (PPARγ agonist used to trigger differentiation of 3T3-L1 cells)-treated group, while simultaneously decreasing the expression of VEGF and resistin. Our obtained results show that VGVAPG peptide sustains 3T3 cells in less undifferentiated state (). However, because of the lack of sufficient data explaining the molecular mechanism of action of VGVAPG peptide in adipose tissue, more studies are necessary on this topic.
Highlights
VGVAPG peptide does not stimulate ROS production and activation of caspase-1 in 3T3-L1 cells
VGVAPG peptide does not induce proliferation of 3T3-L1 cells
VGVAPG peptide decreases differentiation of 3T3-L1 cells into adipocytes
VGVAPG peptide decreases lipid accumulation as measured by ORO assay
VGVAPG peptide increases expression of Pref-1, serpin E1 and adiponectin as compared to rosiglitazone
VGVAPG peptide decreases expression of VEGF and resistin as compared to rosiglitazone
Author statement
Konrad A. Szychowski: Conceptualization, Methodology, Validation, Formal analysis, Investigation, Writing - Review & Editing, Project administration, Data Curation, Writing - Original Draft. Bartosz Skóra.: Data curation, Writing- Original draft preparation. Jakub Tobiasz: Investigation. Jan Gmiński: Supervision Writing - Review & Editing, Funding acquisition.
Acknowledgments
This work was supported by statutory funds from the University of Information Technology and Management in Rzeszow, Poland (DS 503-07-01-21).
Disclosure statement
The authors declare no conflict of interests.
Additional information
Funding
References
- Li DY, Brooke B, Davis EC, et al. Elastin is an essential determinant of arterial morphogenesis. Nature. 1998;393:276–280.
- Senior RM, Griffin GL, Mecham RP, et al. Val-Gly-Val-Ala-Pro-Gly, a repeating peptide in elastin, is chemotactic for fibroblasts and monocytes. J Cell Biol. 1984;99:870–874.
- Keith DA, Paz MA, Gallop PM, et al. Histologic and biochemical identification and characterization of an elastin in cartilage. J Histochem Cytochem. 1977;25:1154–1162.
- Mecham RP, Madaras J, McDonald JA, et al. Elastin production by cultured calf pulmonary artery endothelial cells. J Cell Physiol. 1983;116:282–288.
- Lin S, Sandig M, Mequanint K. Three-dimensional topography of synthetic scaffolds induces elastin synthesis by human coronary artery smooth muscle cells. Tissue Eng - Part A. 2011;17:1561–1571.
- Uitto J. Connective tissue biochemistry of the aging dermis. Age-associated alterations in collagen and elastin. Clin Geriatr Med. 1989;5:127–147.
- Brophy CM, Reilly JM, Smith GJW, et al. The role of inflammation in nonspecific abdominal aortic aneurysm disease. Ann Vasc Surg. 1991;5:229–233.
- Lapis K, Tímár J. Role of elastin-matrix interactions in tumor progression. Semin Cancer Biol. 2002;12:209–217.
- Robert L, Robert AM, Jacotot B. Elastin-elastase-atherosclerosis revisited. Atherosclerosis. 1998;140:281–295.
- Péterszegi G, Robert L. Cell death induced in lymphocytes expressing the elastin-laminin receptor by excess agonists: necrosis and apoptosis. Biomed Pharmacother. 1998;52(9):369–377.
- Barrett AJ, McDonald JK. Nomenclature: protease, proteinase and peptidase. Biochem J. 1986;237(3):935.
- Janoff A, Scherer J. Mediators of inflammation in leukocyte lysosomes. IX. Elastinolytic activity in granules of human polymorphonuclear leukocytes. J Exp Med. 1968;128(5):1137–1155.
- Leake DS, Lieberman GE, Peters TJ. Properties and subcellular localization of adenosine diphosphatase in arterial smooth muscle cells in culture. BBA - Mol Cell Res. 1983;762:52–57.
- Homsy R, Pelletier-Lebon P, Tixier J-M, et al. Characterization of human skin fibroblasts elastase activity. J Invest Dermatol. 1988;91(5):472–477.
- Kao RT, Wong M, Stern R. Elastin degradation by proteases from cultured human breast cancer cells. Biochem Biophys Res Commun. 1982;105(1):383–389.
- Ezure T, Amano S. Increment of subcutaneous adipose tissue is associated with decrease of elastic fibres in the dermal layer. Exp Dermatol. 2015;24(12):924–929.
- Mochizuki S, Brassart B, Hinek A. Signaling pathways transduced through the elastin receptor facilitate proliferation of arterial smooth muscle cells. J Biol Chem. 2002;277(47):44854–44863.
- Floquet N, Héry-Huynh S, Dauchez M, et al. Structural characterization of VGVAPG, an elastin-derived peptide. Biopolymers. 2004;76:266–280.
- Hinek A, Rabinovitch M, Keeley F, et al. The 67-kD elastin/laminin-binding protein is related to an enzymatically inactive, alternatively spliced form of beta-galactosidase. J Clin Invest. 1993;91:1198–1205.
- Skeie JM, Hernandez J, Hinek A, et al. Molecular responses of choroidal endothelial cells to elastin derived peptides through the elastin-binding protein (GLB1). Matrix Biol. 2012;31:113–119.
- Ochieng J, Furtak V, Lukyanov P. Extracellular functions of galectin-3. Glycoconj J. 2002;19:527–535.
- Cantarelli B, Duca L, Blanchevoye C, et al. Elastin peptides antagonize ceramide-induced apoptosis. FEBS Lett. 2009;583(14):2385–2391.
- Bresalier RS, Byrd JC, Wang L, et al. Colon cancer mucin: A new ligand for the β-galactoside-binding protein galectin-3. Cancer Res. 1996;56:4354–4357.
- Wang Y, Nangia-Makker P, Tait L, et al. Regulation of prostate cancer progression by galectin-3. Am J Pathol. 2009;174(4):1515–1523.
- Ochieng J, Warfield P, Green-Jarvis B, et al. Galectin-3 regulates the adhesive interaction between breast carcinoma cells and elastin. J Cell Biochem. 1999;75(3):505–514.
- Pocza P, Süli-Vargha H, Darvas Z, et al. Locally generated VGVAPG and VAPG elastin-derived peptides amplify melanoma invasion via the galectin-3 receptor. Int J Cancer. 2008;122:1972–1980.
- Le Page A, Khalil A, Vermette P, et al. The role of elastin-derived peptides in human physiology and diseases. Matrix Biol. 2019;84:81–96.
- Blood CH, Sasse J, Brodt P, et al. Identification of a tumor cell receptor for VGVAPG, an elastin-derived chemotactic peptide. J Cell Biol. 1988;107:1987–1993.
- Jung S, Rutka JT, Hinek A. Tropoelastin and elastin degradation products promote proliferation of human astrocytoma cell lines. J Neuropathol Exp Neurol. 1998;57:439–448.
- Hance KA, Tataria M, Ziporin SJ, et al. Monocyte chemotactic activity in human abdominal aortic aneurysms: role of elastin degradation peptides and the 67-kD cell surface elastin receptor. J Vasc Surg. 2002;35:254–261.
- Szychowski KA, Gmiński J. Impact of elastin-derived VGVAPG peptide on bidirectional interaction between peroxisome proliferator-activated receptor gamma (Pparγ) and beta-galactosidase (β-Gal) expression in mouse cortical astrocytes in vitro. Naunyn Schmiedebergs Arch Pharmacol. 2019;392:405–413.
- Takamura T, Nohara E, Nagai Y, et al. Stage-specific effects of a thiazolidinedione on proliferation, differentiation and PPARgamma mRNA expression in 3T3-L1 adipocytes. Eur J Pharmacol. 2001;422:23–29.
- Vidal-Puig A, Jimenez-Liñan M, Lowell BB, et al. Regulation of PPAR γ gene expression by nutrition and obesity in rodents. J Clin Invest. 1996;97:2553–2561.
- Gealekman O, Burkart A, Chouinard M, et al. Enhanced angiogenesis in obesity and in response to PPARγ activators through adipocyte VEGF and ANGPTL4 production. Am J Physiol Metab. 2008;295:E1056–64.
- Kadowaki T, Yamauchi T. Adiponectin and adiponectin receptors. Endocr Rev. 2005;26(3):439–451.
- Hudak CS, Sul HS. Pref-1, a gatekeeper of adipogenesis. Front Endocrinol (Lausanne). 2013;4:79.
- Jung UJ, Choi MS. Obesity and its metabolic complications: the role of adipokines and the relationship between obesity, inflammation, insulin resistance, dyslipidemia and nonalcoholic fatty liver disease. Int J Mol Sci. 2014;15:6184–6223.
- Coelho M, Oliveira T, Fernandes R. Biochemistry of adipose tissue: an endocrine organ. Arch Med Sci. 2013;9:191–200.
- Scheja L, Heeren J. The endocrine function of adipose tissues in health and cardiometabolic disease. Nat Rev Endocrinol. 2019;15:507–524.
- Han CY. Roles of reactive oxygen species on insulin resistance in adipose tissue. Diabetes Metab J. 2016;40:272–279.
- Blaise S, Romier B, Kawecki C, et al. Elastin-derived peptides are new regulators of insulin resistance development in mice. Diabetes. 2013;62:3807–3816.
- Bako G, Jacob MP, Fulop T, et al. Immunology of elastin: study of anti-elastin peptide antibodies by DOT immunobinding assay. Immunol Lett. 1987;15:187–192.
- Fülöp T, Wei SM, Robert L, et al. Determination of elastin peptides in normal and arteriosclerotic human sera by ELISA. Clin Physiol Biochem. 1990;8:273–282.
- Fulop T, Jacob MP, Robert L. Determination of anti-elastin peptide antibodies in normal and arteriosclerotic human sera by ELISA. J Clin Lab Immunol. 1989;30:69–74.
- Zebisch K, Voigt V, Wabitsch M, et al. Protocol for effective differentiation of 3T3-L1 cells to adipocytes. Anal Biochem. 2012;425:88–90.
- Gomes A, Fernandes E, Lima JLFC. Fluorescence probes used for detection of reactive oxygen species. J Biochem Biophys Methods. 2005;65:45–80.
- Nicholson DW, Ali A, Thornberry NA, et al. Identification and inhibition of the ICE/CED-3 protease necessary for mammalian apoptosis. Nature. 1995;376(6535):37–43.
- Vega-Avila E, Pugsley MK. An overview of colorimetric assay methods used to assess survival or proliferation of mammalian cells. Proc West Pharmacol Soc. 2011;54:10–14.
- Szychowski KA, Rombel-Bryzek A, Dołhańczuk-Śródka A, et al. Antiproliferative effect of elastin-derived peptide VGVAPG on SH-SY5Y neuroblastoma cells. Neurotox Res. 2019;36:503–514.
- Szychowski KA, Gmiński J. The VGVAPG peptide regulates the production of nitric oxide synthases and reactive oxygen species in mouse astrocyte cells in vitro. Neurochem Res. 2019;44(5):1127–1137.
- Robert L, Jacob MP, Frances C, et al. Interaction between elastin and elastases and its role in the aging of the arterial wall, skin and other connective tissues. A review. Mech Ageing Dev. 1984;28(2–3):155–166.
- Scandolera A, Rabenoelina F, Chaintreuil C, et al. Uncoupling of elastin complex receptor during in vitro aging is related to modifications in its intrinsic sialidase activity and the subsequent lactosylceramide production. PLoS One. 2015;10(6):e0129994. .
- Gayral S, Garnotel R, Castaing-Berthou A, et al. Elastin-derived peptides potentiate atherosclerosis through the immune Neu1-PI3K pathway. Cardiovasc Res. 2014;102:118–127.
- Moynagh PN. The interleukin-1 signalling pathway in astrocytes: A key contributor to inflammation in the brain. J Anat. 2005;207(3):265–269.
- Scheer JM Caspase-1. In: Handbook of proteolytic enzymes. London, UK:Elsevier; 2013. p. 2237–2243.
- Cho K-A, Suh JW, Lee KH, et al. IL-17 and IL-22 enhance skin inflammation by stimulating the secretion of IL-1β by keratinocytes via the ROS-NLRP3-caspase-1 pathway. Int Immunol. 2012;24(3):147–158.
- Barker BR, Taxman DJ, Ting JP-Y. Cross-regulation between the IL-1β/IL-18 processing inflammasome and other inflammatory cytokines. Curr Opin Immunol. 2011;23:591–597.
- Trøseid M, Seljeflot I, Arnesen H. The role of interleukin-18 in the metabolic syndrome. Cardiovasc Diabetol. 2010;9(1):1–8.
- Wang H, Capell W, Yoon JH, et al. Obesity development in caspase-1-deficient mice. Int J Obes. 2014;38(1):152–155.
- Membrez M, Ammon-Zufferey C, Philippe D, et al. Interleukin-18 protein level is upregulated in adipose tissue of obese mice. Obesity (Silver Spring). 2009;17:393–395.
- Patel R, Rinker L, Peng J, et al. Reactive Oxygen Species: The Good and the Bad. In: Filip C, Albu A, editors. Reactive Oxygen Species (ROS) in Living Cells. IntechOpen; 2018. p. 7-20
- Snezhkina AV, Kudryavtseva AV, Kardymon OL, et al. ROS generation and antioxidant defense systems in normal and malignant cells. Oxid Med Cell Longev. 2019;2019:6175804.
- Satta J, Laurila A, Pääkkö P, et al. Chronic inflammation and elastin degradation in abdominal aortic aneurysm disease: an immunohistochemical and electron microscopic study. Eur J Vasc Endovasc Surg. 1998;15:313–319.
- Debret R, Le Naour RR, Sallenave J-M, et al. Elastin fragments induce IL-1beta upregulation via NF-kappaB pathway in melanoma cells. J Invest Dermatol. 2006;126:1860–1868.
- Kobayashi K, Jokaji R, Miyazawa-Hira M, et al. Elastin-derived peptides are involved in the processes of human temporomandibular disorder by inducing inflammatory responses in synovial cells. Mol Med Rep. 2017;16(3):3147–3154.
- Szychowski KA, Gmiński J. The elastin-derived peptide VGVAPG does not activate the inflammatory process in mouse cortical astrocytes in Vitro. Neurotox Res. 2020;37(1):136–145.
- Wang XY, You LH, Cui XW, et al. Evaluation and optimization of differentiation conditions for human primary brown adipocytes. Sci Rep. 2018;8:1–12.
- Green H, Kehinde O. Sublines of mouse 3T3 cells that accumulate lipid. Cell. 1974;1(3):113–116.
- Kuri-Harcuch W, Green H. Adipose conversion of 3T3 cells depends on a serum factor. Proc Natl Acad Sci U S A. 1978;75:6107–6109.
- Kraus NA, Ehebauer F, Zapp B, et al. Quantitative assessment of adipocyte differentiation in cell culture. Adipocyte. 2016;5:351–358.
- Wang Y, Kim K-A, Kim J-H, et al. Pref-1, a preadipocyte secreted factor that inhibits adipogenesis. J Nutr. 2006;136:2953–2956.
- Liang X, Kanjanabuch T, Mao SL, et al. Plasminogen activator inhibitor-1 modulates adipocyte differentiation. Am J Physiol - Endocrinol Metab. 2006;290:103–113.
- Brody R, Peleg E, Grossman E, et al. Production and secretion of adiponectin from 3T3-L1 adipocytes: comparison of antihypertensive drugs. Am J Hypertens. 2009;22(10):1126–1129.
- Fasshauer M, Neumann S, Eszlinger M, et al. Hormonal regulation of adiponectin gene expression in 3T3-L1 adipocytes. Biochem Biophys Res Commun. 2002;290:1084–1089.
- Jemai R, Drira R, Fetoui H, et al. Colocynth (Citrullus colocynthis) flesh extract suppresses adipogenesis by down-regulating adipogenic transcription factors and their target genes in 3T3-L1 preadipocytes. Food Nutr Sci. 2018:1014–1028.
- Stofkova A. Leptin and adiponectin: from energy and metabolic dysbalance to inflammation and autoimmunity. Endocr Regul. 2009;43:157–168.
- Cohen SL, Halaas JL, Friedman JM, et al. Human leptin characterization. Nature. 1996;382:589.
- Pedersen O, Bak JF, Andersen PH, et al. Evidence against altered expression of GLUT1 or GLUT4 in skeletal muscle of patients with obesity for NIDDM. Diabetes. 1990;39:865–870.
- Fasshauer M, Paschke R, Stumvoll M. Adiponectin, obesity, and cardiovascular disease. Biochimie. 2004;86(11):779–784.
- Holmes DIR, Zachary I. The vascular endothelial growth factor (VEGF) family: angiogenic factors in health and disease. Genome Biol. 2005;6:209.
- Dikov MM, Ohm JE, Ray N, et al. Differential Roles of Vascular Endothelial Growth Factor Receptors 1 and 2 in Dendritic Cell Differentiation. J Immunol. 2005;174(1):215–222.
- Hasan AU, Ohmori K, Konishi K, et al. Eicosapentaenoic acid upregulates VEGF-A through both GPR120 and PPARγ mediated pathways in 3T3-L1 adipocytes. Mol Cell Endocrinol. 2015;406:10–18.
- Gong H, Ni Y, Guo X, et al. Resistin promotes 3T3-L1 preadipocyte differentiation. Eur J Endocrinol. 2004;150:885–892.
- Haugen F, Jorgensen A, Drevon CA, et al. Inhibition by insulin of resistin gene expression in 3T3-L1 adipocytes. FEBS Lett. 2001;507(1):105–108.
- Steppan CM, Bailey ST, Bhat S, et al. The hormone resistin links obesity to diabetes. Nature. 2001;409(6818):307–312.
- Patel L, Buckels AC, Kinghorn IJ, et al. Resistin is expressed in human macrophages and directly regulated by PPARγ activators. Biochem Biophys Res Commun. 2003;300(2):472–476.
- Ikeda Y, Tsuchiya H, Hama S, et al. Resistin affects lipid metabolism during adipocyte maturation of 3T3-L1 cells. Febs J. 2013;280(22):5884–5895.
- Way JM, Görgün CZ, Tong Q, et al. Adipose Tissue Resistin Expression is Severely Suppressed in Obesity and Stimulated by Peroxisome Proliferator-activated Receptor γ Agonists. J Biol Chem. 2001;276(28):25651–25653.
- Gelman L, Fruchart JC, Auwerx J. An update on the mechanisms of action of the peroxisome proliferator-activated receptors (PPARs) and their roles in inflammation and cancer. Cell Mol Life Sci. 1999;55:932–943.
- Bost F, Aouadi M, Caron L, et al. The role of MAPKs in adipocyte differentiation and obesity. Biochimie. 2005;87(1):51–56.
- Khatiwala CB, Kim PD, Peyton SR, et al. ECM compliance regulates osteogenesis by influencing MAPK signaling downstream of RhoA and ROCK. J Bone Miner Res. 2009;24(5):886–898.
- Wang W, Lu S, Li T, et al. Inducible activation of ERK5 MAP kinase enhances adult neurogenesis in the olfactory bulb and improves olfactory function. J Neurosci. 2015;35(20):7833–7849.