ABSTRACT
Background
Platinum is a commonly used drug for ovarian cancer (OvCa) treatment, but drug resistance limits its clinical application. This study intended to delineate the effects of adipocytes on platinum resistance in OvCa.
Methods
OvCa cells were maintained in the adipocyte-conditioned medium. Cell viability and apoptosis were detected by CCK-8 and flow cytometry, separately. Proliferation and apoptosis-related protein expression were assayed by western blot. The IC50 values of cisplatin and carboplatin were determined using CCK-8. IGF1 secretion and expression were assayed via ELISA and western blot, respectively. A xenograft model was established, and pathological changes were detected by H&E staining. Proliferation and apoptosis-associated protein expression was assessed via IHC.
Results
Adipocytes promoted the viability and repressed cell apoptosis in OvCa, as well as enhancing platinum resistance, while the addition of IGF-1 R inhibitor reversed the effects of adipocytes on proliferation, apoptosis, and drug resistance of OvCa cells. Treatment with different concentrations of Ojeok-san (OJS) inhibited the adipocyte-induced platinum resistance in OvCa cells by suppressing IGF1. The combined treatment of OJS and cisplatin significantly inhibited tumour growth in vivo with good mouse tolerance.
Conclusion
In summary, OJS inhibited OvCa proliferation and platinum resistance by suppressing adipocyte paracrine IGF1 secretion.
KEYWORDS:
1. Introduction
Ovarian cancer (OvCa) is one of the major cancer-related killers of women [Citation1]. The majority of OvCa patients are discovered at an advanced stage due to the limited clinical signs in the early stages, which presents a significant challenge for treatment [Citation2]. Currently, the most common strategy for OvCa is debulking surgery plus platinum-based chemotherapy [Citation3]. However, chemotherapy resistance often leads to recurrence and poor prognosis, with a 5-year survival rate of advanced OvCa patients remaining largely unchanged [Citation4]. Therefore, exploring molecular mechanisms of chemotherapy resistance in OvCa patients is crucial for guiding therapeutic interventions.
The function and paracrine signalling of adipocytes are altered as a result of the interaction between adipose tissue and cancer cells, which in turn promote tumour growth in the tumour microenvironment [Citation5]. Adipocytes are crucial for OvCa development, such as omentum adipocytes upregulating CD36 in OvCa cells to accelerate uptake of exogenous fatty acids and facilitate OvCa progression and metastasis via positive feedback [Citation6]; adipocytes inducing upregulation and activation of SIK2/p85α-S154 pathway in OvCa cells to mediate downstream signalling (AMPK/ACC) and changes in β-oxidation metabolism [Citation7]; and adipocyte-induced FABP4 expression promoting OvCa cell metastasis and mediating resistance to carboplatin [Citation8]. Although adipocytes are a pivotal element of the OvCa microenvironment, interactions between adipocytes and OvCa cells are undefined. Therefore, we intended to exploit molecular mechanisms underlying platinum resistance in OvCa cells and adipocytes, providing new directions for chemotherapy treatment in OvCa.
IGF1 is a highly conserved single-strand secreted protein of 70 amino acids. It acts as a major regulator of cell proliferation, differentiation, and apoptosis, playing a crucial role in many physiological and pathological processes in humans and other mammals [Citation9–11]. By raising IGF1 expression in matrix cells originating from breast adipose tissue, obesity promotes the local invasion, and losing weight may restore this tumour-stimulating phenotype of breast cancer cells [Citation12]. Liotti et al. [Citation13] found that periprostatic adipose tissue upregulates TUBB2B β-tubulin subtype through paracrine secretion of IGF1, promoting resistance of prostate cancer to docetaxel. These studies suggest that IGF1 is a likely target for inhibiting cancer occurrence and reversing tumour resistance. However, the role of adipocyte paracrine IGF1 in OvCa is not clear.
Ojeok-san (OJS) is a herbal formula [Citation14,Citation15] used in Asian countries, such as Korea, Japan, China, etc. for the treatment of depression, gastrointestinal tract and pain. It has been proven to have anti-inflammatory, immune regulation and other characteristics. For example, Shin et al. [Citation16] reported that OJS can reduce the number of inflammation-related factors in bronchoalveolar lavage fluid, such as interleukin-4, immunoglobulin, etc., and reduce the infiltration of inflammatory cells in the respiratory tract and inhibit pulmonary fibrosis. OJS can also reduce the feeling of mechanical injury induced by interleukin-10 knockout mice and improve the pain-like behaviour of inflammatory bowel disease model [Citation15]. In a colitis-induced colorectal cancer mouse model, OJS can reduce the pain perception of visceral and physical injuries in mice [Citation17]. In clinical practice, the use of Saengmaek-san in the treatment of gastroesophageal reflux-induced chronic cough, while taking OJS, can significantly enhance the therapeutic effect, and no serious adverse events occurred [Citation18]. These studies have shown that OJS has small side effects and plays a role in many aspects and has the potential as a cancer treatment drug.
In this study, we found that adipocyte-conditioned medium (Adipo-CM) promoted OvCa cell proliferation, repressed apoptosis, and enhanced resistance to platinum-based chemotherapy. Further exploration of interaction of OvCa cells with adipocytes revealed that adipocytes enhanced chemotherapy resistance in OvCa cells by paracrine secretion of IGF1, while treatment with Ojeok-san (OJS) increased sensitivity of OvCa cells to platinum drugs. Our study suggested that blocking adipocyte paracrine IGF1 secretion could effectively inhibit OvCa progression and chemotherapy resistance.
2. Materials and methods
2.1. Preparation of OJS
OJS (also known as Ojeok-san, wuji-san, goshaku-san) [Citation19,Citation20] consists of 16 herbs: largehead atractylodes rhizome (Rhizoma Atractylodis Macrocephalae; 7.5 g), ephedra (Herba Ephedrae; 3.75 g), dried tangerine peel (Pericarpium Citri Reticulatae; 3.75 g), officinal magnolia bark (Cortex Magnoliae Officinalis; 3.0 g), Platycodon root (Radix Platycodonis; 3.0 g), orange fruit (Fructus Aurantii; 3.0 g), Chinese angelica (Radix Angelicae Sinensis; 3.0 g), dried ginger (Rhizoma Zingiberis; 3.0 g), Chinese peony (Paeonia lactiflora Pall; 3.0 g), Poria Sclerotium (3.0 g), dahurian angelica root (Radix Angelicae Dahuricae; 2.63 g), common cnidium fruit (Fructus Cnidii; 2.63 g), pinellia tuber (Rhizoma Pinelliae; 2.63 g), cassia bark (Cortex Cinnamomi; 2.63 g), liquorice root (Radix Glycyrrhizae; 2.25 g), and fistular onion stalk (Allii Fistulosi Bulbus; 3.75 g). These herbs were mixed and prepared as an OJS decoction using conventional methods, filtered, and freeze-dried to form an OJS powder formula. The powdered extract was stored at 4°C [Citation17]. The specific preparation method of OJS refers to previous studies [Citation21].
2.2. Cell culture and transfection
SKOV3 cell line was obtained from the American Type Culture Collection (ATCC), and HPAd cells were from Sigma-Aldrich (US). Routine tests were performed to detect mycoplasma contamination in all cell lines. All tumour cell lines were maintained in DMEM with 10% FBS under conditions of 37°C and 5% CO2.
For the collection of CM, HPAd cells were seeded in Petri dishes and cultured for 24 hours. Adipocyte differentiation medium (ScienCell, USA) was added. After 14 days of culture (during culture, the medium was changed every two days), the cells were washed with phosphate-buffered saline (PBS), and then placed in RPMI-1640 medium without phenol red for 24 hours. Here, we directly named the mature adipocytes differentiated from HPAD cells as adipocytes. Following 24 hours, culture medium was collected from adipocytes, centrifuged at 1500 ×g for 5 minutes, and filtered through a 0.22 μm filter. The collected medium was mixed with fresh medium at 1:1 and used to treat SKOV3 cells for 24 hours for in vitro proliferation and apoptosis assays [Citation22].
IGF1 overexpression plasmid (oe-IGF1) and negative control (oe-NC) were purchased from GenePharma (China) and transfected into adipocytes using Lipofectamine 3000 (Invitrogen, USA). After 48 hours, the cells were collected for subsequent experiments.
2.3. Oil red O staining experiment
HPAd cells were cultured in adipogenic differentiation mediums. On the 1st, 7th and 14th day of culture, the medium was removed, and 4% paraformaldehyde was added to fix the cells for 1 hour, and then oil red O working solution (Sigma, USA) was added for 15 min of staining. Afterward, samples were washed with deionized water, dried, and photographed under a fluorescence inverted microscope.
2.4. ELISA
The secretion of IGF1 in adipocyte culture supernatant was measured using ELISA. ELISA assay kits were purchased from Abcam (UK) and ELISA was performed. Each sample was repeated three times.
2.5. Cell viability assay
CCK-8 assay was recommended for viability test. In brief, OvCa cells (2 × 103) were plated into a 96-well plate. Following 24 hours of incubation, the medium or prepared Adipo-CM was added to the wells and incubated for 1–3 days. The medium was then replaced with 100 μL serum-free medium, and 10 μL CCK-8 solution (Solarbio, China) was dropped into each well for 4 hours of incubation. Absorbance was measured by microplate reader at 450 nm.
2.6. Western blot analysis
Cells collected by pre-cooled PBS washing were resuspended in cell lysis buffer (Beyotime, China) with phosphatase and protease inhibitors (Beyotime, China). After vortexing and lysing on ice for 10 minutes, protein samples were prepared. Protein concentration was tested using with BCA kit (Beyotime, China). PAGE gels were prepared and loaded with marker and equal amounts of protein samples for separation. Proteins were transferred from gel to PVDF membrane. The membrane was then soaked in 5% skim milk for 60 minutes. After washing with TBST, membrane was incubated with primary antibody at 4°C overnight. Antibodies against Ki-67 were obtained from Huabio (China), and antibodies against IGF1, PARP, Bcl-2, Bax, and cleaved caspase 3 were obtained from ABclonal (China). After washing with TBST, the membrane was incubated with HRP-conjugated goat anti-rabbit IgG secondary antibody for 2 hours at room temperature. Protein bands were assessed using the BeyoECL Plus ultra-sensitive ECL chemiluminescence kit (Beyotime, China).
2.7. qRT-PCR assay
The qRT-PCR experiment was performed with reference to other people’s research [Citation23]. Briefly, HPAd cells on day 1 and day 7 were collected and lysed by TRIzol reagent (Invitrogen, USA) to obtain total RNA. High-Capacity cDNA Reverse Transcription Kit (ThermoFisher, USA) was used to obtain cDNA; SYBR Green qPCR Master Mix (Universal) (MCE, USA) was used for quantitative real-time PCR. GAPDH was used as an internal reference, and the primer sequence is shown in .
Table 1. Primer sequence.
2.8. H&E staining
Subcutaneous OvCa tumour tissue from mice was fixed with formalin, dehydrated with graded ethanol, and embedded in paraffin after transparentizing with xylene. Tissue sections were cut to 4 μm and then deparaffinized with xylene and rehydrated with graded ethanol. Sections were stained with haematoxylin for 10 minutes, differentiated with 1% ethanol hydrochloric acid for 20 seconds, treated with 1% ammonia water for 30 seconds, and then stained with eosin for 3 minutes. The tissue sections were then dehydrated with graded ethanol, transparentized with xylene, sealed with neutral resin, and observed under a microscope. Images were obtained and analysed under a microscope (Olympus, Japan).
2.9. Immunohistochemistry (IHC) experiment
Subcutaneous OvCa tumour tissue slices underwent xylene deparaffinization and graded ethanol rehydration. Endogenous peroxidase activity was inhibited by incubating the sections with 3% H2O2 at room temperature for 10 minutes after the sections had undergone 20 minutes of antigen retrieval with EDTA. Sections were then sealed with 5% goat serum (Gibco, US) for 20 minutes and incubated with primary antibodies against Ki-67 (Huabio, China), PARP (ABclonal, China), Bcl-2 (ABclonal, China), Bax (ABclonal, China), and cleaved caspase 3 (CST, USA) overnight at 4°C. Samples were incubated with HRP-conjugated goat anti-rabbit IgG antibody (Abcam, UK) at 37°C for 40 minutes. Sections were then stained with DAB, counterstained with haematoxylin for 3 minutes, dehydrated, and sealed. The expression of Ki-67, PARP, Bcl-2, Bax, and cleaved caspase 3 in each group was observed under a microscope (Olympus, Japan).
2.10. Construction of the mouse xenograft Model
Animal research was conducted per procedures approved by the The Second Affiliated Hospital, Zhejiang University, School of Medicine Ethics Committee and in accordance with GB/T35893–2018 Guidelines for Ethical Review of Laboratory Animals. Animal studies were performed in line with the relevant ethical regulations of animal experiments. Female BALB/c nude mice (4–5 weeks old) were accessed from Model Animal Research Center of Nanjing University and fed with irradiated sterile feed according to the requirements of the model. They were fed under SPF conditions, namely 21–23°C, 30%-60% relative humidity, and 12 hours of light and dark, respectively. The mice were divided into three groups: tumour group without drug treatment (Control), cisplatin treatment group (Cisplatin) and cisplatin+OJS co-treatment group (Cisplatin+OJS), 5 mice in each group.SKOV3 cells (7 × 106) and adipocytes (highly differentiated HPAd cells)(7 × 106) were suspended in 200 µL of Matrigel (BD Biosciences, US) and injected into subcutaneous tissue of the right lower limb of the mice. Subcutaneous xenograft growth was monitored with calipers every three days. Tumour volume was computed following the equation: 0.5×L×W2 (where L is the longest dimension of the tumour and W is the widest dimension of the tumour). When the average volume of the xenograft reached 100 mm3, the mice were randomly grouped into groups for drug treatment. The control group was injected with saline, experimental group 1 received intraperitoneal injections of cisplatin (2 mg/kg, Aladdin, China) twice a week, experimental group 2 received intraperitoneal injections of cisplatin (2 mg/kg, Aladdin, China) twice a week and was orally administered OJS (15 mg/kg) by gavage. When the volume of the subcutaneous tumour tissue exceeded 1200 mm3, the animals were euthanized by CO2 asphyxiation, and the tumour tissue was obtained and stored in liquid nitrogen or paraformaldehyde for molecular biology and pathological experiments [Citation3].
2.11. Data analysis
Statistical analysis was performed on GraphPad Prism 8.0 software (GraphPad Software, US). The two-tailed Student’s t-test was utilized to determine the statistical significance of differences between two groups. One-way analysis of variance was to determine the statistical significance of multi-group differences. All data were presented as mean ± standard deviation (n ≥ 3). All experiments were repeated three times. P-value <0.05 indicated that the experimental results are statistically significant.
3. Results
3.1. Adipocytes promote platinum drug resistance in OvCa cells
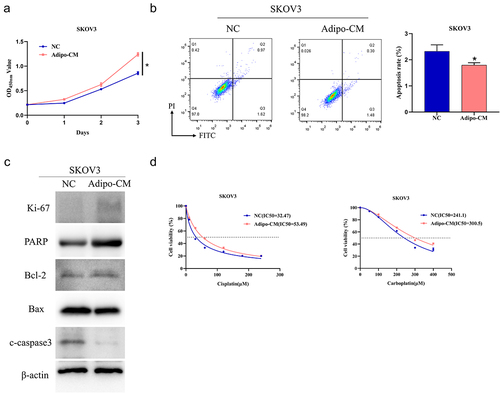
First of all, oil red O staining experiments showed that HPAd cells gradually increased lipid droplets with the increase of induction time, with slightly more lipid droplets on day 7 compared to day 1 and more on day 14 (Supplementary Figure 1A). Further, qRT-PCR was used to detect adipogenic differentiation markers [Citation23]. Compared with day 1 and day 7, the expression of Pref-1 was significantly decreased (0.01 ± 0.00, p < 0.05), and the expression of C/EBPβ, PPARγ, SLC2A4, C/EBPα was significantly increased (2.62 ± 0.09, 48.80 ± 0.67, 343.78 ± 24.75, 58.09 ± 12.35, p < 0.05) (Supplementary ). These results indicated that HPAd cells successfully differentiated into mature adipocytes. To delineate the effect of adipocytes on growth of OvCa cells, we first cultured OvCa cells in Adipo-CM and compared it to cells cultured without Adipo-CM. Cell viability was assayed by CCK-8 and apoptosis by flow cytometry. The addition of Adipo-CM increased the viability of SKOV3 cells (Day 3: NC group, OD = 0.86 ± 0.03; Adipo-CM group, OD = 1.24 ± 0.04, p < 0.05) and decreased level of apoptosis (NC group = 2.32 ± 0.25; Adipo-CM group = 1.80 ± 0.09, p < 0.05) (). Western blot analysis revealed that treatment with Adipo-CM upregulated proliferation-related protein Ki-67, PARP, and the anti-apoptotic protein Bcl-2, while downregulating pro-apoptotic proteins Bax and cleaved caspase 3 (). Finally, we determined the IC50 values of different concentrations of cisplatin (0, 30, 60, 120, 180, 240 μM) and carboplatin (0, 50, 100, 200, 300, 400 μM) in SKOV3 cells with or without Adipo-CM treatment. IC50 values of SKOV3 cells (cisplatin and carboplatin were treated respectively) were substantially elevated after treatment with Adipo-CM (NC group: IC50 = 32.47, 241.1 μM; Adipo-CM group: IC50 = 53.49, 300.5 μM) (). These experiments indicated that adipocytes promoted platinum drug resistance in OvCa cells.
Figure 1. Adipocytes promote platinum drug resistance in OvCa cells.
3.2. Adipocyte-secreted IGF1 promotes platinum resistance in OvCa cells
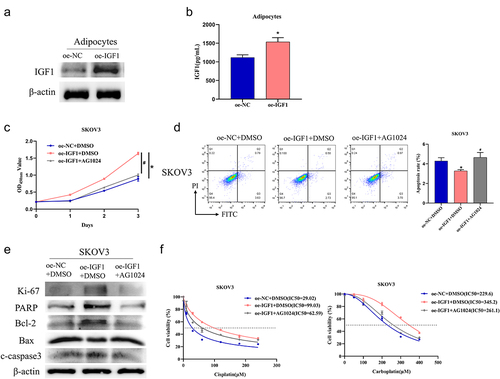
Adipocyte-secreted IGF1 promotes progression of prostate cancer and breast cancer through IGF1-R [Citation12,Citation13,Citation24]. To verify whether a similar phenomenon exists in OvCa, we constructed cell lines based on adipocytes (highly differentiated HPAd cells) with overexpression of IGF1 (oe-IGF1) or control (oe-NC) and detected the expression of IGF1 by western blot analysis. IGF1 was substantially increased in the oe-IGF1 group but not in the oe-NC group (). ELISA experiments reported that IGF1 level in supernatant of oe-IGF1 group was substantially higher than oe-NC group (oe-NC group, 1118.27 ± 70.53; oe-IGF1 group, 1535.8 ± 113.92, p < 0.05) (). We treated SKOV3 cells with AG1024 and highly differentiated HPAd cell culture supernatant from different treatment groups and then assessed cell viability and apoptosis by CCK-8 and flow cytometry. Enforced expression of IGF1 led to increased cell viability (Day 3: NC group, 0.89 ± 0.07; oe-IGF1,1.64 ± 0.04, p < 0.05) and decreased apoptosis (NC group, 4.29 ± 0.34; oe-IGF1,3.28 ± 0.13, p < 0.05) in SKOV3 cells, while adding the IGF1-R inhibitor AG1024 reversed the above experimental results (1.00 ± 0.03/4.65 ± 0.5, p < 0.05) (). Western blot presented that overexpression of IGF1 increased Ki-67, PARP, and Bcl-2, while decreasing Bax and cleaved caspase 3. Adding AG1024 to SKOV3 cells restored the protein expression to the control level (). To verify the influence of adipocyte-secreted IGF1 on platinum drug resistance in OvCa cells, we treated SKOV3 cells with cisplatin (0, 30, 60, 120, 180, 240 μM) and carboplatin (0, 50, 100, 200, 300, 400 μM) and measured cell viability. The results showed that overexpression of IGF1 in HPAd cells increased the IGF1 level in the supernatant, leading to a significant increase in IC50 values of cisplatin and carboplatin in SKOV3 cells (NC group: IC50 = 29.02/229.6; oe-IGF1+DMSO group: IC50 = 99.03/345.2, p < 0.05). Adding AG1024 to SKOV3 cells restored the IC50 values to the level of oe-NC+DMSO (IC50 = 62.59/261.1, p < 0.05) (). These results indicated that adipocyte paracrine IGF1 secretion promoted platinum resistance in OvCa cells.
Figure 2. Adipocytes promote OvCa resistance to platinum drugs through paracrine secretion of IGF1.
3.3. OJS inhibits adipocyte paracrine IGF1 secretion and suppresses OvCa progression
OJS is commonly used in the clinical treatment of gynaecological diseases. Here, we dissected the role of OJS in the development of OvCa. First, we treated adipocytes with different concentrations of OJS (0, 10, 50, 100, 200 μg/mL) and measured IGF1 expression in cells and supernatant by western blot and ELISA, respectively. IGF1 gradually reduced with increasing concentrations of OJS (1421.27 ± 53.67, 1320.93 ± 73.15, 1087.00 ± 47.07, 637.0 ± 87.4, 487.13 ± 41.68, p < 0.05). The optimal concentration was found to be 200 μg/mL, which was used for subsequent experiments ().
Figure 3. OJS inhibits adipocyte paracrine secretion of IGF1 to regulate OvCa progression.
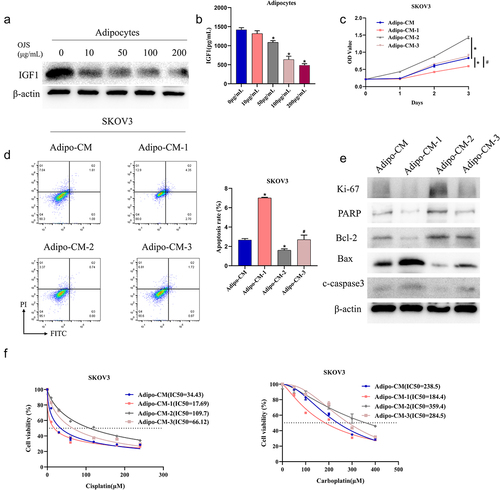
Next, we constructed cell lines based on adipocytes, including the control group, OJS-treated group, oe-IGF1 group, and OJS and oe-IGF1 co-treated group. The supernatants of the four groups were collected and used to treat SKOV3 cells, designated as Adipo-CM, Adipo-CM-1, Adipo-CM-2, and Adipo-CM-3, respectively. Cell viability and apoptosis of OvCa cells were assayed by CCK-8 and flow cytometry, separately. OJS treatment at 200 μg/mL led to reduced cell viability (Day 3: Adipo-CM, 0.83 ± 0.00; Adipo-CM-1, 0.60 ± 0.00, p < 0.05) and elevated apoptosis (Adipo-CM, 2.67 ± 0.15; Adipo-CM-1, 7.02 ± 0.06, p < 0.05) in SKOV3 cells. In addition, OJS rescued effects of oe-IGF1 on cell viability and apoptosis (Adipo-CM-2, 1.43 ± 0.04/1.60 ± 0.16; Adipo-CM-3, 0.89 ± 0.05/2.7 ± 0.47; p < 0.05) (). Western blot revealed that OJS treatment downregulated proliferation-related protein Ki-67, PARP, and anti-apoptotic protein Bcl-2, while upregulating pro-apoptotic proteins Bax and cleaved caspase 3. Furthermore, OJS treatment rescued the effects of oe-IGF1 on these proteins (). To validate the impact of OJS on platinum drug resistance in OvCa cells, we treated SKOV3 cells with varying concentrations of cisplatin and carboplatin and found that OJS treatment led to a significant decrease in IC50 values (Adipo-CM group: IC50 = 34.43/238.5; Adipo-CM-1: IC50 = 17.69/184.4, p < 0.05). OJS was also able to reverse the effects of oe-IGF1 on IC50 values (Adipo-CM-2 group: IC50 = 109.7/359.4; Adipo-CM-3 group: IC50 = 66.12/284.5, p < 0.05) (). These results indicated that OJS inhibited adipocyte-secreted IGF1 and suppressed platinum drug resistance in OvCa cells.
3.4. OJS represses OvCa tumour growth in vivo
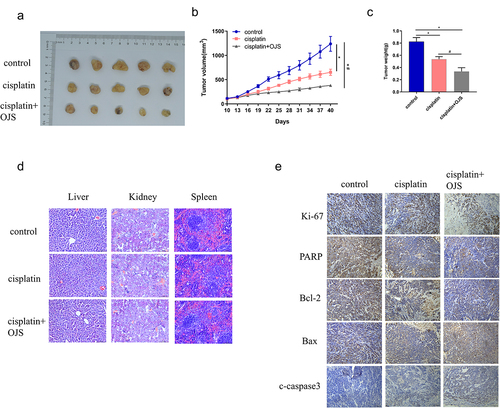
To investigate the influence of OJS on OvCa tumour growth in vivo, we constructed a subcutaneous xenograft mouse model using SKOV3 cells mixed with adipocytes (highly differentiated HPAd cells). We observed the inhibitory effects of OJS and cisplatin on tumour growth in vivo. Cisplatin treatment notably inhibited tumour growth (average tumour volume = 651.43 mm3, average weight = 0.538 g), and co-treatment with OJS and cisplatin resulted in even stronger tumour suppression (average tumour volume = 381.962 mm3, average weight = 0.336 g), with significantly smaller tumour volume (p < 0.05) and weight (p < 0.05) compared to cisplatin treatment alone (). Importantly, neither cisplatin alone nor co-treatment with OJS and cisplatin caused significant damage or morphological changes in the liver, kidney, or spleen, indicating good tolerance in the mice throughout the entire experiment (). IHC presented that cisplatin treatment downregulated proliferation-related protein Ki-67 and anti-apoptotic protein Bcl-2, while upregulating pro-apoptotic proteins Bax and cleaved caspase 3. Furthermore, co-treatment with OJS and cisplatin resulted in more significant changes in these protein expression levels (). Overall, OJS elevated the therapeutic effect of cisplatin in an OvCa xenograft mouse model.
Figure 4. OJS enhances the anticancer activity of cisplatin in SKOV3 and HPAd xenograft models.
4. Discussion
Chemotherapy resistance often leads to unsatisfactory treatment outcomes in OvCa patients receiving platinum-based chemotherapy. With the ongoing advancement in the understanding of OvCa biology, molecular mechanisms of chemotherapy resistance are constantly unearthed and reported. Zhao et al. [Citation25] found that co-expression of FGFR3 and EGFR in OvCa cells enhances cisplatin resistance by activating PI3K/AKT signalling. Increased FANCI is implicated in dismal survival in OvCa patients, as FANCI promotes carboplatin resistance in OvCa by suppressing DNA damage [Citation26]. Resistance of OvCa to platinum-based chemotherapy and the quest for more sustainable ways to improve anticancer efficacy of platinum agents and extend patients’ survival have gained attention from clinicians and researchers.
An expanding corpus of research has linked obesity to the incidence and mortality of female-related cancers. Consequently, the study of the correlation of adipocytes with OvCa becomes a hot topic [Citation3]. Previous studies have shown that adipocytes participate in the occurrence, development, and malignant transformation of OvCa. For instance, adipocytes can activate SphK1, leading to phosphorylation of extracellular signal-regulated kinase (ERK) and promoting epithelial OvCa cell proliferation [Citation27]. Sun et al. [Citation28] also illustrated that the MCP-1/CCR2 axis in adipocytes can promote peritoneal metastasis in OvCa through xenograft and transgenic mouse models. We disclosed that treatment of OvCa cells with Adipo-CM promoted cell proliferation and inhibited apoptosis. Furthermore, adipocytes were found to be associated with chemotherapy resistance in OvCa, as Wang et al. [Citation29] showed that subcutaneous and visceral adipocytes secrete arachidonic acid to enhance OvCa cell resistance to chemotherapy drugs by activating the Akt pathway. Our study using Adipo-CM to treat OvCa cells found that adipocytes could promote platinum resistance in OvCa cells via paracrine secretion of IGF1. This offers novel insights into the mechanism of platinum resistance in OvCa and offers new avenues for reversing platinum drug resistance.
In addition, we found that OJS could reverse the effects of adipocyte paracrine secretion of IGF1 on OvCa cell proliferation, apoptosis, and platinum resistance. In recent years, the use of traditional Chinese medicine formulae for the treatment of cancer has gained more attention and interest. For example, T33, which is composed of Radix Euphorbiae Kansui, Radix Glycyrrhizae, Radix Paeoniae Alba, Rhizoma Pinelliae Preparata, and Radix et Rhizoma Rhei, has significant anti-breast cancer activity. Treatment of HT-29 and Caco2 cells with T33 significantly inhibits cell viability and migration. Tumour volume of breast cancer xenograft mice treated with 200 or 600 mg/kg T33 is notably lower than the control group [Citation30]. Puerariae and Scutellariae and Coptidis Decoction is a classic TCM formula. Lv et al. [Citation31] found that it can downregulate PD-1 expression in CT26 tumours and reshape the gut microbiota and tumour microenvironment, providing a new treatment approach for patients with MSS colorectal cancer. OJS is mainly applied in Asian for treatment of pain, gastrointestinal problems, and depression [Citation17,Citation32]. The use of OJS in cancer therapy is currently the subject of lack of sufficient investigation. Cunningham et al. found that OJS can improve visceral and bodily damage in mice with colon cancer induced by colitis in vitro and vivo [Citation17]. We found that the combination of OJS and cisplatin significantly inhibited OvCa growth in mice but did not have a substantial toxic effect on mice.
In addition, we also found that OJS inhibited platinum resistance in OvCa by regulating adipocyte paracrine IGF1. OJS plays a role by affecting the inflammatory response. For example, OJS can reduce the infiltration of inflammatory cells in the respiratory tract and the number of interleukin-4 and immunoglobulins to inhibit pulmonary fibrosis [Citation16]. IGF1 is also involved in the inflammatory response. Li et al. [Citation33] found that puerarin can inhibit the secretion of interleukin-6 and interleukin-8 by atherosclerotic vascular smooth muscle, mainly through the miR-29b-3p/IGF1 axis to inhibit inflammation. Tofacitinib inhibits the production of pro-inflammatory factors in rheumatoid arthritis synovial fibroblasts, in which IGF1 plays an important role [Citation34]. Therefore, we speculated that OJS may affect the paracrine of IGF1 in adipocytes by affecting inflammatory factors or inflammatory responses. In addition, OJS is composed of 15 drugs, which are rich in chemical composition. For example, the chemical component paeoniflorin has been reported to inhibit the production of inflammatory factors in lipopolysaccharide-stimulated THP-1 cells and to affect NF-κB pathway-related factors [Citation35]. The chemical composition of OJS is complex, and its effect on the paracrine of IGF1 in adipocytes may also be complex, which requires us to continue to explore in subsequent experiments.
In summary, our research showed that adipocytes could promote malignant progression of OvCa through the paracrine secretion of IGF1, while the combination of OJS and platinum drugs could hinder OvCa cell proliferation and induce apoptosis, and OJS could reverse OvCa cell resistance to platinum drugs. This finding may reveal the correlation of adipocytes with OvCa cells, highlight the potential clinical applications of OJS in OvCa treatment, and provide a novel strategy for future clinical use of OJS and platinum drugs in combination. In addition, in the reported studies, OJS showed low cytotoxicity when used in combination with other drugs and was a safe drug. However, the molecular mechanisms underlying the reversal of platinum resistance by OJS in OvCa are still poorly understood, and its clinical application requires further in vivo research and prospective clinical trials for validation. At the same time, in the platinum drug treatment of OvCa, although in the mouse experiment, the combination of OJS and cisplatin did not cause significant damage and morphological changes in the liver, kidney and spleen, more experimental analysis and clinical experiments were needed for further verification. In conclusion, our research results indicated that adipocyte paracrine secretion of IGF1 had a positive effect on the survival and platinum resistance of OvCa, IGF1 secreted by adipocytes may be a potential target for OvCa treatment, which provides ideas for the clinical treatment of OvCa. OJS could act as a new drug for OvCa and provide new ideas for the development of clinical drugs for OvCa. Additionally, given the low toxicity of OJS, OJS may be useful as an adjunctive therapeutic agent for the treatment of OvCa.
Author contributions
Jiong Ma contributed to the study design and wrote the manuscript. Junyan Li conducted the literature search and acquired the data. Xuejun Chen performed data analysis and drafted. Yanyan Ma revised the manuscript and gave the final approval of the version to be submitted.
Ethics approval and consent to participate
The study was approved by the Ethics Committee of The Second Affiliated Hospital, Zhejiang University, School of Medicine.
Supplemental Material
Download TIFF Image (7 MB)Disclosure statement
No potential conflict of interest was reported by the author(s).
Supplementary material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/21623945.2023.2282566
Data availability statement
The data that support the findings of this study are openly available in zenodo at https://doi.org/10.5281/zenodo.8035464, reference number 8,035,464.
Additional information
Funding
References
- Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–11. doi: 10.3322/caac.21660
- Tian W, Lei N, Zhou J, et al. Extracellular vesicles in ovarian cancer chemoresistance, metastasis, and immune evasion. Cell Death Dis. 2022;13(1):64. doi: 10.1038/s41419-022-04510-8
- Zhou S, Wang R, Xiao H. Adipocytes induce the resistance of ovarian cancer to carboplatin through ANGPTL4. Oncol Rep. 2020;44(3):927–938. doi: 10.3892/or.2020.7647
- Wu Y, Wu T, Hu X, et al. Proguanil synergistically sensitizes ovarian cancer cells to olaparib by increasing DNA damage and inducing apoptosis. Int J Med Sci. 2022;19(2):233–241. doi: 10.7150/ijms.67027
- Mukherjee A, Bilecz AJ, Lengyel E. The adipocyte microenvironment and cancer. Cancer Metastasis Rev. 2022;41(3):575–587. doi: 10.1007/s10555-022-10059-x
- Ladanyi A, Mukherjee A, Kenny HA, et al. Adipocyte-induced CD36 expression drives ovarian cancer progression and metastasis. Oncogene. 2018;37(17):2285–2301. doi: 10.1038/s41388-017-0093-z
- Miranda F, Mannion D, Liu S, et al. Salt-inducible kinase 2 Couples ovarian cancer cell Metabolism with survival at the adipocyte-rich metastatic niche. Cancer Cell. 2016;30(2):273–289. doi: 10.1016/j.ccell.2016.06.020
- Mukherjee A, Chiang CY, Daifotis HA, et al. Adipocyte-induced FABP4 expression in ovarian cancer cells promotes metastasis and mediates carboplatin resistance. Cancer Res. 2020;80(8):1748–1761. doi: 10.1158/0008-5472.CAN-19-1999
- Li G, Zhou L, Zhang C, et al. Insulin-Like Growth Factor 1 Regulates Acute Inflammatory Lung Injury Mediated by Influenza Virus Infection. Front Microbiol. 2019;10:2541. doi: 10.3389/fmicb.2019.02541
- Kasprzak A, Szaflarski W. Role of alternatively spliced Messenger RNA (mRNA) isoforms of the insulin-like growth factor 1 (IGF1) in selected human tumors. Int J Mol Sci. 2020;21(19):21. doi: 10.3390/ijms21196995
- Yu H, Rohan T. Role of the insulin-like growth factor family in cancer development and progression. J Natl Cancer Inst. 2000;92(18):1472–1489. doi: 10.1093/jnci/92.18.1472
- Hillers LE, D’Amato JV, Chamberlin T, et al. Obesity-Activated adipose-Derived Stromal cells promote breast cancer growth and invasion. Neoplasia. 2018;20(11):1161–1174. doi: 10.1016/j.neo.2018.09.004
- Liotti A, La Civita E, Cennamo M, et al. Periprostatic adipose tissue promotes prostate cancer resistance to docetaxel by paracrine IGF-1 upregulation of TUBB2B beta-tubulin isoform. Prostate. 2021;81(7):407–417. doi: 10.1002/pros.24117
- Ha H, Lee JK, Lee HY, et al. Genotoxicity assessment of a herbal formula, Ojeok-san. J Ethnopharmacol. 2011;135(2):586–589. doi: 10.1016/j.jep.2011.03.024
- Patton EA, Cunningham P, Noneman M, et al. Acute administration of Ojeok-san Ameliorates pain-like Behaviors in pre-clinical models of inflammatory bowel diseases. Nutrients. 2023;15(7):15. doi: 10.3390/nu15071559
- Shin IS, Lee MY, Jeon WY, et al. Ojeok-san, a traditional Korean herbal medicine attenuates airway inflammation and pulmonary fibrosis induced by repeated ovalbumin challenge. J Ethnopharmacol. 2013;149(1):281–287. doi: 10.1016/j.jep.2013.06.036
- Cunningham P, Sumal A, Patton E, et al. Ojeok-san ameliorates visceral and somatic nociception in a mouse model of colitis induced colorectal cancer. PLoS One. 2022;17(6):e0270338. doi: 10.1371/journal.pone.0270338
- Lyu YR, Kim KI, Yang C, et al. Efficacy and Safety of Ojeok-san Plus Saengmaek-san for gastroesophageal reflux-induced chronic cough: a Pilot, randomized, Double-blind, placebo-controlled trial. Front Pharmacol. 2022;13:787860. doi: 10.3389/fphar.2022.787860
- Kim EJ, Nam D, Ahn BJ, et al. Study to establish Ojeok-san (five accumulation powder: wu ji san) administration criteria and a questionnaire to evaluate the holistic effects of Ojeok-san on patients with low back pain. J Altern Complement Med. 2013;19(11):891–897. doi: 10.1089/acm.2011.0312
- Jeong SJ, Huh JI, Shin HK. Cytotoxicity and sub-acute toxicity in Crl: CD (SD) rats of traditional herbal formula Ojeok-san. BMC Complement Altern Med. 2015;15(1):38. doi: 10.1186/s12906-015-0582-y
- Kim JH, Seo CS, Kim SS, et al. Quality assessment of Ojeok-san, a traditional herbal formula, using high-performance liquid chromatography combined with Chemometric Analysis. J Anal Methods Chem. 2015;2015:1–11. doi: 10.1155/2015/607252
- Wei G, Sun H, Dong K, et al. The thermogenic activity of adjacent adipocytes fuels the progression of ccRCC and compromises anti-tumor therapeutic efficacy. Cell Metab. 2021;33(10):2021–39 e8. doi: 10.1016/j.cmet.2021.08.012
- Magana A, Giovanni R, Essien E, et al. Amniotic growth factors enhanced human pre-adipocyte cell viability and differentiation under hypoxia. J Biomed Mater Res B Appl Biomater. 2022;110(9):2146–2156. doi: 10.1002/jbm.b.35068
- Yang HY, Qu RM, Lin XS, et al. IGF-1 from adipose-derived mesenchymal stem cells promotes radioresistance of breast cancer cells. Asian Pac J Cancer Prev. 2014;15(23):10115–10119. doi: 10.7314/APJCP.2014.15.23.10115
- Zhao J, Tan W, Zhang L, et al. FGFR3 phosphorylates EGFR to promote cisplatin-resistance in ovarian cancer. Biochem Pharmacol. 2021;190:114536. doi: 10.1016/j.bcp.2021.114536
- Li Y, Zhang Y, Yang Q, et al. Silencing of FANCI promotes DNA damage and sensitizes ovarian cancer cells to carboplatin. Curr Cancer Drug Targets. 2022;22(7):591–602. doi: 10.2174/1568009622666220331091709
- Dai L, Wang C, Song K, et al. Activation of SphK1 by adipocytes mediates epithelial ovarian cancer cell proliferation. J Ovarian Res. 2021;14(1):62. doi: 10.1186/s13048-021-00815-y
- Sun C, Li X, Guo E, et al. MCP-1/CCR-2 axis in adipocytes and cancer cell respectively facilitates ovarian cancer peritoneal metastasis. Oncogene. 2020;39(8):1681–1695. doi: 10.1038/s41388-019-1090-1
- Yang J, Zaman MM, Vlasakov I, et al. Adipocytes promote ovarian cancer chemoresistance. Sci Rep. 2019;9(1):13316. doi: 10.1038/s41598-019-49649-1
- Liu YT, Tzang BS, Yow J, et al. Traditional Chinese medicine formula T33 inhibits the proliferation of human colorectal cancer cells by inducing autophagy. Environ Toxicol. 2022;37(5):1007–1017. doi: 10.1002/tox.23460
- Lv J, Jia Y, Li J, et al. Gegen Qinlian decoction enhances the effect of PD-1 blockade in colorectal cancer with microsatellite stability by remodelling the gut microbiota and the tumour microenvironment. Cell Death Dis. 2019;10(6):415. doi: 10.1038/s41419-019-1638-6
- Kim JH, Seo CS, Kim SS, et al. Compositional differences of Ojeok-san (wuji-san) decoctions using pressurized or non-pressurized Methods for variable extraction times. J Pharmacopuncture. 2012;15(2):24–30. doi: 10.3831/KPI.2012.15.2.024
- Li J, Li Y, Yuan X, et al. The effective constituent puerarin, from Pueraria lobata, inhibits the proliferation and inflammation of vascular smooth muscle in atherosclerosis through the miR-29b-3p/IGF1 pathway. Pharm Biol. 2023;61(1):1–11. doi: 10.1080/13880209.2022.2099430
- Liu Y, Peng J, Xiong X, et al. Tofacitinib enhances IGF1 via inhibiting STAT6 transcriptionally activated-miR-425-5p to ameliorate inflammation in RA-FLS. Mol Cell Biochem. 2022;477(10):2335–2344. doi: 10.1007/s11010-022-04444-x
- Cao L, Yang K. Paeoniflorin attenuated TREM-1-Mediated inflammation in THP-1 cells. J Healthc Eng. 2022;2022:7051643. doi: 10.1155/2022/7051643
