Abstract
Cells used in adoptive cell-transfer immunotherapies against cancer include dendritic cells (DCs), natural-killer cells, and CD8+ T-cells. These cells may have limited efficacy due to their lifespan, activity, and immunosuppressive effects of tumor cells. Therefore, increasing longevity and activity of these cells may boost their efficacy. Four cytokines that can extend immune effector-cell longevity are IL-2, IL-7, IL-21, and IL-15. This review will discuss current knowledge on effector-cell lifespans and the mechanisms by which IL-2, IL-7, IL-15, and IL-21 can extend effector-cell longevity. We will also discuss how lifespan and efficacy of these cells can be regulated to allow optimal clinical benefits.
Introduction
The use of immunotherapy as a novel treatment for various cancers has undergone extensive investigation over the past few decades and, more recently, has started to show real promise in clinical application with the introduction of approved immunotherapies. One of the main immunotherapeutic modalities is the adoptive transfer of immune effector cells that can directly or indirectly kill tumor cells. However, little research has been carried out to establish the length of time that these cells remain active. The longevity of these cells is an important factor for immunotherapies to have long lasting effects. A further consideration is that many cancer microenvironments are immunosuppressive and thus adoptively transferred immune effector cells must remain active and survive in this hostile microenvironment.
Immune cell longevity is generally controlled by three major factors: telomere length, regulation of apoptosis, and cell anergy. Telomeres are DNA repeats found at the end of chromosomes. During cell division, dicentric chromosomes break apart as the two centromeres are drawn to opposite poles of the mitotic spindle. The chromosomal ends, if exposed, are unstable and tend to fuse with other broken ends that they come into contact with. This leads to fusion-bridge-breakage cycles resulting in genomic instability. Thus, telomeres protect the chromosomal ends from undergoing this fusion and help maintain their stability. These telomere repeats shorten after each cell replication cycle and eventually deplete leaving the chromosome ends to become exposed. Consequently genome instability occurs, which leads to apoptosis. As a result, cells only have a limited number of times they can divide, the Hayflick Limit.Citation1,2
A wide range of molecules regulate apoptosis. Some are anti-apoptotic, such as heat shock proteins and certain members of the Bcl-2 family. Others are pro-apoptotic, for example p53 and other members of the Bcl-2 family.Citation3
Anergy refers to functional inactivation of T cells and is a result of cellular senescence. Consequently, the immune cells lose their effector function but remain as long-lived cells in a hyporesponsive state.Citation4 The molecular mechanism controlling T cell anergy is thought to be initiated by an imbalance in Ras/MAPK signaling due to suboptimal co-stimulation with CD28 as the T cell receptor (TCR) binds to antigen presented on major histocompatibility complex (MHC). This leads to a disparity in Ca2+ concentration across the TCR membrane resulting in the induction of anergy inducing genes.Citation5
Agents that increase cell longevity may function by affecting these mechanisms that control cell survival.
This review will consider the major cell types used in adoptive cell transfer immunotherapies, their natural lifespans and discuss the key agents that increase the longevity of these cells. The review will also consider the underlying mechanisms by which these agents act.
Cell Types used in Adoptive Therapy
Dendritic cells
DCs are cells of the innate immune system and can be divided into two major subsets: myeloid DCs originate from myeloid progenitors, whereas plasmacytoid DCs originate from lymphoid progenitors. The two subsets exhibit different toll-like receptors (TLRs) on their surface and preferentially secrete different factors. Myeloid DCs secrete mainly IL-12 and plasmacytoid DCs IFNα. DCs start as “immature” cells that circulate around peripheral tissue and when a foreign antigen is recognized by the TLR they mature.Citation6 Immature circulating DCs can survive up to 10–14 d in vivo, with the lifespan of myeloid DCs being relatively shorter than that of plasmacytoid DCs.Citation7,8 DC maturation is induced by a number of factors including TNF-α, CpG, Poly I:C, and LPS.Citation9 The mature DCs stimulate the adaptive immune system by presentation of foreign antigen in the context of MHC I or MHC II to naive CD8+ or CD4+ T cells respectively, causing them to become activated.Citation6 Following antigen presentation, DCs undergo apoptosis.Citation10 The lifespan of DCs decreases following maturation, and mature DCs have been shown to survive up to 3 d in vitro.Citation11
DCs play an integral role in linking the innate and adaptive immune systems, which makes them a suitable choice for cancer immunotherapy. DC based immunotherapy can involve ex vivo generation of DCs presenting tumor antigens to stimulate cytotoxic T cells. Alternatively, it can involve stimulation of antigen uptake by DCs in vivo in a pro-inflammatory environment using tumor cells or antigens linked to DC maturation stimuli.Citation6 One DC based vaccine that has been approved by the Food and Drug Administration (FDA) in the USA is Sipuleucel-T (commercially known as Provenge) for the treatment of metastatic castrate-resistant prostate cancer. This is an ex vivo generated DC vaccine that involves DCs being cultured with GM-CSF to induce their expansion and with the antigen Prostate Acid Phosphatase (PAP). Provenge is administered three times with 2-week intervals between each infusion. Phase III trials of the vaccine have shown a prolonged survival of approximately 4 mo in prostate cancer patients.Citation6,12 Phase III clinical trials for two other DC-based immunotherapy vaccines from ex vivo generation have also shown promise in the treatment of melanoma and prolonging the extent of remission following chemotherapy for follicular lymphoma.Citation13,14
A number of cancers, including multiple myeloma, bladder, prostate, kidney, and breast cancer and have been shown to decrease the antigen-presenting capacity of DCs, rendering adoptive DC-based immunotherapies less effective.Citation15-17 Various factors secreted by tumor cells have been shown to be responsible for this by reducing DC differentiation and maturation. These include: TGF-β, IL-6, IL-10, and VEGF, as well as tumor antigens such as prostate-specific antigen (PSA), which exhibit similar effects.Citation15,18-21 Furthermore, prostate tumor cells have been demonstrated to induce DC apoptosis through promotion of Bcl-2 family proteins.Citation22 Therefore, increasing DC longevity has the potential to be beneficial in DC-based immunotherapeutic treatments for these cancers. Although it is known how long mature DCs survive in mice (upto 3 d), it is currently unknown how long antigen primed mature DCs can survive after adoptive transfer.
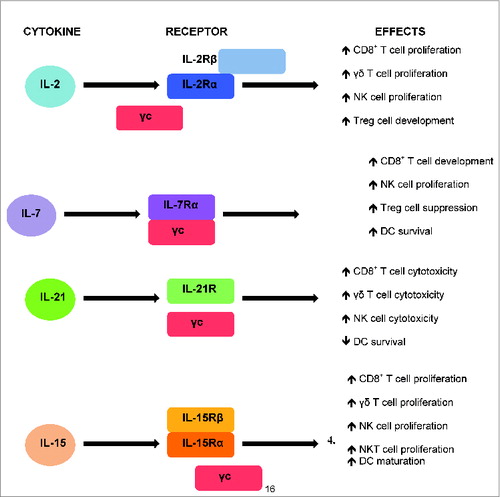
Table 1. Summary table of the currently known mechanisms by which the four cytokines act on immune effector cells
Natural killer cells
Natural Killer (NK) cells are part of the innate immune system and have a short life span. In healthy young adults they have a half-life (t1/2) of less than 10 d, with proliferation rates falling in old age.Citation23 NK cells exhibit an array of receptors, including NKG2D, NKp46, NKp30, NKp44, and DNAM1. Without the need for MHC these receptors recognize “stressed” cells, such as tumor cells, and are cytotoxic toward them.Citation24,25 They do this by recognizing ligands that are expressed on tumor cells.Citation26 In addition, NK cells secrete a number of cytokines such as IFNγ, TNF-α, and GM-CSF. These are involved in the activation of innate and adaptive immune system cells, which further attack cancer cells.Citation27
Some tumor cells have means to evade these mechanisms, largely by reducing their longevity and exerting anergic effects. NK cell abnormalities that have been observed in cancer patients include a decrease in cytotoxicity, defective expression of activating receptors or intracellular signaling molecules, overexpression of inhibitory receptors, defective proliferation, decreased numbers in peripheral blood and in tumor infiltrate, and defective cytokine production.Citation28,29 Factors secreted by tumor cells that exert anergic effects include IL-10, IL-6, IL-1β, PGE2, GM-CSF, and IL-8.Citation30,31 A major factor that induces NK cell apoptosis is TNF-α.Citation30,32 Another mechanism of NK cell immunosuppression seen in prostate cancer is the shedding of soluble ligands, for the killer activatory receptor -NKG2D, such as MICA, which then attract the NKG2D receptors on NK cells and act as a decoy away from the tumor cell, although this does not directly affect the longevity of NK cells.Citation25 In addition to these tumor cell ligands, other ligands are also expressed on tumor cells which act on groups of receptors on NK cells known as inhibitory killer-Ig-Like receptors (inhibitory KIRs).Citation33 A similar family of receptors – the leukocyte – Ig-like receptors (LILRs) are also found on a number of types of effector immune cells.Citation33,34
Adoptive NK cell immunotherapy involves administration of either allogeneic or in vitro generated autologous tumor-specific NK cells, which are then administered to the patient. Early adoptive therapies using NK cells in the 1980s–1990s involved the generation of autologous lymphokine activated killer cells (LAK) cells – these are lymphocytes isolated from patients and stimulated with high doses (1000 I.U. per mL) of IL-2. LAK cells were shown to lyse fresh autologous tumor cells that are resistant to NK cell cytotoxicity.Citation35 However, by the end of the 1990s, data from phase II and III trials of metastatic patients had concluded that the clinical response rate (15–20%) seen with LAK therapy was not superior to that of IL-2 or IL-2 and INF-gamma therapies. The patients also required high doses of systemically administered IL-2 to maintain the survival of the LAK cells and thus toxicity was commonly observed, which gradually resulted in the cessation of these types of therapy.
Since these early trials, various NK cell therapy trials have continued using either autologous or allogeneic NK cells, for a variety of cancers with a variable and often limited benefit (reviewed in Cheng et al, 2013Citation28). One trial with eight patients including seven with metastatic melanoma and one patient with RCC showed that autologous adoptively transferred NK cells could be expanded within the patients but had no anti-tumor activity.Citation36 This was possibly due to the activation agent used in this trial (IL-2) which can upregulate regulatory T cells (Tregs) which can then inhibit NK activity in vivo. Phase II trials are currently ongoing with the results from one completed phase II trial presently published. Findings from the trial showed a lack of NK cell expansion and restricted anti-tumor effects in patients with ovarian and breast cancer.Citation37 This limited response may be due to the immunosuppressive effects of cancer cells on NK cells, as listed above.Citation28,38
Recently, research has also shown that some NK cells can become NK memory cells, which have a longer life span. These cells are capable of antigen-specific recall, a feature that had previously been regarded as unique to the adaptive immune system. NK memory cells, like other memory cells, maintain their existence through self-renewal. They can be induced by cytokines, however, the underlying mechanism behind this is currently not understood.Citation39
Gamma delta T cells
Gamma Delta (γδ) T cells have TCRs that are composed of γ and δ chains, as opposed to α and β chains which are found on most other T cells.Citation40 In mice, most γδ T cells were found to survive up to 4 weeks in vivo.Citation41 Furthermore, γδ T cells can also exhibit a memory phenotype (CD45RO+) and are therefore capable of long-term survival over years.Citation42 This differentiation of γδ T cells into memory cells is induced by IL-21.Citation43 The majority of γδ T cells express Vγ9Vδ2 TCRs. They can recognize their targets, such as tumor cells, independent of MHC-mediated presentation and are cytotoxic toward them. Additionally, γδ T cells secrete interferon-γ, a cytokine involved in antitumor immune responses.Citation44 A numbers of studies, both in vitro and in vivo have demonstrated the effects of Vγ9Vδ2 T cells against a number of tumors including many different carcinomas, melanoma, myeloma, lymphoma, and neuroblastoma.Citation44,45
Vγ9Vδ2 T cells express the NK cell receptor NKG2D, which is involved in tumor recognition. Therefore, shedding of NKG2D ligands, as is seen in prostate cancer, may have a similar effect on γδ T cells as seen in NK cells, deterring the immune effector cells away from the tumor cells.Citation46 Whether tumor cells have further effects on reduction of γδ T cell longevity is yet to be confirmed.
Phase I/II clinical trials involving adoptive cell transfer of ex vivo expanded γδ T cells have been carried out for lymphoid malignancies, prostate cancer, melanoma, breast cancer, and colon cancer. These trials have all involved administration of γδ T cells in combination with either zoledronate or IL-2. Results from the trials have shown the therapies to be generally well tolerated and demonstrate potential antitumor activity.Citation45
CD4+ and CD8+ T cells
CD4+ and CD8+ T cells form a major component of the adaptive immune system. Through tumor antigens presented by antigen presenting cells (APCs), they can be primed to proliferate and secrete cytokines that also aid the activation of other cell types such as NK cells, neutrophils or APCs. Adoptive transfer strategies have manly focused on CD8+ T-cells that are directly responsible for tumor cell killing, although CD4+ T cells have also been shown to have class II restricted and non-restricted cytotoxic activities against tumor cells, particularly where recipients are initially rendered lymphopenic. CD8+ T cells, also known as cytotoxic T cells, recognize their targets by binding to antigen associated with MHC class I on the surface of APCs, such as DCs. Naïve CD8+ T cells are quiescent, though their exact lifespan is undetermined, and enter the cell cycle following this antigen interaction.Citation47,48 These activated CD8+ T cells induce cytolysis of the target cells and secrete cytokines such as TNF-α and IFNγ.Citation49 Following activation, most effector CD8+ T cells undergo apoptosis after approximately 2 weeks, with a small proportion of cells surviving to become CD8+ memory T cells capable of survival over years, largely mediated by the presence of the forkhead transcription factor, FOXO1.Citation50,51 There is however, evidence that adoptive transfer of antigen primed naïve T cells yields better efficacies in reduction of tumor volume than transfer with central memory T cells, due to naïve cells having a greater potential for clonal expansion than the memory cells.Citation52
Tumor cells have been shown to secrete TGF-β, which can induce CD8+ effector T cells to express FOXP3. This is a transcription factor that stimulates the CD8+ effector T cells to differentiate into Tregs cells. Treg cells suppress other effector CD8+ T cells resulting in a decrease in their response to tumor cells.Citation53 Multiple mechanisms are involved in this suppression, notably through induction of apoptosis and secretion of the immunosuppressive cytokines IL-10, TGF-β, and IL-35.Citation54 Furthermore, tumor cells can further suppress CD8+ T cells by directly secreting these cytokines that induce cell anergy.Citation55
CD4+, CD8+ T cells and whole T cell populations have been adoptively transferred into patients for cancer immunotherapy. Four main types of T-cells have been used (reviewed inCitation56):
Autologous TILs (Tumor infiltrating lymphocytes) – The majority of trials with these cells have been on patients with metastatic melanoma. Tils are isolated from fresh tumor biopsy specimens, expanded with IL-2 and then infused intravenously into patients. Prior chemotherapy to induce lymphodepletion is important for efficacy of these transferred cells as seen in object clinical responses, and also their longevity.Citation57 After cell infusion, patients are typically given IL-2 (either high or low dose) to maintain the activity of the TILs.
Autologous whole T cells or CD8+ T cells taken from the periphery and selected for their tumor antigen specificity or stimulated with tumor antigens presented by APCs – This approach involves the expansion of tumor antigen specific CD4+ or CD8+ T cells by using APCs loaded with one or more tumor antigens. The tumor specific T cells are then infused into the patients together with IL-2. Although the cells produced are more specific to the cancer in question, the technique has not given rise to response rates above 10%, possibly due to the short longevity of the antigen specific T cells.
T cells transduced with receptors specific for selected tumor antigens. This technique involves taking peripheral PBMCs and then transducing the T cells with TCRs specific for selected tumor antigens and then expanding these cells. The technique has been used for a number of cancers including melanoma, head and neck cancer, and colorectal cancer using tumor antigens specific to these cancers e.g., MART1 in melanoma and CEA in colorectal cancer. This approach has led to good clinical response rates (above 30%) with certain antigens such as MART1 and NY-ESO-1 used in melanoma patients, however, the limitation of the cells in their recognition of specific antigens has led to only selected patients benefiting from this approach.Citation58
Chimeric antigen receptors (CARs)Citation59 – These have been produced by expansion of CD4+ or CD8+ T cells that are then transduced with a receptor complex consisting of a (scFv) extracellular domain of a tumor specific antibody, linked through hinge and transmembrane domains of either CD4+ or CD8+ to a cytoplasmic signaling region. The first generation of these CARs used the CD3 receptor ζ chain alone, but more recently, second and third generation CARs have been engineered with one or two additional intracellular costimulatory signaling domains (e.g, CD28, OX40, or 41BB). The additional domains have allowed a greater cytotoxic activity of these cells combined with a greater lifespan. Also CD4+ T cells can be given the ability to lyse tumor cells in an MHC class II independent manner when transfected with a CAR complexCitation60 Gamma delta cells have also been transduced with CARs directed to CD19 to allow significant expansion in response to CD19 thus removing the limitation that only one gamma delta subset is currently expandable (with aminobisphosphonates such as zolendrenic acid.).Citation61
The best clinical outcomes have so far come from autologous TILs with partial or complete clinical response rates (CRRs) of over 50% in melanomaCitation56 and more recently, using CARs with a CD19 directed antibody region to treat B cell leukaemias, where CRRs over 80% have been observed.Citation62 On the basis of these outcomes – a CAR therapy, CTL0019 – a CAR with CD19 joined to intracellular regions of CD3 ζ and 41BB has been granted FDA approval as a breakthrough therapy. However, so far, treatments with adoptively transferred T cells have been mainly restricted to “immunogenic” cancers where T cells can be directed against tumor associated antigens. The lifespan of TILs and genetically engineered T cells has been documented in a few studies in patients – both TILs and CAR-T cells can persist for upto a year.Citation63,64 The persistence of TILs has been shown to be related to the length of their telomeres.Citation65
Agents that can Increase Immune Cell Longevity
IL-2
IL-2 is a cytokine normally secreted by activated T cells and to a lesser extent by activated DCs (Fig. 1). It binds to the IL-2 receptor (IL-2R), which is made up of 3 subunits: an α chain (IL-2Rα, also known as CD25), a β chain (IL-2Rβ, also known as CD122), and the common gamma chain (γc also known as CD132). γc is shared with a number of other cytokines, namely IL-4, IL-7, IL-9, IL-15, and IL-21.Citation66 IL-2 can boost the expansion of activated CD8+ T cells following acute viral infection. This expansion has been shown to be approximately three times less in IL-2 knockout mice, with similar results seen in IL-2R deficient CD8+ T cells.Citation67,68 Exposure of naive CD8+ T cells to high concentrations of IL-2 has also been demonstrated to result in a large proliferation of these cells, both in vitro and in vivo.Citation69 IL-2 similarly stimulates the expansion of NK and γδ T cells.Citation70,71
These properties of IL-2 made it one of the first cytokines used to boost immune cells and thus enhance adoptive cell therapy. Exogenous administration of IL-2 has been found to increase persistence of adoptively transferred CD8+ and γδ T cells.Citation72,73 However, IL-2 is not without its problems and despite the action of IL-2 to stimulate proliferation of CD8+ T cells, it may also have negative effects by increasing the number of Treg cells.Citation74
IL-2 is approved by the FDA in the USA for the treatment of metastatic melanoma and renal cell carcinoma, and by the NHS in the UK for renal cell carcinoma (commercially known as Proleukin). However, this is not a mainstay treatment due to limited efficacy, expense and associated toxicity.Citation75,76 These factors are all due to the pharmacokinetic properties of IL-2. In studies in renal metastatic patients, the half-life was 2.8–5.1 h and the tmax was 4 h. Therefore patients need to be dosed daily. Typical doses of IL-2 needed for durable clinical response rates (i.e., partial or complete regression) in renal carcinoma or melanoma are 700,000 international units (I.U)/kg (delivered every 8 h intravenously (0.04 mg/kg).Citation77 These doses are known to cause significant expansion of Treg cells in patients.Citation78
IL-7
IL-7 is a cytokine important in early T cell development and in their homeostasis. It is normally produced by thymic and bone marrow stroma. IL-7 signals through the IL-7 receptor (IL-7R), a heterodimer comprised of IL-7Rα (also known as CD127) and γc. Administration of the cytokine can boost T cell proliferation and IL-7 knockout mice have arrested development of T cells.Citation79,80 Therapy with IL-7 has been shown to increase the numbers of peripheral T cells, predominantly through an increase in homeostatic peripheral expansion in both mice and non-human primate models.Citation81–83 Furthermore, IL-7 has been found to increase the survival of CD8+ memory T cells as well as effector cells, a trait not seen with IL-2.Citation84 A further advantage of IL-7 over the use of IL-2 is that IL-7 is able to down regulate Treg activity, a factor involved in suppression of cytotoxic T cell activity.Citation85
Considering the use of IL-7 in cancer immunotherapy, administration of the cytokine in tumor-bearing mice was shown to prolong their survival, and this correlated with an increased in activated DC numbers, T cell numbers in lymphoid tissues and activated effector T cells in the tumor microenvironment.Citation86 Two phase I human trials, the first in patients with metastatic cancer and the second in patients with different types of refractory cancers, showed the cytokine to be well tolerated and lead to a dose-dependent proliferation of CD8+ T cells.Citation87,88 Doses of the cytokine required for T cell proliferation are from 10 to 60 μg/kg In addition, adoptively transferred CD8+ T cells have a greater antitumor efficacy when combined with IL-7 due to the decrease in Treg cells, and activated CD8+ T cells that express IL-7R are associated with greater memory CD8+ T cell development.Citation89,90 In humans, expansion in CD8+ T cells has been found to start 4 d after administration of IL-7 and this increase persists for approximately 14 d, despite the half-life of the cytokine being 7–23 h and decreasing to baseline after 72 h.Citation91 IL-7 is also capable of increasing the survival of NK cells with increased numbers of NK cells seen after 7 d incubation with the cytokine.Citation92 The lower concentrations of IL-7 needed for clinical efficacy, together with the longer half-life, and the lack of effect on Treg cell expansion makes IL-7 a promising cytokine to use in future clinical trials.
IL-21
IL-21 is a cytokine that is secreted by activated CD4+ T cells and NKT cells.Citation93 The IL-21 receptor (IL-21R) is composed of an IL-21R subunit and γc. IL-21R is most closely related to IL-2Rβ and IL-4Rα.Citation94 CD8+ T cell development is not affected in IL-21 knockout mice, suggesting the cytokine is not important in their development.Citation95 However, administration of IL-21 has been shown to enhance proliferation of these cells when in combination with either IL-7 or IL-15.Citation96 IL-21 can also increase to cytotoxicity of CD8+ T cells, as well as γδ T cells.Citation97,98 Similarly, IL-21 knockout mice do not show halted development of NK cells thus suggesting IL-21 is not important for their development.Citation95 Nevertheless, IL-21 has been demonstrated to enhance the growth of immature NK cells at low doses, an effect that, interestingly, reverses to inhibition of proliferation at high doses of IL-21.Citation99 Considering mature NK cells, IL-21 has been shown to decrease their proliferation but increase their cytotoxicity and IFNγ production resulting in greater efficacy.Citation100-102
Due to this increase in CD8+ T and NK cell cytotoxicity, a number of phase I and phase II clinical trials to study IL-21 in the treatment of advanced-stage melanoma or renal cell carcinoma patients have taken place (reviewed inCitation103). From these studies, the half-life of the protein ranges from 1.1 to 4.2 h with a maximal tolerated dose of 30 μg/kg. Many of these trials have shown complete responses in a very small number of patients (typically upto 5%) and partial responses in up to 25% of the patients. Results from these trials have also shown the cytokine to be well tolerated with an increase in NK and CD8+ T cell function through increased IFNγ and perforin production.Citation104,105
Despite these effects on CD8+ T and NK cells, IL-21 has been shown to inhibit DC activation and maturation and induces apoptosis of these cells via STAT3 signaling.Citation107,108 Thus IL-21 is detrimental toward DC function.
IL-15
IL-15 is a cytokine that stimulates the proliferation of activated T cells and is involved in the maturation and survival of NK cells.Citation109 IL-15 knockout mice have been shown to have decreased numbers of total CD8+ T cells, memory phenotype CD8+ T cells, NK cells, and NKT cells.Citation110 In addition, studies have shown that IL-15 expands populations of NK, NKT and CD8+ T cells and induces maturation of DCs with enhanced IFNγ secretion.Citation111-114 CD8+ T cells incubated with IL-15 were shown to retain their effector phenotype for up to 60 d in incubation with IL-15.Citation113 IL-15 does not affect Tregs and promotes the long-term maintenance of CD8+ memory T cells.Citation115,116
IL-15 acts by binding to a receptor composed of a β subunit (IL-2R/15Rβ) that is shared with the IL-2 receptor, the γc, and a distinct α subunit (IL-15Rα) giving the receptor specificity to the IL-15 cytokine.Citation109
Two preclinical studies involving murine models have shown that administration of IL-15 is effective against colon carcinoma with both studies observing prolonged survival of mice with the cancer.Citation117,118 A study investigating the effects of IL-15 in rhesus macaques showed the cytokine to be generally well tolerated.Citation115 The development of GMP grade IL-15 in recent yearsCitation119 has enabled the initiation of Phase I clinical trials on patients with metastatic melanoma and metastatic renal cell carcinoma – although results for these are yet to be reported.Citation109 The pharmacokinetic properties of IL-15 as reported in macaques is similar to that of IL-2 and IL-21 with a half-life of 1 h – although MTDs have not yet been established, doses of 50 μg/kg were used in the animals without life limiting toxicities.
In a study where the cytokines IL-15, IL-2, IFN-γ, IL-12, and IL-21 were individually incubated in co-culture with non-adherent peripheral blood mononuclear cells (PBMCs) containing NK and CD8+ immune effector cell populations and prostate cancer cell lines, results showed IL-15 to be the only agent that caused significant expansion of the PBMCs and killing of tumor cells. This suggests that IL-15 can activate the antitumor immune response in the presence of prostate cancer cells whereas IL-2, IFNγ, IL-12, and IL-21 cannot, despite having potent activity on selected immune effector cell populations in the absence of tumor cells. Therefore, IL-15 may be of particular importance for tumor immunotherapy, playing a protective role against the development of tumors.Citation120
Underlying Mechanisms that Increase Immune Cell Longevity
Telomerase activation
IL-2, IL-7, IL-21, and IL-15 have all been shown to upregulate telomerase activity in various cell types, thereby preventing telomere loss and allowing cells to divide more times than they would normally do so (Table 1). IL-2 has been demonstrated to increase telomerase activity in NK cells indicated by an increase in levels of human telomerase reverse transcriptase (hTERT) mRNA, a catalytic component of telomerase.Citation121 IL-21 has also been demonstrated to increase NK cell longevity by maintaining telomere length, suggesting telomerase activity is taking place.Citation122 Considering CD8+ T cells, IL-15 has been shown to increase CD8+ memory T cell longevity by inducing telomerase activity through JAK3 and PI3K signaling pathways.Citation123,124 A further study, looking at both IL-7 and IL-15 showed an upregulation of naïve CD8+ T cells when incubated with these cytokines, with a higher response seen with IL-15.Citation125
Stimulation of telomerase activity in immune cells makes these cytokines potentially harmful to administer in cancers such as leukemia, where there is already uncontrolled telomerase activity occurring in T cells. This has been shown with IL-2, which promotes tumor growth in chronic adult T cell leukemia (ATL) cells. The signaling pathways involved were found to be the JAK/STAT pathway and the JAK/PI3K/AKT/HSP90/mTORC1 pathway in these ATL cells.Citation126
Due to the similarity between morphology of these cytokines and their effects on telomerase activity, one can postulate that they are acting via similar mechanisms. As mentioned, the JAK/STAT pathway has been identified as playing an important role. This pathway occurs within the cells and is activated by the interaction of the cytokine with its corresponding receptor. This interaction results in autophosphorylation of Janus Kinase (JAK), which subsequently phosphorylates Signal Transducer and Activator of Transcription (STAT). The phosphorylated STAT then dimerizes and translocates into the nucleus where it binds to DNA and promotes gene transcription. JAK1 and 3, and STAT3 and 5, have been found to be the predominant activated subtypes by all four cytokines. However, IL-21 is known to signal primarily through the STAT3 component of the JAK/STAT pathway with less STAT5 involvement, whereas IL-15 signals primarily through STAT5. Both STAT3 and STAT5 are directly involved in telomerase transcription.Citation126,127
Despite these predictions from the involvement of shared signaling pathways, further research is required to clarify the effects of these cytokines on telomerase in both CD8+ and NK cells. Currently, only IL-15 and IL-7 have been shown to enhance telomerase activity in CD8+ cells with no published research describing their effects on telomerase activity in NK cells. Conversely, and IL-2 and IL-21 have been shown to stimulate telomerase activity in NK cells with no studies reporting their effects on telomerase in CD8+ T cells. Furthermore, whether there is upregulation of telomerase activity in DCs or γδ T cells is still unknown. Another important consideration is whether these cytokines affect telomerase in different cancer cells types as well as the immune system cells. As discussed, IL-2 increases telomerase in leukemia cells, promoting tumor growth.Citation126 However, IL-15 only stimulates telomerase in immune effector cells in the presence of prostate cancer cells without any effect on prostate cancer cell proliferation.Citation120 Further research is required to determine effects of the different cytokines on different cancers.
Another possibility to enhance immune effector cell longevity through telomerase activation would be via transfection with telomerase genes in order to potentially immortalize adoptively transferred cells. Telomerase gene transfection has been carried out in DCs with hTERT transfection by both plasmid transfection and adenoviral gene transfer. This produced immortalized DCs that were shown to induce a cytotoxic T cell response both in vitro and in vivo.Citation128,129
Inhibition of apoptosis
The major pathway targeted by these cytokines affecting apoptosis is through the anti-apoptotic Bcl-2 protein. Studies have shown IL-2, IL-7, and IL-15 all to decrease apoptosis and enhance survival of both NK and CD8+ T cells by upregulation of Bcl-2.Citation88,130–134 Another mechanism by which IL-7 has been found to decrease CD8+ T cell apoptosis is through diminishing programmed cell death protein 1 (PD-1) expression in CD8+ T cell populations in vivo.Citation135 A further study in mice has shown IL-15 to block sepsis-induced apoptosis of DCs, as well as NK and CD8+ T cells.Citation136
Despite the effect of IL-21 to increase CD8+ T cell cytotoxicity, the cytokine has been found to stimulate apoptosis of these cells through downregulation of Bcl-2. This pro-apoptotic effect on CD8+ T cells is a unique feature to the cytokines discussed.Citation137 The induction of apoptosis in DCs by IL-21 is induced via STAT3 and Bim in vivo. Interestingly, this effect was repressed by granulocyte-macrophage colony-stimulating factor (GM-CSF) due to competition between GM-CSF-induced STAT5 and IL-21-induced STAT3.Citation108
Reversal of anergy
In addition to telomerase activation and inhibition of apoptosis in the immune effector cells, the third mechanism to increase immune cell longevity is through reversal of anergy. This has been shown to be the case in T cells (CD3+) following stimulation with IL-2 in vitro, which involved JAK3 and mTOR signaling.Citation138 Furthermore, downregulation of IL-2 production in mice by injecting PD-1 resulted in induction of CD8+ T cell anergy.Citation139 Additional effects of the cytokines discussed on immune effector cell anergy have not yet been published and this mechanism of inducing increased cell longevity requires further clarification.
Translation into Clinical Practice
Adoptive cell transfer as a cancer immunotherapy is still in its infancy in relation to routine use in clinical practice. However, further research and clinical trials show real promise for effective and targeted anticancer therapies. Selected treatments have already gained approval in clinical practice, for example, the FDA approved use of Provenge™ for prostate cancer in the USA. A number of trials combining Provenge with other immunotherapeutic or conventional chemotherapeutic/radiotherapeutic modalities are currently taking place for example combinations of Provenge with either ipilimumab or IL-7 (clinicaltrials.gov).
More recently, as the functions of cytokines are becoming better understood, and are being used as adjuvants for adoptive cell therapy trials to improve their efficacy and diminish the negative effects seen such as immunosuppression. Understanding which cytokine, or combination has the greatest effect on boosting immune cell longevity will ultimately lead to anticancer therapy with high-efficacy and minimal side effects.
It is also important to consider the mechanism of action behind these cytokines, as this too has important clinical applications. Importantly, knowing that these cytokines boost telomerase activity suggests that any potential use of these cytokines should not be in combination with telomerase inhibitors which are another class of drug gaining popularity as anticancer drugs. Furthermore, an understanding of underlying pathways paves the way to even more direct targeting of immune effector cells, for example transfection with telomerase to potentially immortalize adoptively transferred cells, together with the introduction of cytokine cassettes with one or more of the above mentioned cytokines.
However, a key translational step will be to control the activity of the cells that have an increase in lifespan so that toxic side effects, such as cytokine storms and autoimmunity can be minimized. In order to do this, one must also consider the artificial engineering of cells described here, that can be regulated by transfection with suicide genes such as those for herpes simplex virus thymidine kinase (TK)Citation140 or CD20Citation141 or the use of transfected extracellular FK506 binding domains with Caspase-9 signaling motifs to cause cell death upon treatment with FK506.Citation142
Concluding Remarks
A major disadvantage of current adoptive cell transfer cancer immunotherapies is their unpredictable lifespan and the ability of cancer cells to evade attack from cells of the immune system. Increasing cell proliferation with non-cell based strategies may help overwhelm the cancer and therefore decrease this immune suppression making the cell-based immunotherapy more effective. There are four major agents that have been shown to have these effects: IL-2, IL-7, IL-15, and IL-21. These four cytokines affect the immune cells with similar mechanisms: first through stimulation of telomerase activity, and second through inhibition of apoptosis, resulting in an increase in cell longevity. Reversal of anergy may also be another mechanism through which these cytokines act. Further research is required in order to validate these effects and the effects of these cytokines on γδ T cells need further clarification. Beyond this, it will be important to determine exactly how much these cytokines are increasing cell longevity and how long the effects last for. The consequences of prolonged longevity of these cells should also be considered. If activity and excessive cytokine production from these cells occurs, there must be checkpoints in place to attenuate this to prevent toxicity to the patient. This will help to establish which agent, or combination of agents, is the most effective in boosting immune cells without affecting cancer cells.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- Xu L, Li S, Stohr BA. The role of telomere biology in cancer. Ann Rev Pathol 2013; 8:49-78; PMID:22934675; http://dx.doi.org/10.1146/annurev-pathol-020712-164030
- Harley CB. Telomere loss: mitotic clock or genetic time bomb? Mutat Res 1991; 256:271-82; PMID:1722017; http://dx.doi.org/10.1016/0921-8734(91)90018-7
- Bree RT, Stenson-Cox C, Grealy M, Byrnes L, Gorman AM, Samali A. Cellular longevity: role of apoptosis and replicative senescence. Biogerontology 2002; 3:195-206; PMID:12232501; http://dx.doi.org/10.1023/A:1016299812327
- Schwartz RH. T cell anergy. Annu Rev Immunol 2003; 21:305-34; PMID:12471050; http://dx.doi.org/10.1146/annurev.immunol.21.120601.141110
- Crespo J, Sun H, Welling TH, Tian Z, Zou W. T cell anergy, exhaustion, senescence, and stemness in the tumor microenvironment. Curr Opin Immunol 2013; 25:214-21; PMID:23298609; http://dx.doi.org/10.1016/j.coi.2012.12.003
- Palucka K, Banchereau J. Cancer immunotherapy via dendritic cells. Nat Rev Cancer 2012; 12:265-77; PMID:22437871; http://dx.doi.org/10.1038/nrc3258
- Chino T, Draves KE, Clark EA. Regulation of dendritic cell survival and cytokine production by osteoprotegerin. J Leukoc Biol 2009; 86:933-40; PMID:19641036; http://dx.doi.org/10.1189/jlb.0708419
- Colonna M, Trinchieri G, Liu YJ. Plasmacytoid dendritic cells in immunity. Nat Immunol 2004; 5:1219-26; PMID:15549123; http://dx.doi.org/10.1038/ni1141
- Abediankenari S, Yousefzadeh Y, Azadeh H, Vahedi M. Comparison of several maturation inducing factors in dendritic cell differentiation. Iran J Immunol 2010; 7:83-8; PMID:20574121
- Granucci F, Zanoni I. The dendritic cell life cycle. Cell Cycle 2009; 8:3816-21; PMID:19887908; http://dx.doi.org/10.4161/cc.8.23.9998
- Hou WS, Van Parijs L. A Bcl-2-dependent molecular timer regulates the lifespan and immunogenicity of dendritic cells. Nat Immunol 2004; 5:583-9; PMID:15133508; http://dx.doi.org/10.1038/ni1071
- Higano CS, Schellhammer PF, Small EJ, Burch PA, Nemunaitis J, Yuh L, Provost N, Frohlich MW. Integrated data from 2 randomized, double-blind, placebo-controlled, phase 3 trials of active cellular immunotherapy with sipuleucel-T in advanced prostate cancer. Cancer 2009; 115:3670-9; PMID:19536890; http://dx.doi.org/10.1002/cncr.24429
- Schwartzentruber DJ, Lawson D, Richards J, Conry RM, Miller D, Triesman J, Gailani F, Riley LB, Vena D, Hwu P. A phase III multi-institutional randomized study of immunization with the gp100: 209–217(210M) peptide followed by high-dose IL-2 compared with high-dose IL-2 alone in patients with metastatic melanoma. J Clin Oncol Meeting Abstracts 2009; 27:CRA9011
- Flowers CR. BiovaxID idiotype vaccination: active immunotherapy for follicular lymphoma. Expert Rev Vaccines 2007; 6:307-17; PMID:17542746; http://dx.doi.org/10.1586/14760584.6.3.307
- Ratta M, Fagnoni F, Curti A, Vescovini R, Sansoni P, Oliviero B, Fogli M, Ferri E, Della Cuna GR, Tura S et al. Dendritic cells are functionally defective in multiple myeloma: the role of interleukin-6. Blood 2002; 100:230-7; PMID:12070032; http://dx.doi.org/10.1182/blood.V100.1.230
- Troy AJ, Davidson PJ, Atkinson CH, Hart DN. CD1a dendritic cells predominate in transitional cell carcinoma of bladder and kidney but are minimally activated. J Urol 1999; 161:1962-7; PMID:10332481; http://dx.doi.org/10.1016/S0022-5347(05)68864-7
- Gabrilovich DI, Corak J, Ciernik IF, Kavanaugh D, Carbone DP. Decreased antigen presentation by dendritic cells in patients with breast cancer. Clin Cancer Res 1997; 3:483-90; PMID:9815709
- Yamaguchi Y, Tsumura H, Miwa M, Inaba K. Contrasting effects of TGF-beta 1 and TNF-alpha on the development of dendritic cells from progenitors in mouse bone marrow. Stem Cells 1997; 15:144-53; PMID:9090791; http://dx.doi.org/10.1002/stem.150144
- Steinbrink K, Wolfl M, Jonuleit H, Knop J, Enk AH. Induction of tolerance by IL-10-treated dendritic cells. J Immunol 1997; 159:4772-80; PMID:9366401
- Gabrilovich D, Ishida T, Oyama T, Ran S, Kravtsov V, Nadaf S, Carbone DP. Vascular endothelial growth factor inhibits the development of dendritic cells and dramatically affects the differentiation of multiple hematopoietic lineages in vivo. Blood 1998; 92:4150-66; PMID:9834220
- Aalamian M, Tourkova IL, Chatta GS, Lilja H, Huland E, Huland H, Shurin GV, Shurin MR. Inhibition of dendropoiesis by tumor derived and purified prostate specific antigen. J Urol 2003; 170:2026-30; PMID:14532846; http://dx.doi.org/10.1097/01.ju.0000091264.46134.b7
- Pirtskhalaishvili G, Shurin GV, Esche C, Cai Q, Salup RR, Bykovskaia SN, Lotze MT, Shurin MR. Cytokine-mediated protection of human dendritic cells from prostate cancer-induced apoptosis is regulated by the Bcl-2 family of proteins. Br J Cancer 2000; 83:506-13; PMID:10945499; http://dx.doi.org/10.1054/bjoc.2000.1289
- Zhang Y, Wallace DL, de Lara CM, Ghattas H, Asquith B, Worth A, Griffin GE, Taylor GP, Tough DF, Beverley PC et al. In vivo kinetics of human natural killer cells: the effects of ageing and acute and chronic viral infection. Immunology 2007; 121:258-65; PMID:17346281; http://dx.doi.org/10.1111/j.1365-2567.2007.02573.x
- Vivier E, Tomasello E, Baratin M, Walzer T, Ugolini S. Functions of natural killer cells. Nat Immunol 2008; 9:503-10; PMID:18425107; http://dx.doi.org/10.1038/ni1582
- Vivier E, Raulet DH, Moretta A, Caligiuri MA, Zitvogel L, Lanier LL, Yokoyama WM, Ugolini S. Innate or adaptive immunity? The example of natural killer cells. Science 2011; 331:44-9; PMID:21212348; http://dx.doi.org/10.1126/science.1198687
- Bottino C, Castriconi R, Moretta L, Moretta A. Cellular ligands of activating NK receptors. Trends Immunol 2005; 26:221-6; PMID:15797513; http://dx.doi.org/10.1016/j.it.2005.02.007
- Yokoyama WM, Kim S, French AR. The dynamic life of natural killer cells. Annu Rev Immunol 2004; 22:405-29; PMID:15032583; http://dx.doi.org/10.1146/annurev.immunol.22.012703.104711
- Cheng M, Chen Y, Xiao W, Sun R, Tian Z. NK cell-based immunotherapy for malignant diseases. Cell Mol Immunol 2013; 10:230-52; PMID:23604045; http://dx.doi.org/10.1038/cmi.2013.10
- Sutlu T, Alici E. Natural killer cell-based immunotherapy in cancer: current insights and future prospects. J Intern Med 2009; 266:154-81; PMID:19614820; http://dx.doi.org/10.1111/j.1365-2796.2009.02121.x
- Jewett A, Tseng HC. Tumor induced inactivation of natural killer cell cytotoxic function; implication in growth, expansion and differentiation of cancer stem cells. J Cancer 2011; 2:443-57; PMID:21850212; http://dx.doi.org/10.7150/jca.2.443
- Thomas GR, Chen Z, Leukinova E, Van Waes C, Wen J. Cytokines IL-1 alpha, IL-6, and GM-CSF constitutively secreted by oral squamous carcinoma induce down-regulation of CD80 costimulatory molecule expression: restoration by interferon gamma. Cancer Immunol Immunother 2004; 53:33-40; PMID:14551747; http://dx.doi.org/10.1007/s00262-003-0433-4
- Jewett A, Cavalcanti M, Bonavida B. Pivotal role of endogenous TNF-alpha in the induction of functional inactivation and apoptosis in NK cells. J Immunol 1997; 159:4815-22; PMID:9366406
- Lanier LL. Up on the tightrope: natural killer cell activation and inhibition. Nat Immunol 2008; 9:495-502; PMID:18425106; http://dx.doi.org/10.1038/ni1581
- Brown D, Trowsdale J, Allen R. The LILR family: modulators of innate and adaptive immune pathways in health and disease. Tissue Antigens 2004; 64:215-25; PMID:15304001; http://dx.doi.org/10.1111/j.0001-2815.2004.00290.x
- Grimm EA, Mazumder A, Zhang HZ, Rosenberg SA. Lymphokine-activated killer cell phenomenon. Lysis of natural killer-resistant fresh solid tumor cells by interleukin 2-activated autologous human peripheral blood lymphocytes. J Exp Med 1982; 155:1823-41; PMID:6176669; http://dx.doi.org/10.1084/jem.155.6.1823
- Parkhurst MR, Riley JP, Dudley ME, Rosenberg SA. Adoptive transfer of autologous natural killer cells leads to high levels of circulating natural killer cells but does not mediate tumor regression. Clin Cancer Res 2011; 17:6287-97; PMID:21844012; http://dx.doi.org/10.1158/1078-0432.CCR-11-1347
- Geller MA, Cooley S, Judson PL, Ghebre R, Carson LF, Argenta PA, Jonson AL, Panoskaltsis-Mortari A, Curtsinger J, McKenna D et al. A phase II study of allogeneic natural killer cell therapy to treat patients with recurrent ovarian and breast cancer. Cytotherapy 2011; 13:98-107; PMID:20849361; http://dx.doi.org/10.3109/14653249.2010.515582
- Galluzzi L, Vacchelli E, Eggermont A, Fridman WH, Galon J, Sautes-Fridman C, Tartour E, Zitvogel L, Kroemer G. Trial watch: adoptive cell transfer immunotherapy. Oncoimmunology 2012; 1:306-15; PMID:22737606; http://dx.doi.org/10.4161/onci.19549
- Min-Oo G, Kamimura Y, Hendricks DW, Nabekura T, Lanier LL. Natural killer cells: walking three paths down memory lane. Trends Immunol 2013; 34:251-8; PMID:23499559; http://dx.doi.org/10.1016/j.it.2013.02.005
- Carding SR, Egan PJ. Gammadelta T cells: functional plasticity and heterogeneity. Nat Rev Immunol 2002; 2:336-45; PMID:12033739; http://dx.doi.org/10.1038/nri797
- Tough DF, Sprent J. Lifespan of gammadelta T cells. J Exp Med 1998; 187:357-65; PMID:9449716; http://dx.doi.org/10.1084/jem.187.3.357
- Anane LH, Edwards KM, Burns VE, Zanten JJCSVv, Drayson MT, Bosch JA. Phenotypic characterization of γδ T cells mobilized in response to acute psychological stress. Brain Behav Immun 2010; 24:608-14; PMID:20060888; http://dx.doi.org/10.1016/j.bbi.2010.01.002
- Eberl M, Engel R, Beck E, Jomaa H. Differentiation of human gamma-delta T cells towards distinct memory phenotypes. Cell Immunol 2002; 218:1-6; PMID:12470608; http://dx.doi.org/10.1016/S0008-8749(02)00519-1
- Gomes AQ, Martins DS, Silva-Santos B. Targeting gammadelta T lymphocytes for cancer immunotherapy: from novel mechanistic insight to clinical application. Cancer Res 2010; 70:10024-7; PMID:21159627; http://dx.doi.org/10.1158/0008-5472.CAN-10-3236
- Nicol AJ, Tokuyama H, Mattarollo SR, Hagi T, Suzuki K, Yokokawa K, Nieda M. Clinical evaluation of autologous gamma delta T cell-based immunotherapy for metastatic solid tumours. Br J Cancer 2011; 105:778-86; PMID:21847128; http://dx.doi.org/10.1038/bjc.2011.293
- Raulet DH, Gasser S, Gowen BG, Deng W, Jung H. Regulation of ligands for the NKG2D activating receptor. Annu Rev Immunol 2013; 31:413-41; PMID:23298206; http://dx.doi.org/10.1146/annurev-immunol-032712-095951
- Zhang X, Sun S, Hwang I, Tough DF, Sprent J. Potent and selective stimulation of memory-phenotype CD8+ T cells in vivo by IL-15. Immunity 1998; 8:591-9; PMID:9620680; http://dx.doi.org/10.1016/S1074-7613(00)80564-6
- Surh CD, Sprent J. Homeostasis of Naive and Memory T Cells. Immunity 2008; 29:848-62; PMID:19100699; http://dx.doi.org/10.1016/j.immuni.2008.11.002
- Wong P, Pamer EG. CD8 T cell responses to infectious pathogens. Annu Rev Immunol 2003; 21:29-70; PMID:12414723; http://dx.doi.org/10.1146/annurev.immunol.21.120601.141114
- Michelini RH, Doedens AL, Goldrath AW, Hedrick SM. Differentiation of CD8 memory T cells depends on Foxo1. J Exp Med 2013; 210:1189-200; PMID:23712431; http://dx.doi.org/10.1084/jem.20130392
- Sprent J, Surh CD. T cell memory. Annu Rev Immunol 2002; 20:551-79; PMID:11861612; http://dx.doi.org/10.1146/annurev.immunol.20.100101.151926
- Hinrichs CS, Borman ZA, Cassard L, Gattinoni L, Spolski R, Yu Z, Sanchez-Perez L, Muranski P, Kern SJ, Logun C et al. Adoptively transferred effector cells derived from naïve rather than central memory CD8+ T cells mediate superior antitumor immunity. Proc Natl Acad Sci 2009; 106:17469-74; PMID:19805141; http://dx.doi.org/10.1073/pnas.0907448106
- Shafer-Weaver KA, Anderson MJ, Stagliano K, Malyguine A, Greenberg NM, Hurwitz AA. Cutting edge: tumor-specific CD8+ T cells infiltrating prostatic tumors are induced to become suppressor cells. J Immunol 2009; 183:4848-52; PMID:19801511; http://dx.doi.org/10.4049/jimmunol.0900848
- Schmidt A, Oberle N, Krammer PH. Molecular mechanisms of treg-mediated T cell suppression. Front Immunol 2012; 3:51; PMID:22566933; http://dx.doi.org/10.3389/fimmu.2012.00051
- Shimabukuro-Vornhagen A, Draube A, Liebig TM, Rothe A, Kochanek M, von Bergwelt-Baildon MS. The immunosuppressive factors IL-10, TGF-beta, and VEGF do not affect the antigen-presenting function of CD40-activated B cells. J Exp Clin Cancer Res 2012; 31:47; PMID:22592077; http://dx.doi.org/10.1186/1756-9966-31-47
- Wu R, Forget M-A, Chacon J, Bernatchez C, Haymaker C, Chen JQ, Hwu P, Radvanyi LG. Adoptive T-cell therapy using autologous tumor-infiltrating lymphocytes for metastatic melanoma: current status and future outlook. Cancer J 2012; 18:160-75; PMID:22453018; http://dx.doi.org/10.1097/PPO.0b013e31824d4465
- June CH. Adoptive T cell therapy for cancer in the clinic. J Clin Invest 2007; 117:1466-76; PMID:17549249; http://dx.doi.org/10.1172/JCI32446
- Kershaw MH, Westwood JA, Darcy PK. Gene-engineered T cells for cancer therapy. Nat Rev Cancer 2013; 13:525-41; PMID:23880905; http://dx.doi.org/10.1038/nrc3565
- Kershaw MH, Devaud C, John LB, Westwood JA, Darcy PK. Enhancing immunotherapy using chemotherapy and radiation to modify the tumor microenvironment. OncoImmunol 2013; 2:e25962; PMID:24327938; http://dx.doi.org/10.4161/onci.25962
- Hombach A, Heuser C, Marquardt T, Wieczarkowiecz A, Groneck V, Pohl C, Abken H. CD4+ T cells engrafted with a recombinant immunoreceptor efficiently lyse target cells in a MHC antigen- and fas-independent fashion. J Immunol 2001; 167:1090-6; PMID:11441120; http://dx.doi.org/10.4049/jimmunol.167.2.1090
- Deniger DC, Switzer K, Mi T, Maiti S, Hurton L, Singh H, Huls H, Olivares S, Lee DA, Champlin RE, Cooper LJN. Bispecific T-cells expressing polyclonal repertoire of endogenous γδ T-cell receptors and introduced CD19-specific chimeric antigen receptor. Mol Ther 2013; 21:638-47; PMID:23295945; http://dx.doi.org/10.1038/mt.2012.267
- Mantripragada K, Reagan JL, Quesenberry PJ, Fast LD. Advances in cellular therapy for the treatment of leukemia. Discov Med 2014; 17:15-24; PMID:24411697
- Morgan RA, Dudley ME, Wunderlich JR, Hughes MS, Yang JC, Sherry RM, Royal RE, Topalian SL, Kammula US, Restifo NP et al. Cancer regression in patients after transfer of genetically engineered lymphocytes. Science 2006; 314:126-9; PMID:16946036; http://dx.doi.org/10.1126/science.1129003
- Ritchie DS, Neeson PJ, Khot A, Peinert S, Tai T, Tainton K, Chen K, Shin M, Wall DM, Honemann D et al. Persistence and efficacy of second generation CAR T cell against the LeY antigen in acute myeloid leukemia. Mol Ther 2013; 21:2122-9; PMID:23831595; http://dx.doi.org/10.1038/mt.2013.154
- Shen X, Zhou J, Hathcock KS, Robbins P, Powell DJ, Jr., Rosenberg SA, Hodes RJ. Persistence of tumor infiltrating lymphocytes in adoptive immunotherapy correlates with telomere length. J Immunother 2007; 30:123-9; PMID:17198091; http://dx.doi.org/10.1097/01.cji.0000211321.07654.b8
- Boyman O, Sprent J. The role of interleukin-2 during homeostasis and activation of the immune system. Nat Rev Immunol 2012; 12:180-90; PMID:22343569; http://dx.doi.org/10.1038/nri3156
- Kundig TM, Schorle H, Bachmann MF, Hengartner H, Zinkernagel RM, Horak I. Immune responses in interleukin-2-deficient mice. Science 1993; 262:1059-61; PMID:8235625; http://dx.doi.org/10.1126/science.8235625
- Bachmann MF, Wolint P, Walton S, Schwarz K, Oxenius A. Differential role of IL-2R signaling for CD8+ T cell responses in acute and chronic viral infections. Eur J Immunol 2007; 37:1502-12; PMID:17492805; http://dx.doi.org/10.1002/eji.200637023
- Cho JH, Boyman O, Kim HO, Hahm B, Rubinstein MP, Ramsey C, Kim DM, Surh CD, Sprent J. An intense form of homeostatic proliferation of naive CD8+ cells driven by IL-2. J Exp Med 2007; 204:1787-801; PMID:17664294; http://dx.doi.org/10.1084/jem.20070740
- Kabelitz D, Kirchner H, Armerding D, Wagner H. Recombinant interleukin 2 rapidly augments human natural killer cell activity. Cell Immunol 1985; 93:38-45; PMID:2581709; http://dx.doi.org/10.1016/0008-8749(85)90386-7
- Kabelitz D. Human gammadelta T lymphocytes for immunotherapeutic strategies against cancer. F1000 Med Rep 2010; 2; PMID:20948839
- Yee C, Thompson JA, Byrd D, Riddell SR, Roche P, Celis E, Greenberg PD. Adoptive T cell therapy using antigen-specific CD8+ T cell clones for the treatment of patients with metastatic melanoma: in vivo persistence, migration, and antitumor effect of transferred T cells. Proc Natl Acad Sci U S A 2002; 99:16168-73; PMID:12427970; http://dx.doi.org/10.1073/pnas.242600099
- Kobayashi H, Tanaka Y, Yagi J, Minato N, Tanabe K. Phase III study of adoptive transfer of gammadelta T cells in combination with zoledronic acid and IL-2 to patients with advanced renal cell carcinoma. Cancer Immunol Immunother 2011; 60:1075-84; PMID:21519826; http://dx.doi.org/10.1007/s00262-011-1021-7
- Zhang H, Chua KS, Guimond M, Kapoor V, Brown MV, Fleisher TA, Long LM, Bernstein D, Hill BJ, Douek DC et al. Lymphopenia and interleukin-2 therapy alter homeostasis of CD4+CD25+ regulatory T cells. Nat Med 2005; 11:1238-43; PMID:16227988; http://dx.doi.org/10.1038/nm1312
- Clement JM, McDermott DF. The high-dose aldesleukin (IL-2) "select" trial: a trial designed to prospectively validate predictive models of response to high-dose IL-2 treatment in patients with metastatic renal cell carcinoma. Clin Genitourin Cancer 2009; 7:E7-9; PMID:19692326; http://dx.doi.org/10.3816/CGC.2009.n.014
- Gogas H, Polyzos A, Kirkwood J. Immunotherapy for advanced melanoma: fulfilling the promise. Cancer Treat Rev 2013; 39:879-85; PMID:23725878; http://dx.doi.org/10.1016/j.ctrv.2013.04.006
- Rosenberg SA, Yang JC, White DE, Steinberg SM. Durability of complete responses in patients with metastatic cancer treated with high-dose interleukin-2: identification of the antigens mediating response. Ann Surg 1998; 228:307-19; PMID:9742914; http://dx.doi.org/10.1097/00000658-199809000-00004
- Sim GC, Martin-Orozco N, Jin L, Yang Y, Wu S, Washington E, Sanders D, Lacey C, Wang Y, Vence L et al. IL-2 therapy promotes suppressive ICOS+ Treg expansion in melanoma patients. J Clin Invest 2014; 124:99-110; PMID:24292706; http://dx.doi.org/10.1172/JCI46266
- von Freeden-Jeffry U, Vieira P, Lucian LA, McNeil T, Burdach SE, Murray R. Lymphopenia in interleukin (IL)-7 gene-deleted mice identifies IL-7 as a nonredundant cytokine. J Exp Med 1995; 181:1519-26; PMID:7699333; http://dx.doi.org/10.1084/jem.181.4.1519
- Moore TA, von Freeden-Jeffry U, Murray R, Zlotnik A. Inhibition of gamma delta T cell development and early thymocyte maturation in IL-7 -- mice. J Immunol 1996; 157:2366-73; PMID:8805634
- Chu YW, Memon SA, Sharrow SO, Hakim FT, Eckhaus M, Lucas PJ, Gress RE. Exogenous IL-7 increases recent thymic emigrants in peripheral lymphoid tissue without enhanced thymic function. Blood 2004; 104:1110-9; PMID:15130942; http://dx.doi.org/10.1182/blood-2003-10-3635
- Fry TJ, Moniuszko M, Creekmore S, Donohue SJ, Douek DC, Giardina S, Hecht TT, Hill BJ, Komschlies K, Tomaszewski J et al. IL-7 therapy dramatically alters peripheral T-cell homeostasis in normal and SIV-infected nonhuman primates. Blood 2003; 101:2294-9; PMID:12411295; http://dx.doi.org/10.1182/blood-2002-07-2297
- Storek J, Lu H, Kalina T, Kiem H. IL-7 improves reconstitution of CD4 T cells, including cytomegalovirus-specific CD4 T cells, in lymphopenic monkeys. J Allergy Clin Immunol 2004; 113:S50
- Melchionda F, Fry TJ, Milliron MJ, McKirdy MA, Tagaya Y, Mackall CL. Adjuvant IL-7 or IL-15 overcomes immunodominance and improves survival of the CD8+ memory cell pool. J Clin Invest 2005; 115:1177-87; PMID:15841203; http://dx.doi.org/10.1172/JCI200523134
- Ruprecht CR, Gattorno M, Ferlito F, Gregorio A, Martini A, Lanzavecchia A, Sallusto F. Coexpression of CD25 and CD27 identifies FoxP3+ regulatory T cells in inflamed synovia. J Exp Med 2005; 201:1793-803; PMID:15939793; http://dx.doi.org/10.1084/jem.20050085
- Li B, VanRoey MJ, Jooss K. Recombinant IL-7 enhances the potency of GM-CSF-secreting tumor cell immunotherapy. Clin Immunol 2007; 123:155-65; PMID:17320482; http://dx.doi.org/10.1016/j.clim.2007.01.002
- Rosenberg SA, Sportes C, Ahmadzadeh M, Fry TJ, Ngo LT, Schwarz SL, Stetler-Stevenson M, Morton KE, Mavroukakis SA, Morre M et al. IL-7 administration to humans leads to expansion of CD8+ and CD4+ cells but a relative decrease of CD4+ T-regulatory cells. J Immunother 2006; 29:313-9; PMID:16699374; http://dx.doi.org/10.1097/01.cji.0000210386.55951.c2
- Sportes C, Hakim FT, Memon SA, Zhang H, Chua KS, Brown MR, Fleisher TA, Krumlauf MC, Babb RR, Chow CK et al. Administration of rhIL-7 in humans increases in vivo TCR repertoire diversity by preferential expansion of naive T cell subsets. J Exp Med 2008; 205:1701-14; PMID:18573906; http://dx.doi.org/10.1084/jem.20071681
- Gattinoni L, Finkelstein SE, Klebanoff CA, Antony PA, Palmer DC, Spiess PJ, Hwang LN, Yu Z, Wrzesinski C, Heimann DM et al. Removal of homeostatic cytokine sinks by lymphodepletion enhances the efficacy of adoptively transferred tumor-specific CD8+ T cells. J Exp Med 2005; 202:907-12; PMID:16203864; http://dx.doi.org/10.1084/jem.20050732
- Powell DJ, Jr., Dudley ME, Robbins PF, Rosenberg SA. Transition of late-stage effector T cells to CD27+ CD28+ tumor-reactive effector memory T cells in humans after adoptive cell transfer therapy. Blood 2005; 105:241-50; PMID:15345595; http://dx.doi.org/10.1182/blood-2004-06-2482
- Mackall CL, Fry TJ, Gress RE. Harnessing the biology of IL-7 for therapeutic application. Nat Rev Immunol 2011; 11:330-42; PMID:21508983; http://dx.doi.org/10.1038/nri2970
- Michaud A, Dardari R, Charrier E, Cordeiro P, Herblot S, Duval M. IL-7 enhances survival of human CD56bright NK cells. J Immunother 2010; 33:382-90; PMID:20386468; http://dx.doi.org/10.1097/CJI.0b013e3181cd872d
- Coquet JM, Kyparissoudis K, Pellicci DG, Besra G, Berzins SP, Smyth MJ, Godfrey DI. IL-21 is produced by NKT cells and modulates NKT cell activation and cytokine production. J Immunol 2007; 178:2827-34; PMID:17312126; http://dx.doi.org/10.4049/jimmunol.178.5.2827
- Parrish-Novak J, Dillon SR, Nelson A, Hammond A, Sprecher C, Gross JA, Johnston J, Madden K, Xu W, West J et al. Interleukin 21 and its receptor are involved in NK cell expansion and regulation of lymphocyte function. Nature 2000; 408:57-63; PMID:11081504; http://dx.doi.org/10.1038/35040504
- Ozaki K, Spolski R, Feng CG, Qi CF, Cheng J, Sher A, Morse HC, 3rd, Liu C, Schwartzberg PL, Leonard WJ. A critical role for IL-21 in regulating immunoglobulin production. Science 2002; 298:1630-4; PMID:12446913; http://dx.doi.org/10.1126/science.1077002
- Zeng R, Spolski R, Finkelstein SE, Oh S, Kovanen PE, Hinrichs CS, Pise-Masison CA, Radonovich MF, Brady JN, Restifo NP et al. Synergy of IL-21 and IL-15 in regulating CD8+ T cell expansion and function. J Exp Med 2005; 201:139-48; PMID:15630141; http://dx.doi.org/10.1084/jem.20041057
- Ma HL, Whitters MJ, Konz RF, Senices M, Young DA, Grusby MJ, Collins M, Dunussi-Joannopoulos K. IL-21 activates both innate and adaptive immunity to generate potent antitumor responses that require perforin but are independent of IFN-gamma. J Immunol 2003; 171:608-15; PMID:12847225; http://dx.doi.org/10.4049/jimmunol.171.2.608
- Thedrez A, Harly C, Morice A, Salot S, Bonneville M, Scotet E. IL-21-mediated potentiation of antitumor cytolytic and proinflammatory responses of human V gamma 9V delta 2 T cells for adoptive immunotherapy. J Immunol 2009; 182:3423-31; PMID:19265120; http://dx.doi.org/10.4049/jimmunol.0803068
- Toomey JA, Gays F, Foster D, Brooks CG. Cytokine requirements for the growth and development of mouse NK cells in vitro. J Leukoc Biol 2003; 74:233-42; PMID:12885940; http://dx.doi.org/10.1189/jlb.0303097
- Kasaian MT, Whitters MJ, Carter LL, Lowe LD, Jussif JM, Deng B, Johnson KA, Witek JS, Senices M, Konz RF et al. IL-21 limits NK cell responses and promotes antigen-specific T cell activation: a mediator of the transition from innate to adaptive immunity. Immunity 2002; 16:559-69; PMID:11970879; http://dx.doi.org/10.1016/S1074-7613(02)00295-9
- Brady J, Hayakawa Y, Smyth MJ, Nutt SL. IL-21 induces the functional maturation of murine NK cells. J Immunol 2004; 172:2048-58; PMID:14764669; http://dx.doi.org/10.4049/jimmunol.172.4.2048
- Roda JM, Parihar R, Lehman A, Mani A, Tridandapani S, Carson WE, 3rd. Interleukin-21 enhances NK cell activation in response to antibody-coated targets. J Immunol 2006; 177:120-9; PMID:16785506; http://dx.doi.org/10.4049/jimmunol.177.1.120
- Hashmi MH, Van Veldhuizen PJ. Interleukin-21: updated review of Phase I and II clinical trials in metastatic renal cell carcinoma, metastatic melanoma and relapsedrefractory indolent non-Hodgkin's lymphoma. Exp Opin Biol Ther 2010; 10:807-17; PMID:20384523; http://dx.doi.org/10.1517/14712598.2010.480971
- Davis ID, Brady B, Kefford RF, Millward M, Cebon J, Skrumsager BK, Mouritzen U, Hansen LT, Skak K, Lundsgaard D et al. Clinical and biological efficacy of recombinant human interleukin-21 in patients with stage IV malignant melanoma without prior treatment: a phase IIa trial. Clin Cancer Res 2009; 15:2123-9; PMID:19276257; http://dx.doi.org/10.1158/1078-0432.CCR-08-2663
- Davis ID, Skrumsager BK, Cebon J, Nicholaou T, Barlow JW, Moller NP, Skak K, Lundsgaard D, Frederiksen KS, Thygesen P et al. An open-label, two-arm, phase I trial of recombinant human interleukin-21 in patients with metastatic melanoma. Clin Cancer Res 2007; 13:3630-6; PMID:17575227; http://dx.doi.org/10.1158/1078-0432.CCR-07-0410
- Thompson JA, Curti BD, Redman BG, Bhatia S, Weber JS, Agarwala SS, Sievers EL, Hughes SD, DeVries TA, Hausman DF. Phase I study of recombinant interleukin-21 in patients with metastatic melanoma and renal cell carcinoma. J Clin Oncol 2008; 26:2034-9; PMID:18347008; http://dx.doi.org/10.1200/JCO.2007.14.5193
- Brandt K, Bulfone-Paus S, Foster DC, Ruckert R. Interleukin-21 inhibits dendritic cell activation and maturation. Blood 2003; 102:4090-8; PMID:12893770; http://dx.doi.org/10.1182/blood-2003-03-0669
- Wan CK, Oh J, Li P, West EE, Wong EA, Andraski AB, Spolski R, Yu ZX, He J, Kelsall BL et al. The cytokines IL-21 and GM-CSF have opposing regulatory roles in the apoptosis of conventional dendritic cells. Immunity 2013; 38:514-27; PMID:23453633; http://dx.doi.org/10.1016/j.immuni.2013.02.011
- Steel JC, Waldmann TA, Morris JC. Interleukin-15 biology and its therapeutic implications in cancer. Trends Pharmacol Sci 2012; 33:35-41; PMID:22032984; http://dx.doi.org/10.1016/j.tips.2011.09.004
- Kennedy MK, Glaccum M, Brown SN, Butz EA, Viney JL, Embers M, Matsuki N, Charrier K, Sedger L, Willis CR et al. Reversible defects in natural killer and memory CD8 T cell lineages in interleukin 15-deficient mice. J Exp Med 2000; 191:771-80; PMID:10704459; http://dx.doi.org/10.1084/jem.191.5.771
- Carson WE, Giri JG, Lindemann MJ, Linett ML, Ahdieh M, Paxton R, Anderson D, Eisenmann J, Grabstein K, Caligiuri MA. Interleukin (IL) 15 is a novel cytokine that activates human natural killer cells via components of the IL-2 receptor. J Exp Med 1994; 180:1395-403; PMID:7523571; http://dx.doi.org/10.1084/jem.180.4.1395
- Gordy LE, Bezbradica JS, Flyak AI, Spencer CT, Dunkle A, Sun J, Stanic AK, Boothby MR, He YW, Zhao Z et al. IL-15 regulates homeostasis and terminal maturation of NKT cells. J Immunol 2011; 187:6335-45; PMID:22084435; http://dx.doi.org/10.4049/jimmunol.1003965
- Lu J, Giuntoli RL, 2nd, Omiya R, Kobayashi H, Kennedy R, Celis E. Interleukin 15 promotes antigen-independent in vitro expansion and long-term survival of antitumor cytotoxic T lymphocytes. Clin Cancer Res 2002; 8:3877-84; PMID:12473603
- Anguille S, Smits EL, Cools N, Goossens H, Berneman ZN, Van Tendeloo VF. Short-term cultured, interleukin-15 differentiated dendritic cells have potent immunostimulatory properties. J Transl Med 2009; 7:109; PMID:20021667; http://dx.doi.org/10.1186/1479-5876-7-109
- Berger C, Berger M, Hackman RC, Gough M, Elliott C, Jensen MC, Riddell SR. Safety and immunologic effects of IL-15 administration in nonhuman primates. Blood 2009; 114:2417-26; PMID:19605850; http://dx.doi.org/10.1182/blood-2008-12-189266
- Kanegane H, Tosato G. Activation of naive and memory T cells by interleukin-15. Blood 1996; 88:230-5; PMID:8704178; http://dx.doi.org/10.1073/pnas.0902637106
- Zhang M, Yao Z, Dubois S, Ju W, Muller JR, Waldmann TA. Interleukin-15 combined with an anti-CD40 antibody provides enhanced therapeutic efficacy for murine models of colon cancer. Proc Natl Acad Sci U S A 2009; 106:7513-8; PMID:19383782; http://dx.doi.org/10.1073/pnas.0902637106
- Yu P, Steel JC, Zhang M, Morris JC, Waldmann TA. Simultaneous blockade of multiple immune system inhibitory checkpoints enhances antitumor activity mediated by interleukin-15 in a murine metastatic colon carcinoma model. Clin Cancer Res 2010; 16:6019-28; PMID:20924130; http://dx.doi.org/10.1158/1078-0432.CCR-10-1966
- Ochoa MC, Mazzolini G, Hervas-Stubbs S, de Sanmamed MF, Berraondo P, Melero I. Interleukin-15 in gene therapy of cancer. Curr Gene Ther 2013; 13:15-30; PMID:23157547; http://dx.doi.org/10.2174/156652313804806561
- Elhage O, Galustian C, Ukimura O, Gill I, Smith R, Dasgupta P. 1789 Selection of optimal cytokine combinations for immunotherapy of prostate cancer. J Urol 2011; 185:e719; PMID:15843522; http://dx.doi.org/10.1016/j.juro.2011.02.2139
- Kawauchi K, Ihjima K, Yamada O. IL-2 increases human telomerase reverse transcriptase activity transcriptionally and posttranslationally through phosphatidylinositol 3'-kinaseAkt, heat shock protein 90, and mammalian target of rapamycin in transformed NK cells. J Immunol 2005; 174:5261-9; PMID:15843522; http://dx.doi.org/10.4049/jimmunol.174.9.5261
- Denman CJ, Senyukov VV, Somanchi SS, Phatarpekar PV, Kopp LM, Johnson JL, Singh H, Hurton L, Maiti SN, Huls MH et al. Membrane-bound IL-21 promotes sustained ex vivo proliferation of human natural killer cells. PLoS One 2012; 7:e30264; PMID:22279576; http://dx.doi.org/10.1371/journal.pone.0030264
- Li Y, Zhi W, Wareski P, Weng NP. IL-15 activates telomerase and minimizes telomere loss and may preserve the replicative life span of memory CD8+ T cells in vitro. J Immunol 2005; 174:4019-24; PMID:15778359; http://dx.doi.org/10.4049/jimmunol.174.7.4019
- Hsu C, Jones SA, Cohen CJ, Zheng Z, Kerstann K, Zhou J, Robbins PF, Peng PD, Shen X, Gomes TJ et al. Cytokine-independent growth and clonal expansion of a primary human CD8+ T-cell clone following retroviral transduction with the IL-15 gene. Blood 2007; 109:5168-77; PMID:17353346; http://dx.doi.org/10.1182/blood-2006-06-029173
- Wallace DL, Berard M, Soares MV, Oldham J, Cook JE, Akbar AN, Tough DF, Beverley PC. Prolonged exposure of naive CD8+ T cells to interleukin-7 or interleukin-15 stimulates proliferation without differentiation or loss of telomere length. Immunology 2006; 119:243-53; PMID:17005004; http://dx.doi.org/10.1111/j.1365-2567.2006.02429.x
- Yamada O, Ozaki K, Akiyama M, Kawauchi K. JAK-STAT and JAK-PI3K-mTORC1 pathways regulate telomerase transcriptionally and posttranslationally in ATL cells. Mol Cancer Ther 2012; 11:1112-21; PMID:22402124; http://dx.doi.org/10.1158/1535-7163.MCT-11-0850
- Konnikova L, Simeone MC, Kruger MM, Kotecki M, Cochran BH. Signal transducer and activator of transcription 3 (STAT3) regulates human telomerase reverse transcriptase (hTERT) expression in human cancer and primary cells. Cancer Res 2005; 65:6516-20; PMID:16061629; http://dx.doi.org/10.1158/0008-5472.CAN-05-0924
- Frolkis M, Fischer MB, Wang Z, Lebkowski JS, Chiu CP, Majumdar AS. Dendritic cells reconstituted with human telomerase gene induce potent cytotoxic T-cell response against different types of tumors. Cancer Gene Ther 2003; 10:239-49; PMID:12637945; http://dx.doi.org/10.1038/sj.cgt.7700563
- Su Z, Dannull J, Yang BK, Dahm P, Coleman D, Yancey D, Sichi S, Niedzwiecki D, Boczkowski D, Gilboa E et al. Telomerase mRNA-transfected dendritic cells stimulate antigen-specific CD8+ and CD4+ T cell responses in patients with metastatic prostate cancer. J Immunol 2005; 174:3798-807; PMID:15749921; http://dx.doi.org/10.4049/jimmunol.174.6.3798
- Cooper MA, Bush JE, Fehniger TA, VanDeusen JB, Waite RE, Liu Y, Aguila HL, Caligiuri MA. In vivo evidence for a dependence on interleukin 15 for survival of natural killer cells. Blood 2002; 100:3633-8; PMID:12393617; http://dx.doi.org/10.1182/blood-2001-12-0293
- Ranson T, Vosshenrich CA, Corcuff E, Richard O, Muller W, Di Santo JP. IL-15 is an essential mediator of peripheral NK-cell homeostasis. Blood 2003; 101:4887-93; PMID:12586624; http://dx.doi.org/10.1182/blood-2002-11-3392
- Brunner S, Herndler-Brandstetter D, Arnold CR, Wiegers GJ, Villunger A, Hackl M, Grillari J, Moreno-Villanueva M, Bürkle A, Grubeck-Loebenstein B. Upregulation of miR-24 is associated with a decreased DNA damage response upon etoposide treatment in highly differentiated CD8+ T cells sensitizing them to apoptotic cell death. Aging Cell 2012; 11:579-87; PMID:22435726; http://dx.doi.org/10.1111/j.1474-9726.2012.00819.x
- Armant M, Delespesse G, Sarfati M. IL-2 and IL-7 but not IL-12 protect natural killer cells from death by apoptosis and up-regulate bcl-2 expression. Immunology 1995; 85:331-7; PMID:7642225; http://dx.doi.org/doi:10.1016/j.molimm.2006.04.029
- Bosque A, Marzo I, Naval J, Anel A. Apoptosis by IL-2 deprivation in human CD8+ T cell blasts predominates over death receptor ligation, requires Bim expression and is associated with Mcl-1 loss. Mol Immunol 2007; 44:1446-53; PMID:16806475; http://dx.doi.org/10.1016/j.molimm.2006.04.029
- Pellegrini M, Calzascia T, Elford AR, Shahinian A, Lin AE, Dissanayake D, Dhanji S, Nguyen LT, Gronski MA, Morre M et al. Adjuvant IL-7 antagonizes multiple cellular and molecular inhibitory networks to enhance immunotherapies. Nat Med 2009; 15:528-36; PMID:19396174; http://dx.doi.org/10.1038/nm.1953
- Inoue S, Unsinger J, Davis CG, Muenzer JT, Ferguson TA, Chang K, Osborne DF, Clark AT, Coopersmith CM, McDunn JE et al. IL-15 prevents apoptosis, reverses innate and adaptive immune dysfunction, and improves survival in sepsis. J Immunol 2010; 184:1401-9; PMID:20026737; http://dx.doi.org/10.4049/jimmunol.0902307
- Barker BR, Parvani JG, Meyer D, Hey AS, Skak K, Letvin NL. IL-21 induces apoptosis of antigen-specific CD8+ T lymphocytes. J Immunol 2007; 179:3596-603; PMID:17785794; http://dx.doi.org/10.4049/jimmunol.179.6.3596
- Dure M, Macian F. IL-2 signaling prevents T cell anergy by inhibiting the expression of anergy-inducing genes. Mol Immunol 2009; 46:999-1006; PMID:18990450; http://dx.doi.org/10.1016/j.molimm.2008.09.029
- Chikuma S, Terawaki S, Hayashi T, Nabeshima R, Yoshida T, Shibayama S, Okazaki T, Honjo T. PD-1-mediated suppression of IL-2 production induces CD8+ T cell anergy in vivo. J Immunol 2009; 182:6682-9; PMID:19454662; http://dx.doi.org/10.4049/jimmunol.0900080
- Marktel S, Magnani Z, Ciceri F, Cazzaniga S, Riddell SR, Traversari C, Bordignon C, Bonini C. Immunologic potential of donor lymphocytes expressing a suicide gene for early immune reconstitution after hematopoietic T-cell-depleted stem cell transplantation. Blood 2002; 101:1290-8; PMID:12393508; http://dx.doi.org/10.1182/blood-2002-08-2351
- Griffioen M, van Egmond EHM, Kester MGD, Willemze R, Falkenburg JHF, Heemskerk MHM. Retroviral transfer of human CD20 as a suicide gene for adoptive T-cell therapy. Haematologica 2009; 94:1316-20; PMID:19734426; http://dx.doi.org/10.3324/haematol.2008.001677
- Di Stasi A, Tey SK, Dotti G, Fujita Y, Kennedy-Nasser A, Martinez C, Straathof K, Liu E, Durett AG, Grilley B et al. Inducible apoptosis as a safety switch for adoptive cell therapy. N Engl J Med 2011; 365:1673-83; PMID:22047558; http://dx.doi.org/10.1056/NEJMoa1106152