Abstract
Chronic pancreatitis (CP) is a risk factor of pancreatic ductal adenocarcinoma (PDAC) and characterized by a pronounced desmoplastic reaction with CD4+ T cells accounting for the majority of the stromal T cell infiltrate. Epithelial-mesenchymal-transition (EMT) is a critical process for metastasis by which epithelial/carcinoma cells become enabled to disseminate probably prior to tumor formation. To investigate whether CD4+ T cells induce EMT in human pancreatic ductal epithelial cells, premalignant H6c7 cells were mono- or co-cultured with human CD4+CD25+CD127−CD49d− regulatory T cells (T-regs) or CD4+CD25− T-effector cells (T-effs) being isolated by negative magnetic bead separation from blood of healthy donors. Particularly in the presence of activated T-effs, H6c7 cells acquired a spindle-shaped morphology, reduced E-cadherin expression, and elevated expression of the mesenchymal proteins vimentin, L1CAM, and ZEB-1. This was accompanied by an increased invasive behavior. Moreover, activated T-effs exerted similar effects in the PDAC cell line T3M4. Blocking of TNF-α and IL-6 being released at greater amounts into supernatants during co-cultures with activated T-effs attenuated the EMT-associated alterations in H6c7 cells. Supporting these findings, EMT-associated alterations (exemplified by reduced E-cadherin expression and enhanced expression of vimentin and L1CAM) were predominantly detected in ductal epithelium of CP tissues surrounded by a dense stroma enriched with CD4+ T cells. Overall this study points to a novel role of CD4+ T cells beyond their immune function in pancreatic tumorigenesis and underscores the view that EMT induction in pancreatic ductal epithelial cells represents an early event in PDAC development being essentially promoted by inflammatory processes.
Abbreviations:
- AUC, area under the curve
- CP, chronic pancreatitis
- EMT, epithelial-mesenchymal-transition
- PanIN, pancreatic intraepithelial neoplasia
- PBMC, peripheral blood mononuclear cells
- PDAC, pancreatic ductal adenocarcinoma
- SD, standard deviation
- T-effs, CD4+CD25− T-effector cells
- T-regs, CD4+CD25+CD127−CD49d− regulatory T cells
Introduction
PDAC is still one of the most malignant tumors with an overall 5-y-survival rate below 10%.Citation1 This is due to a mostly late diagnosis in an already metastasized stage and hence, curative therapeutic options are limited to <20% of the patients. Additionally, PDAC exhibits a profound resistance toward common therapy regimens worsening therapeutic responses and prognosis of these patients, too.Citation2 PDAC as well as its risk factor condition CP are similarly characterized by a pronounced desmoplasia comprising extracellular matrix proteins, myofibroblasts, endothelial cells, and various immune cells such as macrophages and T cells.Citation3–6 Most of the tumor-associated immune cells are supposed to be immunosuppressive and their presence is regarded as an immune escape mechanism of the tumor enabling survival of pre-/neoplastic cells and thereby tumorigenesis.Citation3,7,8 However, besides their immunological functions, immune cells might contribute to the initiation and progression of tumors by other mechanisms, e.g., by promoting angiogenesis or migration and metastasis of tumor cells.Citation3,6,7 Recent reports demonstrated a role of macrophages in EMT being a central process in early tumorigenesis.Citation9–11 During EMT, epithelial and later on carcinoma cells lose their epithelial phenotype and acquire characteristics of mesenchymal cells such as a spindle-shaped morphology, loss of the epithelial surface molecule E-cadherin, and an increased expression of mesenchymal proteins such as vimentin and N-cadherin. These processes can be regulated by various transcription factors such as Snail, Slug, Twist or ZEB-1. Additionally, we and others have shown that the adhesion molecule L1CAM (CD171) is upregulated during EMT, essentially contributing to a migratory/invasive and apoptosis resistant phenotype of tumor cells but also of premalignant ductal epithelial (H6c7) cells.Citation11–13 Moreover, L1CAM overexpressing H6c7 cells became tumorigenic and metastatic in vivo,Citation14 and an elevated L1CAM expression along with high numbers of stromal cells has been associated with advanced tumor stages and poor prognosis of PDAC patients.Citation5,15–19
Altogether, EMT-associated alterations are regarded as prerequisite for cell dissociation from the primary epithelial context, cell dissemination and finally formation of metastasis.Citation20 Findings from an endogenous mouse (PKCY) model demonstrated that a CP promotes induction of EMT even in premalignant cells of pancreatic intraepithelial neoplasias (PanIN) leading to their dissemination before detection of a primary PDAC.Citation21 In line with this, patients with pancreatic cystic lesions have been shown to exhibit elevated numbers of circulating pancreatic epithelial cells in the blood compared to healthy controls.Citation22 These findings support the view that inflammatory processes promote EMT and dissemination of even premalignant cells quite early in pancreatic tumorigenesis. Based on these observations and on the predominance of CD4+ T cells in the stroma of CPs compared to PDACs,Citation6 this study aimed at elucidating the role of two different CD4+ T cell subsets in promoting EMT-associated alterations in premalignant pancreatic ductal epithelial cells. For this purpose, H6c7 cells used as a model system for premalignant pancreatic ductal epithelial cellsCitation23,24 and T3M4 PDAC cells were directly co-cultured with T-regs or T-effs freshly isolated from healthy donors. In these co-cultures, we analyzed the impact of different CD4+ T cell subsets on these premalignant and malignant ductal epithelial cells regarding cell morphology, expression of EMT marker, cell invasion, and identified the EMT-mediating factors. Finally, tissues from CP patients were immunohistochemically analyzed for the presence of CD4+ T cells and FoxP3+ T-regs as well as for the EMT marker E-cadherin, vimentin and L1CAM.
Results
H6c7 cells acquire a spindle-shaped morphology in the presence of activated CD4+ T-effs and T-regs
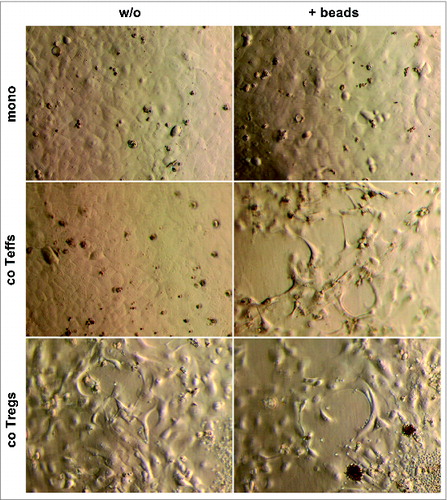
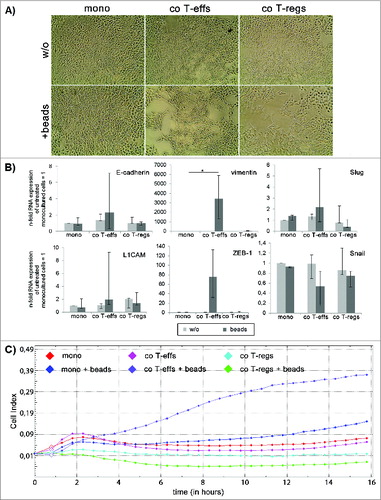
Animal based studies indicate that CP promotes EMT already in the premalignant ductal epithelium of PanINs leading to dissemination of pancreatic epithelial cells with a mesenchymal phenotype.Citation21 Moreover, we previously showed that the majority of CP tissues were characterized by elevated numbers of CD4+ T cells being significantly higher compared to PDAC tissues.Citation6 Altogether this led to the hypothesis that CD4+ T cells might be involved in the induction of EMT in the pancreatic ductal epithelium, particularly under inflammatory conditions paving the way for PDAC development. Thus, in order to analyze the impact of different CD4+ T cell populations on premalignant pancreatic ductal epithelial cells, H6c7 cells were cultured either alone (mono) or in the presence of untouched CD4+CD25+CD127−CD49d− regulatory T cells (co T-regs) or CD4+CD25− T-effector cells (co T-effs) isolated from healthy donors. Characterization of both T cell subsets by flow cytometry confirmed their phenotype as previously reported (Fig. S1).Citation25 To consider different activation stages, particularly of T-effs, all cultures were conducted in the absence or presence of activation beads to mimic antigen stimulation by antigen presenting cells. Under these conditions, T-effs are characterized by elevated expression of CD25, elevated secretion of IL-2 and accordingly high proliferative activity.Citation25 As shown in , monocultured H6c7 cells formed a typical epithelial monolayer with polygonal rounded cells (). In contrast, H6c7 cells co-cultured with bead-activated CD4+ T-effs or T-regs acquired a spindle-shaped morphology which is characteristic for EMT induction ().
Figure 1. H6c7 cells acquire a spindle-shaped morphology in the presence of activated CD4+ T-effs and T-regs. Representative images (n = 7 experiments with T cells isolated from seven individual donors) showing cell morphology of H6c7 cells monocultured (mono) or directly co-cultured with T-effs (co T-effs) or T-regs (co T-regs) for 72 h, either in the absence (w/o) or presence of activation beads (+ beads). Magnification x 400.
Altered expression of EMT markers in H6c7 cells predominates in the presence of activated CD4+ T-effs
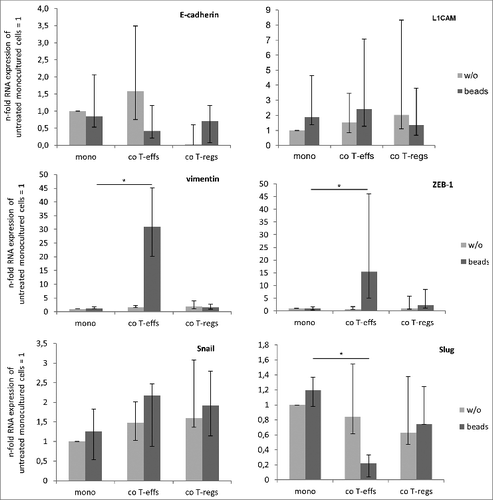
To further validate EMT-associated alterations in H6c7 cells through their exposure to CD4+ T-effs and T-regs, the expression of epithelial E-cadherin, mesenchymal L1CAM, and vimentin as well as the EMT-associated transcription factors ZEB-1, Snail and Slug were analyzed. RT-qPCR revealed most pronounced effects in H6c7 cells when cultured with activated CD4+ T-effs, being in line with the morphological alterations. Thus, a reduced expression of E-cadherin, an enhanced expression of L1CAM and Snail by trend and a significantly increased expression of vimentin (31-fold) and ZEB-1 (15-fold) was observed (). Surprisingly, Slug expression was significantly reduced under these conditions (). Albeit H6c7 cells acquired an elongated mesenchymal cell shape in the presence of T-regs (in the absence and presence of activation beads), too, the effects on EMT marker expression were smaller and less consistent compared to the effects seen after co-culture with activated T-effs. ().
Figure 2. An altered EMT marker expression predominates in H6c7 cells when co-cultured with activated CD4+ T-effs. H6c7 cells were monocultured (mono) or directly co-cultured with T-effs (co T-effs) or T-regs (co T-regs) for 72 h, either in the absence (w/o) or presence of activation beads (+ beads). E-cadherin, L1CAM, vimentin, ZEB-1, Snail and Slug expression were analyzed by RT-qPCR. TBP was used as house-keeping gene for control. Data represent the median values with quartiles (Q0,75 as upper, Q0.25 as lower deviation) of 5–7 independent experiments with T cells isolated from seven individual donors. * = p < 0.05.
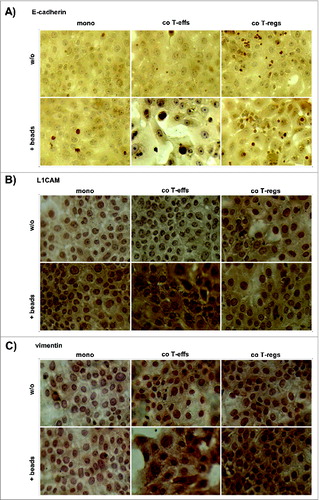
Supporting the PCR based data on EMT marker expression, flow cytometry analyses of EMT marker protein levels revealed that the strongest alterations again occurred in H6c7 cells co-cultured with activated T-effs (). Under these conditions, the percentage of E-cadherin expressing cells was diminished while the percentages of L1CAM and vimentin expressing cells were increased (). To exclude any altered protein expression caused solely by the detachment procedure, mono- and co-cultured H6c7 cells were directly stained in the 96-well-plates after removal of T cells. In accordance with the flow cytometry data, H6c7 cells co-cultured with activated T-effs exhibited the weakest E-cadherin expression () and the strongest expression of L1CAM () and vimentin () compared to H6c7 cells mono-cultured with beads ().
Figure 3. An altered EMT marker expression predominates in H6c7 cells when co-cultured with activated CD4+ T-effs. H6c7 cells were monocultured (mono) or directly co-cultured with T-effs (co T-effs) or T-regs (co T-regs) for 72 h, either in the absence (w/o) or presence of activation beads (+ beads). Flow cytometry analysis of E-cadherin, L1CAM and vimentin expression in detached mono- and co-cultured H6c7 cells. Data are presented as median values with quartiles quartiles (Q0,75 as upper, Q0.25 as lower deviation) of 3–6 independent experiments with T cells isolated from 3–6 individual donors. * = p < 0.05.
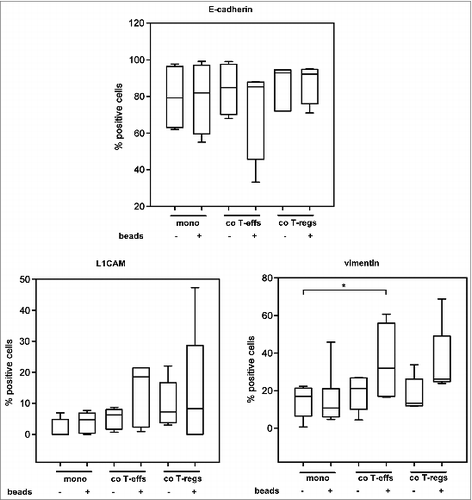
Figure 4. An altered EMT marker expression predominates in H6c7 cells when co-cultured with activated CD4+ T-effs. H6c7 cells were monocultured (mono) or directly co-cultured with T-effs (co T-effs) or T-regs (co T-regs) for 72 h, either in the absence (w/o) or presence of activation beads (+ beads). Immunocytochemistry were used to detect (A) E-cadherin, (B) L1CAM and (C) vimentin in mono- and co-cultured H6c7 cells still adherent in 96-well-plates. Representative stainings of three independent experiments with T cells isolated from three individual donors are shown at 580-fold magnification.
Altogether, these data indicate that premalignant pancreatic ductal epithelial cells acquire a mesenchymal phenotype – exemplified by an elongated cell shape, reduced E-cadherin expression and an enhanced L1CAM and vimentin expression – predominantly in the presence of activated CD4+ T-effs.
H6c7 cells exhibit increased cell invasion after co-culture with activated CD4+ T-effs
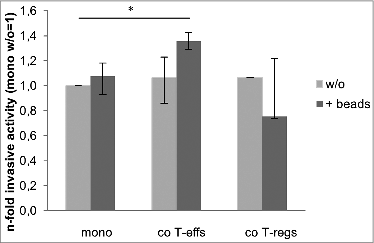
To verify that the altered morphology and EMT marker expression of H6c7 cells upon co-culture with activated T-effs relates to an increased invasive phenotype, cell invasion of mono- and co-cultured H6c7 cells was analyzed by a modified Boyden chamber assay. In the absence of activation beads, co-culture with either CD4+ T cell population slightly increased cell invasion of H6c7 cells by about 10% (). Albeit a weak induction of cell invasion occurred just in the presence of activation beads, H6c7 cells exhibited a significantly increased cell invasion by 36% after co-culture with activated T-effs (). These data indicate that the effects of activated CD4+ T-effs on morphology and EMT marker expression in premalignant pancreatic ductal epithelial cells are associated with greater invasiveness.
Figure 5. Greater invasiveness of H6c7 cells after co-culture with activated CD4+ T-effs. H6c7 cells were mono-cultured (mono) or directly co-cultured with T-effs (co T-effs) or T-regs (co T-regs) for 72 h, either in the absence (w/o) or presence of activation beads (+ beads). Cell invasion of H6c7 cells was determined in a modified boyden chamber on collagen-I coated transwells after 24 h. Median values with quartiles quartiles (Q0,75 as upper, Q0.25 as lower deviation) of % invaded cells from three independent experiments with T cells isolated from three individual donors are shown. * = p < 0.05.
Activated CD4+ T-effs are also potent inducers of EMT in malignant pancreatic ductal epithelial cells
In order to test the ability of CD4+ T cell subsets to induce EMT also in malignant PDAC cells which already exhibit a semi-mesenchymal phenotype per-se, the PDAC cell line T3M4 was mono- or co-cultured with T-effs and T-regs and in the absence or presence of activation beads. As observed in H6c7 cells, T3M4 cells acquired an even more pronounced spindle-shaped morphology upon co-culture with both activated T-effs and T-regs (). Similar to H6c7 cells, RT-PCR analyses revealed a clear upregulation of vimentin (> 3400-fold) and ZEB-1 (75-fold) in T3M4 cells only after co-culture with activated T-effs (). The expression of L1CAM, Snail, and Slug remained almost unaltered while the expression of E-cadherin was even slightly elevated under these conditions. As observed in H6c7 cells, some minor effects of T-regs on EMT marker expression were also observed in T3M4 cells (e.g., increased L1CAM expression) (). Finally, to analyze whether the T-eff mediated alterations in cell morphology and EMT marker expression correlate with an invasive behavior, invasion of T3M4 cells was determined by Boyden chamber assays after 24 h demonstrating a clearly increased cell invasion of T3M4 cells after co-culture with activated CD4+ T-effs. However, invaded cells did not adhere to the collagen matrix of the upper compartment anymore and were felt down in the lower compartment eluding the crystal violet staining (data not shown). Thus, in order to ensure a valid measurement for T3M4 cells, the xCELLigence RTCA DP analyzer® was used which allows monitoring of cell invasion in real time by impedance measurement.Citation26 Compared to premalignant H6c7 cells showing maximal cell invasion after 24 h, T3M4 cells invaded much faster through the collagen-I matrix so that 16 h were chosen as optimal time point (data not shown). Furthermore, only T3M4 cells that had been co-cultured in the presence of activated CD4+ T-effs showed a significantly increased invasive activity through the collagen-I matrix compared to mono-cultured cells in the presence of activation beads (Area under the curve (AUC) T-effs+ beads = 5.79 vs. AUC mono+beads = 2.03; p < 0.05) (). Altogether, these data support the view that activated CD4+ T-effs potently induce EMT and increase cell invasion not only in premalignant but also in malignant pancreatic ductal epithelial cells.
Figure 6. (See previous page). Activated CD4+ T-effs induce EMT and increase cell invasion in T3M4 cells. (A) Representative images (n = 3 experiments with T cells isolated from three individual donors) showing cell morphology of T3M4 cells monocultured (mono) or directly co-cultured with T-effs (co T-effs) or T-regs (co T-regs) for 72 h, either in the absence (w/o) or presence of activation beads (beads). Magnification x 280. (B) E-cadherin, L1CAM, vimentin, ZEB-1, Snail and Slug expression were analyzed by RT-qPCR in the differentially cultured T3M4 cells. TBP was used as house-keeping gene for control. Data represent the median values with quartiles (Q0,75 as upper, Q0.25 as lower deviation) of four independent experiments with T cells isolated from four individual donors. * = p < 0.05. (C) Cell invasion of T3M4 cells was determined in an xCelligence Real time Analyzer with collagen-I coated transwells after 24 h. Since maximum cell invasion was reached after 16 h, cell invasion of mono- and co-cultured T3M4 cells presented as cell indices determined over a time period of 16 h. One representative invasion assay from three independent experiments with T cells isolated from three individual donors is shown.
EMT mediators TNF-α and IL-6 levels are elevated in co-cultures with activated CD4+ T-effs
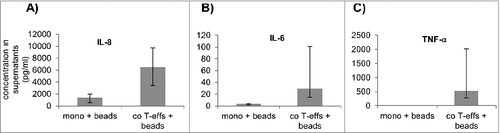
To identify the mediators accounting for the EMT-associated alterations induced by activated CD4+ T-effs, IL-8, IL-12(p70), IL-13, IFNγ, MCSF, GCSF, TGF-β1, TNF-α, and IL-6 were determined in supernatants of mono- and co-cultured H6c7 cells. The amounts of IL-12(p70), IL-13, IFNγ, MCSF, GCSF and TGF-β1 did not show significant alterations upon co-culture with activated CD4+ T-effs when compared to monoculture (data not shown). By contrast, supernatants from H6c7 cells co-cultured with activated CD4+ T-effs contained greater amounts of IL-8 (), IL-6 () and TNF-α (), the latter two of which being established EMT inducing factors. RT-qPCR analysis with RNA from separated co-cultured H6c7 and T cells showed that TNF-α expression was higher in T-effs than in H6c7 cells while IL-6 seemed to originate largely from H6c7 cells because IL-6 levels were greater in co-cultured H6c7 cells than in T-effs (data not shown). Thus, CD4+ T-effs mediated EMT-associated alterations in H6c7 cells may depend on IL-6 and TNF-α released at high amounts upon mutual interaction of CD4+ T-effs and epithelial cells.
Figure 7. Elevated levels of IL-8, IL-6 and TNF-α in co-cultures with activated CD4+ T-effs. Supernatants from monocultured (mono) or directly T-eff co-cultured (co T-effs) H6c7 cells, either in the absence (w/o) or presence of activation beads (+ beads), were analyzed after 72 h for the presence of (A) IL-8, (B) IL-6 and (C) TNF-α. Data are expressed as concentrations (pg/mL) in supernatants and represent median values with quartiles quartiles (Q0,75 as upper, Q0.25 as lower deviation) of three independent experiments with T cells isolated from 3 individual donors.
Elevated levels of TNF-α and IL-6 under co-culture with activated CD4+ T-effs account for the acquisition of the mesenchymal and invasive phenotype of H6c7 cells
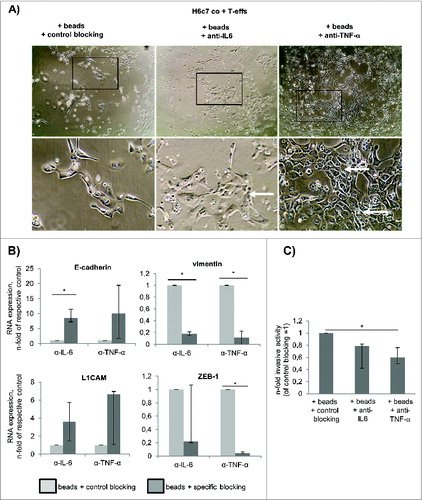
Given that H6c7 cells undergo substantial EMT in the presence of activated T-effs along with secretion of high amounts of IL-6 and TNF-α (), the impact of both factors on these malignancy-associated alterations in H6c7 cells was investigated. Blocking of TNF-α activity by Etanercept clearly impaired the morphological changes in H6c7 cells induced by activated T-effs as more H6c7 cells grew in typical epithelial monolayers. However, several cells still exhibited an elongated cell shape (, right images). Similar effects, albeit less pronounced, were observed in H6c7 cells co-cultured with activated T-effs and anti-IL-6R blocking antibody Tocilizumab (, middle images). When analyzing EMT marker expression by RT-qPCR (), it was noted that blocking of either factor abolished the decline of E-cadherin expression compared to the respective control. Additionally, blocking of IL-6 or TNF-α activity in co-cultures with activated T-effs concomitantly reduced expression of vimentin and ZEB-1 (). Surprisingly, expression of L1CAM was elevated in co-cultured H6c7 cells by either blocking strategy. To elucidate whether this partial EMT reversion results in a diminished invasiveness of H6c7 cells, modified boyden chamber assays with differently co-cultured H6c7 cells were performed. Indeed, blockade of TNF-α activity during co-culture with activated T-effs most potently inhibited H6c7 cell invasion by 40% while blockade of IL-6R caused a decrease of cell invasion by 20% (). These findings match the clear reversion of the mesenchymal phenotype under TNF-α and IL-6 blockade. Altogether, these data indicate that activated CD4+ T-effs promote EMT and cell invasion of premalignant pancreatic ductal epithelial cells depending on the mutual interaction of epithelial and T cells and their release of IL-6 and TNF-α, respectively.
Figure 8. TNF-α and IL-6 account for the acquisition of the mesenchymal and invasive phenotype of H6c7 cells during co-culture with activated CD4+ T-effs. H6c7 cells were directly co-cultured with T-effs (co T-effs) and activation beads (+ beads) for 72 h. As indicated, 10 μg/mL of Rituximab (control blocking) or blocking agents specific for IL-6 (Tocilizumab) or TNF-α (Etanercept) were added during co-culture. (A) Representative images of three independent experiments showing cell morphologies of differentially treated H6c7 cells during T-eff co-culture. Magnification x 280 (upper panel) and x 560 (lower panel). (B) E-cadherin, L1CAM, vimentin and ZEB-1 expression were analyzed by RT-qPCR. TBP was used as house-keeping gene for control. Data represent the median values with quartiles quartiles (Q0,75 as upper, Q0.25 as lower deviation) of 3–4 independent experiments. (C) Cell invasion of differentially treated H6c7 cells was determined in a modified boyden chamber on collagen-I coated transwells after 24 h. Median values with quartiles (Q0,75 as upper, Q0.25 as lower deviation) of % invaded cells from three independent experiments with T cells isolated from three individual donors are shown. * = p < 0.05.
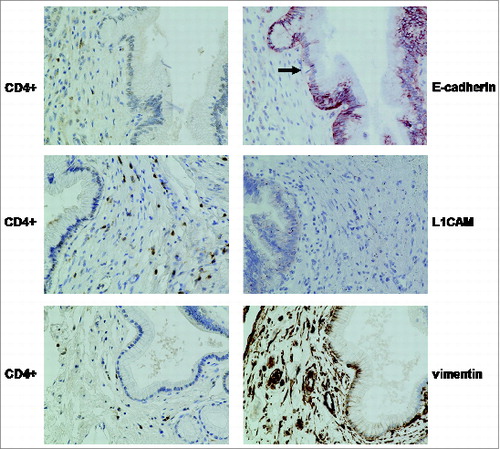
Elevated expression of vimentin and L1CAM and partial loss of E-cadherin in PanINs surrounded by a dense stroma enriched with CD4+ T cells in CP tissues
To evaluate whether such EMT-associated alterations as observed in vitro in the presence of T-effs might also occur in vivo, pancreatic tissues from 15 CP patients were immunohistochemically stained for E-cadherin, vimentin and L1CAM, as well as for CD4+. Additionally, FoxP3 was detected as marker for T-regs. As recently demonstrated,Citation6 14/15 tissues were characterized by high numbers of CD4+ T cells (, ), while FoxP3+ T cells were found at higher levels only in 4/15 CP tissues () indicating that CD4+ cells are presumably CD4+ T-effs predominating T-regs in CP tissues. Although staining of E-cadherin revealed still an overall high expression in PanINs in all tissues analyzed, this epithelial marker was partially lost in those areas of several PanINs that were surrounded by a dense stroma with CD4+ T cells (). While normal pancreatic ducts completely lack L1CAM expression,Citation11 PanIN associated L1CAM expression was present in 10/15 CP tissues and vimentin expression was detectable in PanINs of 8/15 CP tissues (). Although no statistical correlations of EMT markers and the high abundance of CD4+ T cells could be obtained (because the number of CD4+ T cells was constantly scored with 2 in 14/15 tissues), an altered expression profile of vimentin and L1CAM was often found in PanINs when surrounded by a pronounced stroma enriched with CD4+ T cells ().
Table 1. Scoring of CD4+ and FoxP3+ T cells and EMT marker expression in PanINs in pancreatic tissues of CP patients
Figure 9. Distribution of CD4+ T cells and EMT markers in PanINs within CP tissues. Representative E-cadherin, L1CAM and vimentin stainings of CP tissues demonstrate partially reduced E-cadherin expression (indicated by the arrow) and enhanced L1CAM and vimentin expression in PanINs surrounded by a dense stroma enriched with CD4+ T cells (magnification x 400). Note, that besides vimentin expression in PanINs surrounded by a CD4+ T cells containing stroma, the stromal compartment itself was characterized by strong vimentin expression, too.
Discussion
EMT is regarded as a cellular process indispensable for the metastatic cascade, applying also for PDAC.Citation20 Mouse model based studies showed that pancreatic duct cells of PanINs that have undergone EMT disseminate out of the pancreas and seed the liver, a process which is already initiated and enforced by a CP.Citation21 These preclinical findings could be substantiated by detection of such cells in the blood of patients with pancreatic cystic lesions but not in donors lacking any pancreatic disease.Citation22 Thus, metastasis may start prior to formation of the primary tumor or at least prior to its diagnosis underscoring the need to better understand how inflammation promotes EMT in premalignant pancreatic epithelial cells.
The observation that CD4+ T cells are abundant in CP tissues and represent the majority of the T cell infiltrateCitation6 prompted us to elucidate the impact of different CD4+ T cell populations on EMT induction in premalignant pancreatic epithelial cells. We could show that activated CD4+ T-effs most strongly promote EMT-associated alterations in H6c7 cells. Along with the acquisition of an elongated, mesenchymal cell shape, H6c7 cells exhibited an increased expression of mesenchymal proteins such as vimentin and L1CAM, a reduced expression of E-cadherin, and an increased invasive behavior. Moreover, these effects were not restricted to premalignant ductal epithelial cells because similar alterations were observed in the PDAC cell line T3M4 after co-culture with activated CD4+ T-effs. However, downregulation of E-cadherin expression was only observed in premalignant H6c7 but not T3M4 cells which can be explained by the fact that T3M4 cells per-se exhibit a semi-mesenchymal phenotype being characterized by an already reduced E-cadherin expression compared to H6c7 cells. Altogether, these findings point to a novel role of CD4+ T cells in EMT induction and support the view that activated CD4+ T cells can exert protumorigenic effects. Yet, only CD8+ T cells have been shown to induce EMT and cancer stemness in a breast cancer mouse model.Citation27 Besides T cells, macrophages and myofibroblasts, which represent two predominant stroma cell populations in CP and PDAC, are also potent EMT inducers in premalignant and malignant pancreatic ductal epithelial cells.Citation11,12 Based on the different distribution patterns of these cell populations in the stromal compartment of CP and PDAC,Citation6 one might speculate on a temporally different involvement of either population in the EMT process during tumorigenesis (). Thus, CD4+ T cells and macrophages, both being more abundant in CP than in PDAC tissues, might represent the predominant EMT inducers during tumor initiation (early stages) while myofibroblasts, being likewise present in CP but even more in PDAC tissues, are the main promoters of EMT at later stages.Citation6,11,12 As two potent factors mediating the EMT induction in H6c7 cells, we identified IL-6 and TNF-α that were both highly elevated in the supernatants of co-cultures with activated CD4+ T-effs. Neutralizing TNF-α most efficiently reversed the EMT-associated alterations in co-cultured H6c7 cells, an effect that was also observed but less pronounced by neutralizing IL-6. Former studies revealed that TNF-α can initiate EMT by upregulating and/or stabilizing Snail, Twist or by inducing TGF-β1 and subsequent ZEB2 activation.Citation28–30 Since TGF-β1 levels were not elevated during co-culture with activated T-effs, the latter mechanism seems not to be involved in this scenario. Interestingly, the presence of activated T-effs enhanced ZEB-1 expression in H6c7 and T3M4 cells that was abolished by TNF-α or IL-6 blockade, indicating that CD4+ T-effs promote EMT-associated alterations through TNF-α and IL-6 dependent induction of ZEB-1. Due to the fact that TNF-α RNA expression was higher in activated CD4+ T-effs than in H6c7 cells (data not shown) one can envision that T-eff released TNF-α leads to elevated expression and release of IL-6 (and to a lesser extent of TNF-α) in H6c7 cells promoting the acquisition of a mesenchymal and invasive phenotype. Moreover, the superior inhibitory effect of Etanercept on EMT-associated alterations is well in line with the much higher TNF-α level (524.7 pg/mL) in supernatants from co-cultures of activated T-effs and H6c7 cells than those of IL-6 (29.5 pg/mL). Similarly, in mammary epithelial cells undergoing TNF-α dependent EMT, E-cadherin downregulation was mediated by an NF-κB dependent increase of ZEB-1 promoter activity.Citation31 As mentioned above, macrophages which are likewise abundant in CP and PDAC tissues have to be considered as another important source of IL-6 and TNF-α.Citation11 Accordingly, we recently demonstrated that pro- and anti-inflammatory macrophages are potent inducers of EMT in premalignant as well as malignant pancreatic ductal epithelial cells,Citation11 a process which is apparently dependent on TNF-α (unpublished observations). Altogether, these findings strongly support a role of TNF-α and ZEB-1 in inducing EMT of epithelial cells in tumorigenesis. Although performed in an orthotopic PDAC xenograft mouse model lacking T cells, treatment of tumor bearing mice with the anti-TNF-α antibody infliximab potently reduced metastasis, pointing to the importance of TNF−α in the process of tumor cell dissemination.Citation32 Altogether these data underscore the role of TNF-α in the metastatic process of PDAC already commencing with EMT induction in pancreatic ductal epithelial cells of chronically inflamed pancreatic tissue. Elevated TNF-α levels in those tissues may either directly result from infiltrated inflammatory cells such as CD4+ T cells or macrophages, or from an enhanced secretion by the stromal and/or the epithelial/carcinoma cells upon their reciprocal interaction, as seen in our experimental setting.
Figure 10. Simplified overview on the mutual epithelia-stroma-interaction promoting EMT and malignancy during inflammation-associated PDAC development. (A) Under physiological conditions, pancreatic ducts are embedded in a homeostatic stromal microenvironment comprising fibroblasts, T cells, and macrophages and exhibit an epithelial and polarized phenotype. (B) Upon chronic inflammation, predominantly CD4+ T effs and macrophages enrich in the pancreatic tissue leading to increased level of inflammatory mediators such as TNF-α and IL-6. This may either directly result from the infiltrated inflammatory cells or from an enhanced secretion by stromal and/or the epithelial cells upon their reciprocal interaction. As a result, elevated TNF-α and IL-6 levels promote EMT in pancreatic ducts leading to loss of E-cadherin expression, upregulation of mesenchymal proteins such as vimentin and L1CAM. This probably paves the way for an early dissemination of epithelial cells.Citation21,22 Elevated epithelial L1CAM expression, in turn, impairs the proliferation of CD4+ T-effs and promotes infiltration of CD4+CD25+CD127-CD49d- T-regs into the pancreas as well as the generation of immunosuppressive CD4+CD25-CD69+ T cells. These various subsets of regulatory T cells contribute to the manifestation of an immunosuppressive microenvironment, e.g., through TGF-β1. Besides its immunoregulatory function, TGF-β1 can directly induce EMT in epithelial/carcinoma cells but may also indirectly contribute to EMT by promoting transdifferentiation of pancreatic stellate cells into myofibroblasts and modulating the phenotype of macrophages. (C) Manifestation of these mechanisms shapes the stromal compartment of PDAC diminishing the percentage of CD4+ T-effs while increasing the percentages of T-reg subsets as well as myofibroblasts. The predominance of these stromal cell types results in a further elevation of TGF-β1 which augments an immunosuppressive microenvironment and EMT induction in carcinoma cells ultimately supporting malignant progression of PDAC.
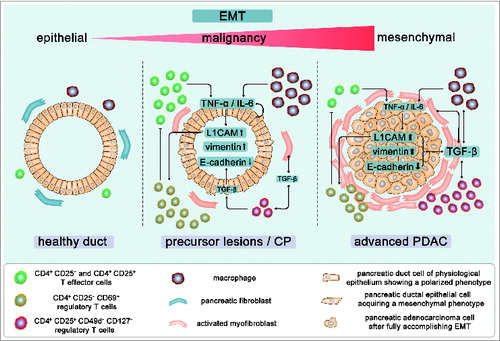
The mutual interaction of CD4+ T cells and pancreatic ductal epithelial cells in turn can also impact on the phenotype and function of the immune cells. Thus, we recently showed that increased L1CAM expression promotes migration/infiltration of CD4+CD25+CD127−CD49d− T-regs into the pancreas, impairs the proliferation of T-effs and fosters the generation of immunosuppressive CD4+CD25−CD69+ T cells.Citation25,33 Based on these findings, the following scenario emerges contributing to inflammation-associated pancreatic tumorigenesis: during pancreatic inflammation, infiltrating CD4+ T-effs promote EMT through TNFα and IL-6 in pancreatic ductal epithelial cells along with an increase of L1CAM expression. L1CAM in turn diminishes proliferation of T-effs and concomitantly promotes enrichment of various subsets of T-regs, the latter adding to an immunosuppressive microenvironment, e.g., through TGF-β1. TGF-β1 which is not only released by T-regs but also by tumor cells and other stromal cells contributes to PDAC initiation and progression by exerting pleiotropic effects. Besides its immunoregulatory function, TGF-β1 is a potent inducer of EMT and can promote this process in a Smad-dependent as well as -independent manner, as being shown for induction of L1CAM.Citation12 Additionally, TGF-β1 can indirectly foster EMT by promoting transdifferentiation of pancreatic stellate cells into myofibroblasts,Citation3 and by modulating the phenotype of macrophages.Citation34,35 As outlined before, T-regs enrich in PDAC tissues so that one can speculate about a role of T-regs in EMT induction by TGF-β1 release at later stages of PDAC development. The slight effects on EMT induction in pancreatic ductal epithelial cells after co-culture with T-regs might point to this scenario. However, keeping in mind that T-regs isolated by the untouched magnetic bead procedure as used in our study do not exhibit 100% purity, one cannot exclude the possibility that the observed effects are mediated by remaining T-effs.
Supporting our in vitro findings of the co-culture experiments, we could demonstrate in vivo that PanINs in CPs already start to loose E-cadherin expression while expression of vimentin and L1CAM, both being absent in normal pancreatic ducts (unpublished observations),Citation36 was upregulated. Albeit not being statistically significant, these alterations were predominantly noted in PanINs surrounded by a dense stroma containing high numbers of CD4+ T cells. Notably, expression of all three markers was not concomitantly altered in the same PanIN as mesenchymal proteins were already detectable despite maintained E-cadherin expression or vice versa.
Overall, by identifying EMT induction in premalignant and malignant pancreatic ductal epithelial cells as a novel property of CD4+ T cells, we provide evidence for an important role of these cells beyond their known immune function in chronic inflammation and in pancreatic tumorigenesis (see ). These data obtained with human cells and patient-derived material are further supported by findings from animal studies. Zhang et al. recently showed that ablation of CD4+ T cells in mice harboring a pancreas specific krasG12D mutation did not develop PanIN lesions despite being susceptible to caerulein-induced pancreatitis. The low-grade PanINs did not further progress due to massive apoptosis induction pointing to further protumorigenic functions of CD4+ T cells beyond EMT induction.Citation37,38
Together with recent findings on circulating pancreatic epithelial cells in blood from patients with pancreatic cystic lesions,Citation22 our study strongly supports the view that inflammatory cells – among them CD4+ T cells – confer pancreatic ductal epithelial cells the ability to disseminate and metastasize probably already prior to visible tumor formation.
Materials and Methods
H6c7 cells and cell culture
The human pancreatic ductal epithelial cell line H6c7 (gift by M.S. Tsao, Ontario Cancer Institute, Toronto, Canada) were kept in H6c7-medium (50% RPMI 1640 medium (Biochrom, F1215) and 50% KSF-medium (Life Technologies, 17005-059), 0.5% L-glutamine (Biochrom, K0283), 50 μg/mL bovine pituitary extract (Life Technologies, 13028-014), 5 ng/mL epidermal growth factor (Life Technologies, PHG0311) and 5% fetal calf serum (Biochrom, S0115). The human pancreatic ductal epithelial cell line T3M4 (Panc89) was kindly provided by H. Kalthoff (Institute of Experimental Cancer Research, Kiel, Germany) and kept in T3M4-medium which is composed of RPMI-1640 medium supplemented with 1% L-glutamine, 10% FCS and 1% sodium pyruvate (Biochrom, L0473).
Isolation of peripheral blood T-regs and T-effs
Human peripheral blood mononuclear cells (PBMCs) were obtained from healthy volunteers by Pancoll (Pan-Biotech, PO4-60500) density-gradient centrifugation of heparinized venous blood. Informed consent was obtained from all donors. To enrich untouched CD4+CD25+CD127−CD49d− T-regs and CD4+ CD25− T-effs, a negative selection procedure using magnetic cell separation (T-Cell Isolation Kit II Miltenyi Biotec, 130-091-155) was performed.Citation25 Purity of both T cell subsets was determined by immunofluorescence staining and subsequent flow cytometry.Citation25 CD4+CD25− T-effs used for co-culture experiments showed a purity of > 95% and CD4+CD25+CD127−CD49d− T-regs of > 75% (Miltenyi Biotec; CD127 Biotin 130-094-891; CD49-d Biotin 130-093-280).
Direct co-culture of H6c7 cells and T cells
The ratios of T cells and epithelial cells for co-cultures have been established in former studies, Citation25 so that 5 × 104 H6c7 or T3M4 cells were seeded in 125 μL H6c7-medium or T3M4-medium/well into 96-well-round bottom plates (Greiner, GR/650180). After 24 h, medium was removed and 1×104 freshly isolated T-regs or T-effs, diluted in 125 μL X-Vivo medium/well (Lonza, BE04-418F) were added, either in the absence or presence of 5 × 103 T cell activation/expansion beads (Miltenyi Biotec, 130-091-441) which had been prepared according to the manufacturer's instructions. For monoculture, H6c7- and T3M4-medium, respectively, was replaced by 125 μL X-Vivo medium without T cells but with or without T cell activation/expansion beads. After 72 h, T cells were separated from adherent H6c7 and T3M4 cells, respectively, by extensive washing with MACS-buffer before epithelial/carcinoma cells were detached with accutase (Millipore, SCR005) for further analysis. In some experiments, activity of IL-6 or TNF-α was neutralized. For this purpose, 10 μg/mL anti-IL-6R antibody Tocilizumab (Roche PZN: 07286896) or 10 μg/mL of the soluble TNF-α receptor Etanercept (Pfizer, PZN: 09221984) were added directly to the cells when co-cultures were started. As control, 10 μg/mL Rituximab (Roche PZN: 02338298), an antibody specific for CD20 which is expressed neither on pancreatic epithelial cells nor by T cells, were used.
Immunofluorescence staining and flow cytometry
Detection of EMT markers in H6c7 cells by immunofluorescence stainings and flow cytometry were conducted using the following specific primary mouse anti-human mAbs: mIgG1 anti-vimentin-Alexa488 (clone V9, Santa Cruz Biotechnology, sc-6260 AF488); mIgG1 anti-L1CAM (clone L1-9.3 kindly provided by Prof. Gerd Moldenhauer, German Cancer Research Center, Heidelberg, Germany) and mIgG1 anti-E-cadherin-AlexaFluor-647 (clone 67A4, BioLegend, 324112). Control stainings were performed with mIgG1-Alexa488 (Santa Cruz Biotechnology, sc-3890), unconjugated mIgG1 (BioLegend, 400108) and mIgG1-AlexaFluor-647 (BioLegend, 400130). Staining of the surface molecules E-cadherin and L1CAM was performed by incubation of cells with specific mAbs for 15 min at 4°C in the dark and at a concentration as recommended by the manufacturer or in case of L1CAM and its respective isotype control which were applied at a concentration of 1 μg/mL. Detection of the anti-L1CAM antibody was performed using a biotin conjugated rabbit–anti-mouse antibody (BioLegend, 315-066-003) and Streptavidin-PerCP/Cy5.5 (BioLegend, 405214). After the last washing step of extracellular staining, intracellular vimentin was detected. For this purpose, cells were treated with fixation/permeabilization solution (eBioscience, 00-5223-56) for 10 min at room temperature (RT) in the dark, then washed with permeabilization buffer (eBioscience, 00-8333-56) and followed by incubation with Alexa488-conjugated anti-vimentin mAb or its isotype control diluted in permeabilization buffer as recommended by the manufacturer for 10 min at RT and in the dark. After all staining steps, cells were washed with MACS-buffer (PBS + 0.5% BSA and 2 mM EDTA) and fixed with 1% formaldehyde (BÜFA Chemicals, 21110).
Immunocytochemical stainings in H6c7 cells
To detect EMT markers in mono- and co-cultured H6c7 cells grown in 96-well-round bottom plates (Greiner, GR/650180), medium was removed and 200 μL PBS/well were added to the plates. Then, T-effs and T-regs were removed by placing the plates 15 min on ice. After washing with PBS, cells were fixed with 4.5% paraformaldehyde for 15 min at RT. The following primary mouse monoclonal antibodies were used according to established protocols based on stainings with appropriate positive controls and isotype controls showing no or only faint background staining at the final concentrations: anti-E-cadherin (1:100 clone 32A8, Cell Signaling, 5296), anti-L1CAM (1:100 clone L1-9.3) and anti-vimentin (1:50 clone V9, Santa Cruz Biotechnology, sc-6260). Then, stainings were performed as described for immunohistochemistry (see below). Evaluation was done using an EvosxL Core microscope (AMG, Bothell, USA).
RNA isolation and RT-qPCR
Total RNA of mono- and co-cultured H6c7 and T3M4 cells was isolated with the total RNA kit peqGOLD (PeqLab, 12-6834-02) and subjected to reversed transcription according to the manufacturer's instructions (Fermentas, EP0442, RO0192, SO132, EO0384). All primers were purchased from Realtime Primers (via Biomol). The primer sequences were: E-cadherin: FW: 5′-TCTTCCCC-GCCCTGCCAATC-3′, RV: 5′-GCCTCTCTCGAGTCCCCTAG-3′; L1CAM: FW: 5′-TCACGGGCAACAACAGCAACT-3′, RV: 5′-CGGCTTCCTGTCAATCATGCT-3′; Slug: FW: 5′-ATATTCGGACCCACACATTACCT-3′, RV: 5′-GCAAATGCTCTGTT-GCAGTGA-3′; Snail: FW: 5′-CTGCTCCACAAGCACCAAGAGTC-3′, RV: 5′-CCAGC-TGCCCTCCCTCCAC-3′; TBP: FW: 5′-GCTG-GCCCATAGTGATCTTT-3′, RV: 5′-CTTCACACGCCAAGAAACAG-3′; vimentin: FW: 5′-TGGCACGTCTTGACCTTGAA-3′, RV: GGTCATCGTG-ATGCTGAGAA-3′; ZEB-1: FW: 5′- TCCATGCTTAAGAGCGCTAGCT-3′, RV: 5′- ACCGTAGTTGAG-TAGGTGTATGCCA-3′. The PCR was performed as duplicate analyses with a LightCycler 480 (Roche, 06991076702) for maximum 40 cycles. The amount of gene of interest mRNA was normalized to the mRNA amount of the control gene TBP.
Cell invasion assay
After 72 h mono- or co-culture, T cells were removed from the co-cultures before H6c7 and T3M4 cells, respectively, were detached by accutase. After washing in PBS, cell invasion of mono- and co-cultured H6c7 cells through a collagen-I matrix was analyzed in a modified boyden chamber as described.Citation12 Cell invasion of T3M4 cells was determined by the xCELLigence RTCA DP analyzer® allowing real time acquisition of impedance alterations which correlate with cell invasion. Impedance is expressed as relative units, the cell index (CI). Prior to assembly of the CIM-Plates 16, the underside of the upper compartment was coated with 30 μL of collagen-I (400 μg/mL) and allowed to dry. The assembled CIM-Plates 16 were equilibrated in medium containing 1% FCS for 1 h at which the background measurement was performed. Then 40,000 cells were loaded per well into CIM-Plates 16 and allowed to invade for 24 h in medium containing 1% FCS. Data acquisition was performed at 30 min intervals and analyzed with the RTCA software (version 1.2.1.1002, Roche). Besides showing the graph from one representative experiment, data from three independent experiments are shown as mean ± SD of the AUC. As maximum cell invasion was observed after 16 h, AUC was calculated at this time point representing the amount of invaded cells within this time period.
Determination of EMT-associated mediators in cell culture supernatants
Detection of human TGF-β1 in cell culture supernatants was performed using the TGF-β1 ELISA kit (MyBioSource via EMCA Bioscience, MBS029781) and IL-6, IL-8, IFNγ, IL-13, IL-12(p70), MCSF, GCSF, and TNF-α were determined using a Luminex Screening Assay (R&D Systems, A3C8CE39), each following the manufacturer's recommendations. Fluorometric measurement was done on a Bio-Plex 200 reader using the corresponding Bio-Plex manager software (kindly provided by the Department of Immunobiology, Research Center Borstel, Borstel, Germany.
CP tissues
Processing, conservation and diagnostic evaluation of CP tissues were carried out at the Institute of Pathology, University Tübingen. Selection of CP tissues was based on the presence of PanIN lesions. The research was approved by the ethics committee of the University Hospital Tübingen (470/210BO1).
Immunohistochemistry
Immunohistochemical stainings were performed with formalin fixed and paraffin embedded sections. CD4 (clone 4B12, Leica Novocastra, PA0427) and vimentin (clone Vim3B4, Dako, M7020) stainings were performed at the Institute of Pathology, UKSH Campus Kiel using an automated routine procedure. Stainings of L1CAM (clone L1-14.10, kindly provided by Prof. Gerd Moldenhauer) and FoxP3 (clone hFoxy, ebioscience, 14-5779-80) were performed as described.Citation13,26 For staining of E-cadherin, sections were deparaffinized and rehydrated as described.Citation13 Then, antigen retrieval was performed with 1:10 diluted citrate buffer at pH 6.0 for 20 min. After washing, sections were incubated with a mouse anti-human E-cadherin monoclonal antibody (mAb) (1:100; clone 32A8, Cell Signaling, 5296) diluted in 1 % BSA and 0.3 % Triton-X-100/PBS at 4°C overnight. After washing, sections were incubated with a biotin-conjugated secondary anti-mouse IgG antibody (1:200; Jackson ImmunoResearch via Dianova, 315-066-003) diluted in PBS + 0.3% Triton-X-100 for 30 min at RT. After washing, sections were incubated with peroxidase conjugated streptavidin (1:500; Jackson ImmunoResearch via Dianova, 016-030-084) diluted in PBS + 0.3% Triton-X-100 for 30 min. Substrate reaction was performed with AEC Substrate (Dako, K3464) for 10 min. After washing, cells were stained in Mayer`s Haemalaun (Merck, 109249) for 2 min. After washing in water for 10 min, sections were covered with Kaiser`s glycerine gelatine (Roth, 6474.1). Usage of control antibodies revealed no or only weak background staining.
Evaluation
Stained CP sections were evaluated twice in a blinded manner by scoring the extent of distribution (given as %-positivity of the whole section). In case of two discrepant results, sections were evaluated by a second investigator. All duct cell-related parameters are mainly based on the evaluation of PanINs. The stromal compartment was defined as the whole pancreatic sample area excluding acinar cells, epithelial cells, lymphatic tissue and blood vessels. Each parameter was evaluated using a 5-score system (1 = negative, 2 = <10% positive, 3 = 11–50% positive, 4 = 51–99% positive, 5 = 100% positive). The created groups were dichotomized by median and refitted into two groups of < median (score 1) and ≥ median (score 2). Evaluation of the sections was carried out using an Axioplan 2.0 microscope (Zeiss, Jena, Germany). Pictures were taken using a Keyence BZ9000 microscope (Keyence, Neu-Isenburg, Germany).
Statistical analysis
Relationships between data from immunohistochemical stainings of CP tissues were categorized and presented as absolute and relative frequencies which were compared between groups by Fisher's exact test. In vitro data are summarized as median values with quartiles and were analyzed by using Kruskal–Wallis one way analysis of variance or Mann–Whitney rank-sum test, as appropriate. All statistical tests were two-sided at a level of significance of 5%, without adjustment for multiple testing. Statistical analyses were conducted using SPSS Version 17.0 (IBM, Ehningen, Germany) and SigmaPlot Software 12.5 (Systat Software GmbH, Erkrath, Germany).
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Supplementary_files.zip
Download Zip (97.9 KB)Acknowledgments
The authors thank Maike Witt-Ramdohr and Dagmar Leisner for excellent technical assistance.
Funding
Funding is acknowledged by the Deutsche Forschungsgemeinschaft (DFG)-funded Cluster of Excellence “Inflammation at Interfaces,” and the DFG-funded Pancreas Cancer Consortium-Kiel (DW, HS, SS).
References
- Siegel R, Naishadham D, Jemal, A. Cancer statistics, 2013. CA Cancer J Clin 2013; 63:11-30; PMID:23335087; http://dx.doi.org/10.3322/caac.21166
- Schneider G, Siveke JT, Eckel F, Schmid RM. Pancreatic cancer: basic and clinical aspects. Gastroenterology 2005; 128:1606-25; PMID:15887154; http://dx.doi.org/10.1053/j.gastro.2005.04.001
- Kleeff J, Beckhove P, Esposito I, Herzig S, Huber PE, Löhr JM, Friess H. Pancreatic cancer microenvironment. Int J Cancer 2007; 121:699-705; PMID:17534898; http://dx.doi.org/10.1002/ijc.22871
- Ino Y, Yamazaki-Itoh R, Shimada K, Iwasaki M, Kosuge T, Kanai Y, Hiraoka N. Immune cell infiltration as an indicator of the immune microenvironment of pancreatic cancer. Br J Cancer 2013; 108:914-23; PMID:23385730; http://dx.doi.org/10.1038/bjc.2013.32
- Protti MP, De Monte L. Immune infiltrates as predictive markers of survival in pancreatic cancer patients. Front Physiol 2013; 4:210; PMID:23950747; http://dx.doi.org/10.3389/fphys.2013.00210
- Helm O, Mennrich R, Petrick D, Goebel L, Freitag-Wolf S, Röder C, Kalthoff H, Röcken C, Sipos B, Kabelitz D et al. Comparative characterization of stroma cells and ductal epithelium in chronic pancreatitis and pancreatic ductal adenocarcinoma. Plos One 2014; 9:e94357; PMID:24797069; http://dx.doi.org/10.1371/journal.pone.0094357
- Zou W. Immunosuppressive networks in the tumour environment and their therapeutic relevance. Nat Rev Cancer 2005; 5:263-74; PMID:15776005; http://dx.doi.org/10.1038/nrc1586
- Fogar P, Basso D, Fadi E, Greco E, Pantano G, Padoan A, Bozzato D, Facco M, Sanzari MC, Teolato S et al. Pancreatic cancer alters human CD4+ T lymphocyte function: a piece in the immune evasion puzzle. Pancreas 2011; 40:1131-37; PMID:21792088; http://dx.doi.org/10.1097/MPA.0b013e31822077b8
- Bonde AK, Tischler V, Kumar S, Soltermann A, Schwendener RA. Intratumoral macrophages contribute to epithelial-mesenchymal transition in solid tumors. BMC Cancer 2012; 12:35; PMID:22273460; http://dx.doi.org/10.1186/1471-2407-12-35
- Lin CY, Lin CJ, Chen KH, Wu JH, Huang SH, Wang SM. Macrophage activation increases the invasive properties of hepatoma cells by destabilization of the adherens junction. FEBS Lett 2006; 580:3042-50; PMID:16678166; http://dx.doi.org/10.1016/j.febslet.2006.04.049
- Helm O, Held-Feindt J, Grage-Griebenow E, Reiling N, Ungefroren U, Vogel I, Krüger U, Becker T, Ebsen M, Röcken C et al. Tumor associated macrophages exhibit pro- and anti-inflammatory properties by which they impact on pancreatic tumorigenesis. Int J Cancer 2014; 135:843-61; PMID:24458546; http://dx.doi.org/10.1002/ijc.28736
- Geismann C, Morscheck M, Koch D, Bergmann F, Ungefroren H, Arlt A, Tsao MS, Bachem MG, Altevogt P, Sipos B et al. Up-regulation of L1CAM in pancreatic duct cells is transforming growth factor beta1- and slug-dependent: role in malignant transformation of pancreatic cancer. Cancer Res 2009; 69:4517-26; PMID:19435915; http://dx.doi.org/10.1158/0008-5472.CAN-08-3493
- Sebens Muerkoster S, Werbing V, Sipos B, Debus MA, Witt M, Grossmann M, Leisner D, Kötteritzsch J, Kappes H, Klöppel G et al. Drug-induced expression of the cellular adhesion molecule L1CAM confers anti-apoptotic protection and chemoresistance in pancreatic ductal adenocarcinoma cells. Oncogene 2007; 26:2759-68; PMID:17086212; http://dx.doi.org/10.1038/sj.onc.1210076
- Schäfer H, Geismann C, Heneweer C, Egberts JH, Korniienko O, Kiefel H, Moldenhauer G, Bachem MG, Kalthoff H, Altevogt P et al. Myofibroblast-induced tumorigenicity of pancreatic ductal epithelial cells is L1CAM dependent. Carcinogenesis 2012; 33:84-93; PMID:22095073; http://dx.doi.org/10.1093/carcin/bgr262
- Ben Q-W, Wang J-C, Liu J, Zhu Y, Yuan F, Yao W-Y, Yuan Y-Z. Positive expression of L1-CAM is associated with perineural invasion and poor outcome in pancreatic ductal adenocarcinoma. Ann Surg Oncol 2010; 17:2213-21; PMID:20162456; http://dx.doi.org/10.1245/s10434-010-0955-x
- Kurahara H, Shinchi H, Mataki Y, Maemura K, Noma H, Kubo F, Sakoda M, Ueno S, Natsugoe S, Takao S. Significance of M2-polarized tumor-associated macrophage in pancreatic cancer. J Surg Res 2009; 167:e211-19; PMID:19765725; http://dx.doi.org/10.1016/j.jss.2009.05.026
- Kurahara H, Takao S, Maemura K, Mataki Y, Kuwahata T, Maeda K, Sakoda M, Iino S, Ishigami S. M2-polarized tumor-associated macrophage infiltration of regional lymph nodes is associated with nodal lymphangiogenesis and occult nodal involvement in pN0 pancreatic cancer. Pancreas 2013; 42:155-59; PMID:22699204; http://dx.doi.org/10.1097/MPA.0b013e318254f2d1
- Hiraoka N, Onozato K, Kosuge T, Hirohashi S. Prevalence of FOXP3+ regulatory T cells increases during the progression of pancreatic ductal adenocarcinoma and its premalignant lesions. Clin Cancer Res 2006; 12:5423-34; PMID:17000676; http://dx.doi.org/10.1158/1078-0432.CCR-06-0369
- Ikemoto T, Yamaguchi T, Morine Y, Imura S, Soejima Y, Fujii M, Maekawa Y, Yasutomo K, Shimada M. Clinical roles of increased populations of Foxp3+CD4+ T cells in peripheral blood from advanced pancreatic cancer patients. Pancreas 2006; 33:386-90; PMID:17079944; http://dx.doi.org/10.1097/01.mpa.0000240275.68279.13
- Thiery JP. Epithelial-mesenchymal transitions in tumour progression. Nat Rev Cancer 2002; 2:442-54; PMID:12189386; http://dx.doi.org/10.1038/nrc822
- Rhim AD, Mirek ET, Aiello NM, Maitra A, Bailey JM, McAllister F, Reichert M, Beatty GL, Rustgi AK, Vonderheide RH et al. EMT and dissemination precede pancreatic tumor formation. Cell 2012; 148:349-61; PMID:22265420; http://dx.doi.org/10.1016/j.cell.2011.11.025
- Rhim AD, Thege FI, Santana SM, Lannin TB, Saha TN, Tsai S, Maggs LR, Kochman ML, Ginsberg GG, Lieb JG et al. Detection of circulating pancreas epithelial cells in patients with pancreatic cystic lesions. Gastroenterology 2014; 146:647-51; PMID:24333829; http://dx.doi.org/10.1053/j.gastro.2013.12.007
- Liu N, Furukawa T, Kobari M, Tsao MS. Comparative phenotypic studies of duct epithelial cell lines derived from normal human pancreas and pancreatic carcinoma. Am J Pathol 1998; 153:263-69; PMID:9665487; http://dx.doi.org/10.1016/S0002-9440(10)65567-8
- Ouyang H, Mou L, Luk C, Liu N, Karaskova J, Squire J, Tsao MS. Immortal human pancreatic duct epithelial cell lines with near normal genotype and phenotype. Am J Pathol 2000; 157:1623-31; PMID:11073822; http://dx.doi.org/10.1016/S0002-9440(10)64800-6
- Grage-Griebenow E, Jerg E, Gorys A, Wicklein D, Wesch D, Freitag-Wolf S, Goebel L, Vogel I, Becker T, Ebsen M et al. L1CAM promotes enrichment of immunosuppressive T cells in human pancreatic cancer correlating with malignant progression. Mol Oncol 2014; 8:982-97; PMID:24746181; http://dx.doi.org/10.1016/j.molonc.2014.03.001
- Limame R, Wouters A, Pauwels B, Fransen E, Peeters M, Lardon F, De Wever O, Pauwels P. Comparative analysis of dynamic cell viability, migration and invasion assessments by novel real-time technology and classic endpoint assays. PLoS One 2012; 7:e46536; PMID:23094027; http://dx.doi.org/10.1371/journal.pone.0046536
- Santisteban M, Reiman JM, Asiedu MK, Behrens MD, Nassar A, Kalli KR, Haluska P, Ingle JN, Hartmann LC, Manjili MH et al. Immune-induced epithelial to mesenchymal transition in vivo generates breast cancer stem cells. Cancer Res 2009; 69:2887-95; PMID:19276366; http://dx.doi.org/10.1158/0008-5472.CAN-08-3343
- Wang H, Wang HS, Zhou BH, Li CL, Zhang F, Wang XF, Zhang G, Bu XZ, Cai SH, Du J. Epithelial-mesenchymal transition (EMT) induced by TNF-α requires AKT/GSK-3β-mediated stabilization of snail in colorectal cancer. PLoS One 2013; 8:e56664; PMID:23431386; http://dx.doi.org/10.1371/journal.pone.0056664
- Li CW, Xia W, Huo L, Lim SO, Wu Y, Hsu JL, Chao CH, Yamaguchi H, Yang NK, Ding Q et al. Epithelial-mesenchymal transition induced by TNF-α requires NF-κB-mediated transcriptional upregulation of Twist1. Cancer Res 2012; 72:1290-300; PMID:22253230; http://dx.doi.org/10.1158/0008-5472.CAN-11-3123
- Techasen A, Namwat N, Loilome W, Duangkumpha K, Puapairoj A, Saya H, Yongvanit P. Tumor necrosis factor-α modulates epithelial mesenchymal transition mediators ZEB2 and S100A4 to promote cholangiocarcinoma progression. J Hepatobiliary Pancreat Sci 2014 May 27; 21(9):703-11 [Epub ahead of print]; PMID:24867797; http://dx.doi.org/10.1002/jhbp.125
- Chua HL1, Bhat-Nakshatri P, Clare SE, Morimiya A, Badve S, Nakshatri H. NF-kappaB represses E-cadherin expression and enhances epithelial to mesenchymal transition of mammary epithelial cells: potential involvement of ZEB-1 and ZEB-2. Oncogene 2007; 26:711-24; PMID:16862183; http://dx.doi.org/10.1038/sj.onc.1209808
- Egberts JH, Cloosters V, Noack A, Schniewind B, Thon L, Klose S, Kettler B, von Forstner C, Kneitz C, Tepel J et al. Anti-tumor necrosis factor therapy inhibits pancreatic tumor growth and metastasis. Cancer Res 2008; 68:1443-50; PMID:18316608; http://dx.doi.org/10.1158/0008-5472.CAN-07-5704
- Grage-Griebenow E, Schäfer H, Sebens S. The fatal alliance of cancer and T cells: how pancreatic tumor cells gather immunosuppressive T cells. OncoImmunology 2014; 25:e29382; PMID:25114835; http://dx.doi.org/10.4161/onci.29382
- Condeelis J, Pollard JW. Macrophages: obligate partners for tumor cell migration, invasion, and metastasis. Cell 2006; 124:263-6; PMID:16439202; http://dx.doi.org/10.1016/j.cell.2006.01.007
- Rivera LB, Bergers G. Location, location, location: macrophage positioning within tumors determines pro- or antitumor activity. Cancer Cell 2013; 24:687-9; PMID:24332035; http://dx.doi.org/10.1016/j.ccr.2013.11.014
- Bergmann F, Wandschneider F, Sipos B, Moldenhauer G, Schniewind B, Welsch T, Schirrmacher P, Klöppel G, Altevogt P, Schäfer H et al. Elevated L1CAM expression in precursor lesions and primary and metastatic tissues of pancreatic ductal adenocarcinoma. Oncol Rep 2010; 24:909-15; PMID:20811670; http:dx.doi.org/10.1038/onc.2012.44.
- Zhang Y, Yan W, Mathew E, Bednar F, Wan S, Collins MA, Evans RA, Welling TH, Vonderheide RH, di Magliano MP. CD4+ T lymphocyte ablation prevents pancreatic carcinogenesis in mice. Cancer Immunol Res 2014; 2:423-35; PMID:24795355; http://dx.doi.org/10.1158/2326-6066.CIR-14-0016-T
- Zhang Y, McAllister F, di Magliano MP. Immune cells in pancreatic cancer. OncoImmunology 2014; 3:e29125; PMID:25083330; http://dx.doi.org/10.4161/onci.29125
