Abstract
IL-15 regulates the development, survival, and proliferation of multiple innate and adaptive immune cells and plays a dual role, inducing both tumor cell growth and antitumor immunity. However, the role of IL-15 in inflammation-induced cancer remains unclear. To explore this, we have compared the colon carcinoma burden of Il15−/− and Il15rα−/− mice with wild type (WT) mice after induction of colitis-associated colon carcinogenesis utilizing the AOM/DSS model. Compared to WT mice, Il15−/− but not Il15rα−/− mice showed reduced survival, along with higher tumor incidence, colon weight, and tumor size. This suggests that low affinity IL-15 signaling via the shared IL-2Rβ/γc decreases the risk for developing colitis-associated cancer. CD11c-Il15 mice, in which IL-15 expression is reconstituted in Il15−/− mice under the control of the CD11c-promoter, showed that selective reconstitution of IL-15 in antigen-presenting cells restored the CD8+ T and NK cell compartments, serum levels of IFNγ, G-CSF, IL-10, and CXCL1 and reduced tumor burden. After demonstrating IL-15 expression in human colorectal cancer (CRC) cells in situ, we investigated the role of this cytokine in the modulation of key colonic oncogenic pathways in the tumor. While these pathways were found to be unaltered in the absence of IL-15, tumor transcriptome analysis showed that the loss of IL-15 upregulates key inflammatory mediators associated with colon cancer progression, such as IL-1β, IL-22, IL-23, Cxcl5, and Spp1. These findings provide evidence that IL-15 suppresses colitis-associated colon carcinogenesis through regulation of antitumor cytotoxicity, and modulation of the inflammatory tumor micromilieu.
Abbreviations
| AOM | = | Azoxymethane |
| CAC | = | Colitis associated carcinoma |
| CRC | = | Colorectal cancer |
| DSS | = | Dextran sulfate sodium |
| EAE | = | Experimental autoimmune encephalomyelitis |
| IBD | = | Irritable bowel disease |
| IEL | = | Intestinal epithelial cell |
| IFN | = | Interferon |
| IL | = | Interleukin |
| LN | = | Lymph node |
| NK | = | Natural killer cell |
| SCRC | = | Spontaneous colorectal cancer |
| TNM | = | TNM classification of malignant tumors |
| WT | = | Wild type |
Introduction
IL-15 has garnered attention as a potential therapeutic agent in cancer immunotherapy, with multiple studies attributing antitumor effects to IL-15, along with enhanced NK and CD8+ T cell cytotoxicity.Citation1 IL-15 is a pleiotropic cytokine constitutively expressed by dendritic cells (DC), macrophages, fibroblasts, and epithelial cells,Citation2 which following inflammatory stimuli is upregulated in macrophages,Citation3 enterocytes and CD11c+CD11b+/− DCs.Citation4 IL-15 signals via a hetero-trimer composed of the IL-2 receptor β (IL-2Rβ), the common gamma chain (γc) and the unique high affinity receptor α chain (IL-15Rα),Citation5-7 with the latter presenting IL-15 in trans to IL-2Rβ/γc on neighboring cells.Citation8,9 IL-15- and IL-15Rα-deficient mice exhibit a common phenotype that has demonstrated an essential role of IL-15 in both the development and homeostasis of memory CD8+ T cells, natural killer cells (NK), invariant NKT (iNKT) cells and intestinal intraepithelial lymphocytes (IEL).Citation10,11
Conversely, IL-15 has been shown to be pro-tumorogenic, through promotion of tumor growth, invasion and metastasis, while also protecting tumor cells from apoptosis.Citation12-16 Furthermore, in humans, overexpression of IL-15 promotes development of large granular lymphocytic leukemia.Citation12,17 Thus, the clinical value of targeting IL-15 in cancer immunotherapy remains uncertain and may be highly context-dependent. Of interest to this study, it is ill understood whether IL-15 is required for the development or suppression of inflammation-induced cancer.
Cytokines produced by tumor-infiltrating immune cells modify tumor growth and survival, promote angiogenesis and inhibit antitumor immune responses to promote the development of pre-malignant cells. The balance between pro- and anti-inflammatory activities is critical for an efficient antitumor immune response,Citation18 with dysregulation of IL-6, IL-17, IL-23, and IL-22 cytokine signaling pathways playing an important inflammatory role in the development and progression of intestinal tumors.Citation19 Furthermore, genetic variations within pro- and anti-inflammatory interleukins may confer risk of developing or impact survival rates in colon cancer.Citation20,21 Among these, single nucleotide polymorphisms (SNPs) from IL-3, IL-6R, IL-8 and, interestingly, IL-15 were found to be associated with an increased colon cancer risk,Citation22 while IL-1 or IL-1 receptor antagonist polymorphisms negatively impact on CRC survival and recurrence.Citation23,24
The role of the inflammatory milieu is of particular importance in CRC as the risk of developing dysplasia and CRC positively correlates with the duration and degree of inflammation in patients with irritable bowel disease (IBD).Citation25 While there is abundant evidence indicating the causal relationship of chronic mucosal inflammation with CRC,Citation26 the mechanism(s) underlying this correlation, namely the role of IL-15-mediated signaling, remain largely unknown.
In light of the complex and dichotomous role of IL-15 in an oncology setting, summarized above, this current study seeks to clarify the contribution of IL-15 in a well-defined experimental model of inflammation-induced CRC. For this, a single injection of the carcinogen azoxymethane (AOM) was combined with the dextran sulfate sodium (DSS) mouse model of chronic colon inflammation.Citation27,28 This generates a relatively non-invasive and reproducible model of colitis-associated carcinoma (CAC). Utilizing this model, we have investigated the role that IL-15 plays on tumor development and growth in vivo using IL-15- or IL-15Rα- deficient mice as well as a newly generated strain of Il15−/− mice, in which IL-15 is selectively expressed in predominantly antigen presenting cells under the control of the CD11c-promoter. In this model, we demonstrate that IL-15 deficiency increases tumor burden as a result of deficient NK and CD8+ T cell immunity and a tumor-supporting inflammatory milieu, indicating that intestinal homeostasis and suppression of inflammation induced-tumorigenesis are dependent on IL-15.
Results
IL-15 deficiency promotes inflammation-induced colorectal tumorigenesis in mice
To determine the role of IL-15 in inflammation-induced colorectal tumorigenesis we utilized the AOM/DSS model. Briefly, mice were injected with AOM followed by three cycles of 1% DSS in drinking water.Citation27,28 Firstly, we compared the survival and colon carcinoma burden between Il15−/− and Il15rα−/− to WT mice after induction of colitis-associated colon carcinogenesis.
IL-15- but not IL-15Rα-deficient mice, had significantly reduced survival rates compared to WT mice (), with 25% of Il15−/− mice dying after completion of the second DSS administration cycle. Tumor incidence was 100% in Il15−/− mice, and colon weight and tumor size was significantly higher in Il15−/− compared to Il15rα−/− and WT mice (). Adenomas greater than 4mm in diameter were exclusively found in mice with disrupted IL-15 signaling (Il15−/− 34% and Il15rα−/− 22%) ().
Figure 1. Increased tumorigenesis in Il15−/− mice in a colitis-associated cancer model. Survival rates (A) of WT, Il15−/− and Il15rα−/− mice subjected to AOM/DSS treatment, were monitored. Colon weight (B), numbers of tumors per mouse (C), size (D) and location (E) of tumors were determined. Data represented as mean ± SEM of two independent experiments (WT n = 14, Il15−/− n = 11, Il15rα−/− n = 13). (F) Representative images of longitudinally cut colons from WT (n = 2), Il15−/− (n = 2) and Il15rα−/− (n = 2). (G) Representative H&E images of formalin-fixed tissues from proximal, mid and distal colonic regions. Dotted lines indicate adenomas with high-grade dysplasia. Insert enlarges a cancerous gland invading muscularis mucosa, with the overlying epithelium which is dysplastic. Scale bar: 600 μm, within the insert 200 μm. Mann–Whitney tests were used for statistical analysis.
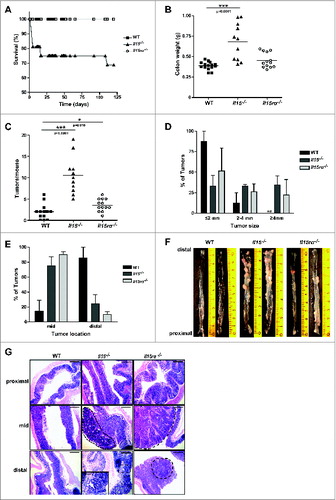
Interestingly the majority of tumors in both Il15−/− and Il15rα−/− mice were localized within the mid and distal region of the colon (), mirroring the predominant occurrence of colorectal tumors in humans, raising the question whether tumor development in some intestinal compartments is more IL-15-dependent than others. All Il15−/− and a significant number of Il15rα−/− mice developed tumors with low- and high-grade dysplasia (). Although invasive intramucosal carcinomas were observed in all genotypes, pT1 stage adenocarcinomas were found exclusively in Il15−/− mice (). IL-15 can reduce mucosal damage through a reduction in intestinal epithelial cell (IEC) apoptosis,Citation29 and in our AOM/DSS treated Il15−/− and Il15rα−/− mice we found significantly increased apoptosis by cleaved caspase-3 immunostaining in both tumor free epithelium (Fig. S1A) and adenomas (Fig. S1B) compared with WT mice. Furthermore Il-15tg mice showed levels of apoptosis comparable with that of WT mice (Fig. S1B and S1C). We observed no significant differences in the proliferative index in adenomas between the different strains (data not shown).
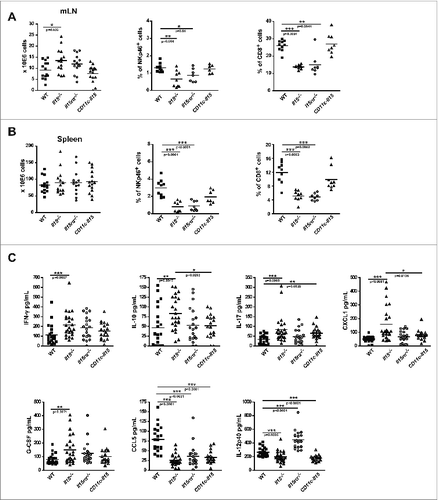
Naïve Il15−/− and Il15rα−/− mice exhibit consistently reduced numbers of NK and CD8+ T cells in both spleen and mesenteric LN,Citation10,11 and this phenotype is maintained after AOM/DSS induction (). The number of CD4+ T cells and B220+ B cells are slightly but significantly increased both in percentage (CD4+ T cells 0.37% ± 0.02 vs. 0.30% ± 0.02, p = 0.0002, B220+ B cells 0.438% ± 0.04 vs. 0.385% ± 0.0.046, p = 0.0188) and absolute number (CD4+ T cells 4.38 ± 1.64 vs. 2.92 ± 1, p = 0.0434, B220+ B cells 5.49 ± 2.22 vs. 3.41 ± 0.96, p = 0.0314) was observed in Il15−/− mLN compared with WT. Furthermore, compared with WT mice, the sera of Il15−/− mice showed higher concentrations of IFNγ, IL-10, IL-17, CXCL1, and G-CSF with decreased levels of CCL5 and IL-12p40 (). Serum levels of eotaxin, IL-1α, IL-1β, IL-2, GM-CSF, IL-3, IL-4, IL-6, IL-12p70, IL-13, MCP1, MIP1α, and MIP1β, were not found to be significantly different between the groups (Fig. S2).
Figure 2. CD11c-Il15 mice showed restored CD8+ T and NK cells and serum levels of IFNγ, G-CSF, IL-10 and CXCL1. Mesenteric lymph nodes (mLN) and spleens were isolated from mice after completion of the AOM/DSS protocol. Total cell numbers and percentage of CD8+ T cells and NK cells were analyzed by flow cytometry in mLNs (A) and spleens (B). Lines show mean values with each point representing an individual mouse from three independent experiments (left panels) (WT n = 14, Il15−/− n = 14, Il15ra−/− n = 14, CD11c-Il15 n = 15) and two experiments (all other panels) (WT n = 9, Il15−/− n = 9, Il15ra−/− n = 7, CD11c-Il15 n = 5-8). Mann–Whitney tests were used for statistical analysis. (C) Sera was collected from mice after completion of the AOM/DSS protocol and cytokine/chemokines were quantified using the Bio-Plex array system (Bio-Rad). Lines indicate mean values with each point representing an individual mouse from two independent experiments (WT n = 22, Il15−/− n = 24, Il15rα−/− n = 20, CD11c-Il15 n = 18). Mann–Whitney tests were used for statistical analysis.
These data demonstrate that IL-15 deficiency profoundly affects survival and accelerates tumor growth and development. In contrast, loss of IL-15Rα showed a less pronounced effect on tumor burden, indicating that intact IL-15 signaling via IL-2Rβ/γc may partially compensate for absent IL-15Rα-mediated signaling with respect to controlling tumor growth.
CD11c-restricted IL-15 expression shows reduced tumor formation
IL-15 is constitutively expressed and highly inducible in DC and trans-presented to CD8+ T and NK cells which induces proliferation and activation of both cell populations.Citation8,9 We therefore sought to determine, whether recovery of NK and CD8+ T cell populations in Il15−/− mice would be sufficient to inhibit tumor growth in the AOM/DSS model. To address this, we generated a novel CD11c-Il15 mouse strain, in which IL-15 expression is reconstituted in antigen presenting cells of Il15−/− mice under the control of the CD11c-promoter (strain 65). (Polansky et al., in preparation).
As expected these mice have a recapitulated NK and CD8+ T cell compartment with percentages of NK and CD8+ T cells comparable with WT mice, along with essentially unchanged relative percentages of total immune cells (). Moreover, the serum levels of IFNγ, IL-10, CXCL1, and G-CSF in these mice approached that of WT levels (). In particular, IL-10 and CXCL1 levels are significantly different in the sera of Il15−/− and CD11c-Il15 mice.
Interestingly, CD11c-Il15 mice showed a re-established control of tumor growth after AOM/DSS induction in comparison with the global IL-15 deficient mouse strain. Selective IL-15 expression in CD11c+ cells resulted in a tumor burden comparable to that of WT mice and while tumor size was similar the macroscopically assessed tumor number was significantly decreased in comparison to Il15−/− mice (). Histological analysis confirmed a decrease in the number of tumor lesions, reduced dysplasia severity and an absence of invasive carcinoma in CD11c-Il15 mice compared with Il15−/− mice following AOM/DSS ().
Figure 3. Restored IL-15 expression reduces tumor formation. Colons were isolated from mice following completion of the AOM/DSS protocol. Numbers of tumors per mouse (A) and size (B) were determined. In A, each point represents an individual mouse and in B data represent mean ± SEM of three independent experiments (WT n = 31, Il15−/− n = 32, CD11c-Il15 n = 28, Il15tg n = 11). Mann–Whitney test was used for statistical analysis. (C) Representative images of colons from WT (n = 2), Il15−/− (n = 2) and CD11c-Il15 (n = 2). (D) Representative H&E images of formalin-fixed tissues from proximal, mid and distal colon. Dotted line indicates the muscularis mucosa with the overlying colon adenoma showing high-grade dysplasia. Dashed lines enclose colon adenomas with high-grade dysplasia. Scale bar: 600 μm. (E) Percentages of tumor-types (low-grade dysplasia, high-grade dysplasia, intramucosal carcinoma, adenocarcinoma) in Il15−/− vs. CD11c-Il15 mice (Il15−/− n = 26, CD11c-Il15 n = 12).
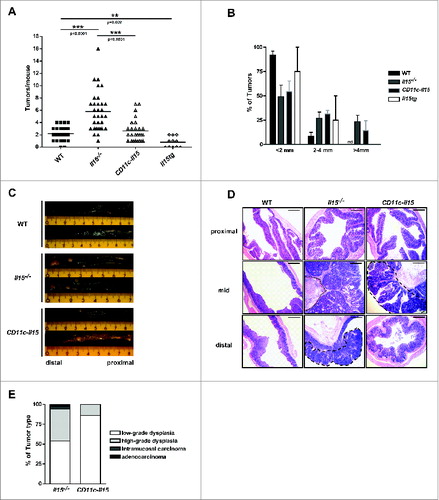
These data demonstrate that the restoration of IL-15-dependent CD8+ T and NK cell immune responses reduce tumor formation, congruent with similar effects of IL-15-mediated signaling in other tumor models.Citation1,30-32 To determine if the antitumor immunity-restoring effect of IL-15 can be further enhanced if IL-15 is ubiquitously overexpressed, AOM/DSS was induced in Il15tg mice.Citation33 These mice had a significantly reduced tumor load compared to both CD11c-Il15, and WT mice (). Thus, as expected, antitumor immunity is maximal if IL-15 is expressed, secreted, and/or trans-presented by both immune and non-immune cells. However, IL-15 overexpression restricted to antigen presenting cells, e.g., in DC therapy strategies, in a physiological situation where other cells are present that constitutively express IL-15, may well suffice to induce therapeutic antitumor immunity.
IL-15 is expressed in human colorectal cancer
In addition to its antitumor effect, IL-15 has also been reported to show tumor growth-enhancing propertiesCitation16 and is also expressed by epithelial cell populations.Citation14,34 We therefore sought to determine if IL-15 expression was altered in a pathophysiological setting. IL-15 expression was profiled in a large cohort of patients with CRC, including 10 cases of CAC and tissue arrays encompassing 74 cases of spontaneous colorectal carcinoma (SCRC).
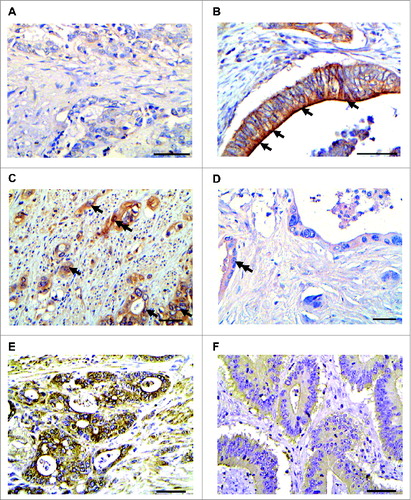
In almost all of the examined CRC patients, CAC and SCRC cells exhibited IL-15 immunoreactivity with varying extent and intensity ( and Table S1 and S2). IL-15 localization was mainly membranous/cytoplasmic () with little IL-15 nuclear staining () the latter having been reported previously for macrophages.Citation35 However, no significant correlation was found between the semi-quantitative IL-15 protein expression score and the TNM stage (Table S1). This shows that while IL-15 protein is prominently expressed in human CRC, its relationship with cytokine expression and tumor characteristics remains unclear.
Figure 4. IL-15 is expressed in human colorectal cancer. (A and B) IL-15 immunostaining in CAC cases. In A, faint cytoplasmic IL-15 immunostaining in CAC4 (IL-15 Score:1). In B, arrows denote cancerous glands with intense membranous/cytoplasmic immunostaining. (CAC n = 10). (C and D) Representative images of CAC expressing intense cytoplasmic/membranous IL-15 immunostaining (C, CAC6, Score:9), SCRC carcinoma exhibiting faint IL-15 immunostaining (D, SColon48, Score:2), Case SColon6 (E), exhibiting intense IL-15 immunostaining (low IL-15 score; score:9) and case SColon16 (F) displaying faint IL-15 immunostaining (high IL-15 score; score:2). (CAC n = 10 and Scolon n = 74). Arrows indicate cancerous glands with intense membranous/cytoplasmic and double arrows depict nuclear IL-15 immunostaining. Scale bar: 50 μm.
Key colonic oncogenic pathways do not appear to be altered by IL-15
As a first step toward dissecting the potential dual role of IL-15 in tumor immunology, we asked whether IL-15 impacts on the expression of key colonic oncogenic pathways. To this purpose, we analyzed the protein expression of key molecules known to associate with murine colon tumorigenesis,Citation36 namely β-catenin, p53 and p21WAF1 in colon tumors from WT, Il15−/−, Il15rα−/−, and CD11c-Il15 mice following AOM/DSS induction.
All colon adenomas of AOM/DSS-treated mice exhibited cytoplasmic/nuclear β-catenin staining, irrespective of their genotype and IL-15 expression status (Fig. S3A). In all genotypes, p53 immunostaining was limited and faint, accompanied by strong p21WAF1 staining within colon adenomas, again without appreciable differences between the various mice strains examined (Fig. S3B). Collectively, these ex vivo-results from established colon tumors in IL-15 deletion mouse strains suggest that IL-15 does not induce classical pathways implicated in murine colon tumorigenesis.
Tumor transcriptional profile analysis correlate IL-15 loss with an increase in pro-inflammatory cytokines associated with colon cancer progression
To further determine the role of IL-15 in colon cancer, we looked at its ability to modulate the inflammatory tumor microenvironment. We utilized microarray analysis of tumor, perilesional colon, and non-tumor-affected colon tissue from IL-15 disrupted and WT AOM/DSS mice. Principal component analysis (PCA) based on 2D scaling divided samples into three different groups of comparable genes expression data space: group 1 included tumor tissue from Il15−/− mice, group 2 included near-tumor and tumor tissue from WT and group 3 included tumor-free tissue from WT and Il15−/− mice and the Il15−/− near-tumor tissue (Fig. S4A and S4B).
Interestingly, while tumor-free and perilesional colon showed no major gene expression differences between WT and Il15−/− mice, 1856 genes were dysregulated in Il15−/− compared with WT tumors: 201 genes with fold change of ≥2, of which 106 were upregulated and 95 downregulated ( and Fig. S4C and Table S3). These results suggest that IL-15 loss exerts a major transcriptional impact on the tumor, on tumor-infiltrating cells, or both.
Figure 5. Differentially-expressed genes profiles in WT and Il15−/− colon tissues. RNA from no tumor, near tumor and tumor colon tissue from WT and Il15−/− mice were used for gene expression analysis by microarrays. (A) LIMMA analysis of differentially expressed genes showing a q value < 0.05 and a log2 fold change (FC) ≥2. Pie charts show the number of significant genes (yellow) and the number significant genes that exhibit a fold change ≥2 (green). The green gene pool was used to determine the proportion of upregulated (red) and downregulated (blue) genes. (B) Fold change of top 50 upregulated and downregulated genes. Comparative heat map shows the fold change of the gene expression between WT and Il15−/− mice from no tumor, near tumor and tumor tissue from colon sections. Red and blue color intensity indicates the degree of upregulated and downregulated genes, respectively (WT n = 3, Il15−/− n = 3). The lists of genes are shown in Table S3. (C) qRT-PCR analysis showing the level of mRNA expression of indicated genes in normal and tumor tissue from WT and Il15−/− mice relative to Hprt. Data are shown as mean ± SEM of WT n = 14-17, Il15−/− n = 14-17. Results are from three independent experiments. (D) Mean fold change increase of expression levels of genes in Il15−/− vs. WT tumor tissues normalized to their respective non-lesional tissue. Mann–Whitney tests were used for statistical analysis.
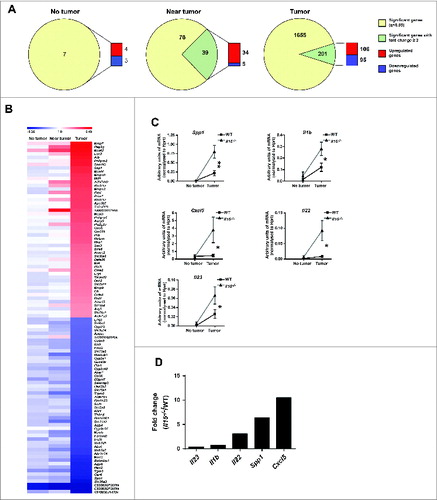
The upregulated genes in Il15−/− tumors were grouped into functionally different clusters: hydrolases/proteases (e.g., mast cell proteases and matrix metalloproteases), cytokines and cytokine/cytokine receptor interactions (e.g., IL-1α, IL-1β, chemokine CXCL14, CXCL5, and SPP1), signaling pathways implicated in cell proliferation/growth/Wnt signaling, apoptosis, oxidation/reduction reactions, and transcription factors and membrane components (Fig. S4C and Table S4). Within the top-50 upregulated genes, the majority were found in the hydrolases/proteases cluster, followed by the cytokine/cytokine receptors interaction gene cluster. Since the upregulation of hydrolases/proteases could be a secondary effect associated with tissue remodeling induced by the increased tumor growth in Il15−/− mice, we focused on the upregulation of cytokine/chemokine expression as a potentially direct IL-15-dependent phenomenon.
To confirm the microarray results, the expression levels of selected cytokine and chemokine genes that were exclusively upregulated in tumor tissue were quantified by qRT-PCR. This confirmed that not only Spp1 but also Il1b and Cxcl5 expression were strongly and significantly upregulated in Il15−/− tumors compared to WT tumors and non-tumor bearing, AOM/DSS-treated colon (). In addition, mRNA expression of the TH17 cytokines, Il22 and Il23 in lesional tissue, albeit transcribed at low levels, was significantly upregulated in Il15−/− compared to WT tumors (). These gene expression profiling data from IL-15-deficient mice suggest that IL-15 impacts on the expression of key inflammatory mediators associated with colon cancer progression and that IL-15 deficiency facilitates the creation of a tumor growth-enhancing cytokine/chemokine tumor microenvironment.
Discussion
Collectively, the current study contributes to dissecting the as yet unclear, dual role of IL-15 as a potential regulator of both pro- and antitumor immunity. We have shown that the deletion of IL-15 has a dual effect in colitis-associated murine colon carcinoma: while IL-15 deletion impairs NK/CD8+ T cell-mediated anti-colon carcinoma immunity, this may also create a cytokine/chemokine microenvironment that facilitates tumor growth.
IL-15 deficient mice have been used extensively to delineate the multifaceted role that IL-15 plays in inflammatory conditions. Most recently, deletion of IL-15 has been associated with increased disease severity in the experimental autoimmune encephalomyelitis (EAE) model of multiple sclerosis with increased CD8+ T cell influx and cytokines levels such as IL-6,Citation37 and in the TAX-LUC model of human lymphoma characterized instead by a reduction of CD8+ infiltrating T cells and IL-6.Citation38 Therefore, these findings highlight the duality of IL-15 function with respect to the CD8+ T cell compartment and the influence on IL-6 levels. Furthermore, the contribution of IL-15 toward the final disease outcome is dependent on the inflammatory context, with deletion of the cytokine in a Toxoplamsa gondii infection model resulting in reduced disease severity and mortality, with lower levels of IL-6, TNFα and IL-1β produced compared to WT mice.Citation4 Of interest to this present study, IL-15 deletion has been investigated in the DSS colitis model, with IL-15 deletion resulting in a protective effect with lower numbers of lamina propria CD8+ T cells, NK cells, and pro-inflammatory cytokines.Citation39
Our data provide novel insight into the role of IL-15 in colorectal carcinogenesis, by showing that in mice, disruption of the IL-15 signaling pathway correlates with enhanced tumorigenesis which is marked by increased serum levels of IFNγ, IL-10, IL-17, CXCL1, and G-CSF. In a different model using metastatic murine CT26 or MC38 colon carcinoma, IL-15 alone or in combination with other molecules exert a protective role in cancer.Citation32,40 Additionally, in human tissue we observed markedly increased IL-15 expression in colon neoplastic cells suggesting a regulatory effect of IL-15 in CRC.
Under physiologic conditions IL-15 exerts a tumor suppressive function; however in transformed tumor epithelial cells IL-15 promotes their survival ultimately exerting a tumor-promoting role. This provides an explanation for the initially confounding findings of Kuniyasu and colleagues (2001) supporting an oncogenic role for IL-15 by promoting the proliferation of IECs and the production of angiogenic factors.Citation14,16 Interestingly, the antitumor effect of IL-15 appears to display only partial dependence on IL-15Rα signaling. Our data show that Il15rα−/− mice are significantly less susceptible to tumor development than Il15−/−, suggestive of alternative and redundant IL-15 signaling via IL-2Rβ and IL-2Rγ pathways.
IL-15 is expressed by epithelial and non-epithelial cells and the increased incidence and accelerated onset of tumorigenesis in Il15−/− mice potentially results from a combination of epithelial damage and non-epithelial contributions and deregulated colon repair. IL-15 in chronic colitis, besides acting as a pro-inflammatory cytokine reduces mucosal damage by preventing IEC apoptosis.Citation29 Neutralization of IL-15 in chronic DSS-induced colitis increased the score of disease.Citation29 Furthermore, it has been shown that IL-15 is required for intestinal epithelial barrier integrity by regulating tight junction formation in an IL-2Rβ-dependent manner.Citation41 In this present study we find increased apoptosis in IL-15 deficient mice, with no change in proliferation, supporting the previously described role for IL-15 in colon repair.Citation29
We show that selective recovery of IL-15 expression in CD11c+ cells of Il15−/− mice significantly lowers tumor burden and decreases the severity of dysplasia compared with Il15−/− mice. CD11c is a marker of DC, which are known to trans-present IL-15 to NK and CD8+ cytotoxic lymphocytes, augmenting the “tumor-suppressor” activity of the latter.Citation1,42 Our data suggest that during AOM/DSS (a model of carcinogenesis) mice deficient in IL-15 are more susceptible to tumor development as a consequence of an increased inflammatory promoting effect on tumor growth. In our hands the restoration of CD8+ T cells and NK antitumor immunity is sufficient to overcome the protumorigenic effects of the inflammatory milieu seen in the IL-15 deficient mice. However, we do recognize that reconstitution of IL-15 in antigen presenting cells could have an additional effect on other immune cells such as macrophages. In tumor transplantation model Gillgrass and colleagues recently demonstrated that Il15−/− CD4+ T cells display a Th2 phenotype, which may be capable of polarizing macrophages to a pro-metastatic M2 phenotype while lung macrophages of Il15tg mice produce high levels of nitric oxide and IL-12, indicative of an anti-metastatic M1 polarized phenotype.Citation43,44
The increased tumorgenesis in Il15−/− mice was found to be accompanied by increased levels of specific cytokines in serum, among them, IL-17, IFNγ and IL-10. TH17 cytokines are expressed by TH17 cells and by innate lymphoid cells (ILCs) in response to IL-23 in the inflamed colon,Citation45 furthermore IL-17 has been described to promote angiogenesis and tumor growthCitation46 while IL-22 plays a dual protective and pro-inflammatory role in intestinal inflammation.Citation47,48 Interestingly, IL-22 was recently found to play a significant role for CAC developmentCitation49 and in the absence of IL-15, we have detected an induction of IL-23 and IL-23-induced cytokines. We observed an increase in IL-22 transcripts in colonic tumor together with IL-17A and IFNγ levels in sera of Il15−/− mice. In comparison with WT, we also observed a significant decrease in IL-12(p40), a shared subunit between IL-23 and IL-12 possibly indicating a more prominent role for the cytokine in this setting. Our results compliment previous studies demonstrating IL-17 correlation with worse prognosis in patients affected by colon carcinomas,Citation50 furthermore, increased IL-17 levels have been described in the mouse colonic cancer model.Citation51 Taken together, decreased IL-15 correlates with an increase in IL-22 as well as IL-17 and IFNγ, cytokines that promote active chronic colitis and progression to colon cancer. Our results therefore, support an inhibitory effect of IL-15 on type 17-responses.
The IL-15 signaling in the presence of its receptor, IL-15Rα regulates CCL5 production by T cells, DC and myeloid cells.Citation52-54 In accordance, in our model, the T and NK cell recruiting chemokine, CCL5, is strongly reduced in the sera of Il15−/− mice compared to the WT level, therefore inhibiting antitumor immunity.Citation55,56
The role of IL-15 on regulatory T cells (Tregs) is complex and controversial. The increased levels of IL-10, the main suppressive cytokine produced by Tregs, in the sera of IL-15 deficient AOM/DSS mice agrees with the inhibitory role of IL-15 on intestinal regulatory activities shown by Depaolo and colleagues.Citation57 Our data suggest that the loss of IL-15 leads to increased levels of the regulatory IL-10 and the pro-tumoral chemokine, CXCL1Citation58 which in turn results in suppression of antitumor immune responses and promotes tumor progression. Moreover, this is significantly inhibited when CD11c+ cells produce IL-15 selectively.
Microarray analysis revealed that IL-15 deficiency in tumor tissue correlates with an increased expression of inflammatory promoting genes, such as proteases, cytokines/chemokines/cytokines receptors. Collectively the results obtained from our analysis indicate that since IL-15 deficiency promotes colon-associated carcinogenesis, classical tumor markers are amplified, but that specific inflammatory mediators are induced in an IL-15-dependent manner. To confirm the latter hypothesis, it will be interesting to examine in subsequent studies whether, for example, a signaling milieu dominated by Spp1, Il1b, Cxcl5, Il22, and/or Il23, does indeed promote colon tumor development/growth. On this matter, currently we can only speculate that the differences observed in cytokine expression in the tumor microenvironment are the final result of multiple events and indirectly controlled by IL-15. IL-22, IL-23, and IL-1β have been shown to cross-regulate their expression, be pro-inflammatory and exert both tumor inhibitory and promoting effects in colon cancer and are most likely the product of infiltrating immune cells, while Spp1 and CXCL5 are likely the result of increased epithelial activity and/or colonic tumor cells. We therefore suggest that the dysregulation of inflammatory cells accompanying tumor growth seen in IL-15 deficient mice results in the skewing of the cytokine milieu.
In summary, based on the data presented here we conclude that IL-15 plays a crucial and multifaceted role in gut homeostasis. This study sheds light on the role of IL-15 in colorectal carcinogenesis, providing supporting data for the employment of IL-15 for immune boosting in cancer immunotherapy, although the anti-apoptotic function of IL-15 on epithelial tumor cells should be carefully considered.
Materials and Methods
Mice
6–10 week old aged-matched female mice were used for all experiments. C57BL/6j (WT), Il15−/−10, IL15tg (strain B1)Citation33, Il15rα−/−11, and CD11c-Il15 were used for the study, with all mice on the C57BL/6 background. CD11c-Il15 mice were generated by breeding transgenic mice, which express IL-15 under the CD11c promoter, with Il15−/− mice (Polansky et al., in preparation). Mice were maintained in specific pathogen-free conditions at the Research Center Borstel animal facilities with all in vivo procedures performed in compliance with national animal care and institutional guidelines. Protocols were approved by the “Ministerium für Landwirtschaft, Umwelt und ländliche Räume des Landes Schleswig-Holstein” (# AZ V312-72241.123-3 (12-2/10)).
Human tissue samples
Formalin-fixed paraffin-embedded specimens from 10 patients with CAC along with tissue arrays encompassing 74 cases with SCRC were examined. Patients had not received any immuno-, chemo- or, radiotherapy interventions prior to surgical resection of the lesions. All samples were obtained from the Laboratory of Histology and Embryology, Athens Medical School according to local ethical committee approvals.
AOM/DSS protocol
Colitis-induced tumorigenesis was induced by a single intraperitoneal injection of AOM (Sigma–Aldrich) (10 mg/kg) 7 d prior to three 5-d cycles of 1% (w/v) DSS (MP Biomedicals, molecular weight of 36,000–50,000) in the drinking water. DSS was alternated with 14-d cycles of drinking water. At the endpoint (day 100–120) colons were harvested, incised longitudinally, and washed with PBS. Colon weight was measured, photographs were taken (Sony Cyber-shot DSC-W55S Compact 7.2 Mpix), tumors were counted and their diameter was measured.
FACS analysis
Cells were isolated from spleen and mesenteric lymph nodes, resuspended in FACS-buffer (2% newborn calf serum, 0.1% NaN3, 2 mM EDTA in PBS), stained with mAb against CD8α (clone: 53-6.7, 100701), NKp46 (clone: 29A1.4, 137601) (all Biolegend), for 20 min at 4°C and subsequently washed with FACS buffer containing 1 μg/mL propidium iodide (Sigma–Aldrich). Cells were analyzed using a FACSCalibur flow cytometer (BD Biosciences) and Flowjo software (Treestar Inc.).
Cytokines and chemokines measurement
Cytokines (IL-1α, IL-1β, IL-2, IL-3, IL-4, IL-5, IL-6, IL-9, IL-10, IL-12 (p40), IL-12 (p70), IL-13, IL-17A, Eotaxin, G-CSF, GM-CSF, IFNγ, KC, MCP-1 (MCAF), MIP-1α, MIP-1β, CCL5, and TNF-α) were quantified from mouse sera using the Bio-Plex Mouse Cytokine Group I 23-plex Assay (Bio-Rad) according to manufacturer's instructions.
Histopathological analysis of mouse tissue
Colon tissue was fixed in 3.7% buffered formalin and embedded in paraffin and sections examined following H&E staining. Colitis severity was evaluated (designated as histological activity index) according to Ullman and Itzkowitz grading: grade 1: inactive colitis; grade 2: mild colitis; grade 3: moderate colitis; grade 4: severe colitis. Assessment of dysplasia was based on the two-tiered (low- and high-grade) system.Citation59 Histopathological changes were evaluated by three molecular pathologists blinded to the identity of the treatment group.
Immunohistochemical analysis
Immunohistochemical staining was performed on paraffin-embedded tissues as previously described,Citation60 using the following antibodies against: IL-15 (500-p15, Peprotech; 1:500), β-catenin (E-5, sc7963, Santa Cruz; 1:100), p53 (NCL-p53-CM5p, Novocastra; 1:400), p21 (F-5, sc-6246, Santa Cruz; 1:200), cleaved caspase-3 (9661L, Cell Signaling; 1:100). Heat-mediated antigen retrieval was performed in a microwave using 10 mM citric acid (pH 6.0) for 25 min. For the immunohistochemical analysis the UltraVision LP Detection System (#TL-060-HD, Thermo Scientific) was employed according to the manufacturer's instructions. A mixed score (MS) combining a staining index (SI) and a labeling index (LI) was used for the evaluation of the IL-15 expression. The SI is subdivided into four ordinal values: 0: negative, 1: weak, 2: moderate, 3: strong. LI is defined as follows: 0: negative, 1: 1–20%, 2: 21–50%, 3: 51–100% positive cells. We classified IL-15 score as follows: 0–2: low, 3–4: medium, 6–9: high. The MS is obtained by multiplying SI with LI (MS = SI × LI). Evaluation of p53, p21, β-catenin, and cleaved caspase-3 was performed as previously described.Citation60-64 Previously characterized cases served as positive controls for p53 and p21.Citation60-62 Three independent observers carried out slide examination, with minimal inter-observer variability.
RNA extraction and microarray analysis
Colon tissue (30 mg) from WT and Il15−/− mice was homogenized in 600 μL of RLT buffer using the Omni TH2 homogenizer (Omni International). RNA extraction was performed using the RNeasy mini Kit (Qiagen) as per manufacturer's instructions. First strand cDNA was synthesized using 2 μg of RNA, oligo-dT primer (5′-TTTTGTACAAGC(TTT)10-3′) and the Superscript III reverse transcriptase (Life Technologies) according to manufacturer's instructions.
Gene expression from non-tumor, near tumor and tumor sections from 3 WT and 3 Il15−/− mice were examined by microarray analysis using the Affymetrix Genechip® Mouse Gene 1.0 ST array (28,853 gene transcripts). RNA quality control, gene chip hybridization and data acquisition were performed at the Expression Core Facility, TU Munich. Arrays data were normalized using the Exon Robust Multichip Average (RMA) algorithm from the software Partek Genomic Suite (Partek Inc.) and analyzed with the Linear Model for Microarray Data (LIMMA) to identify differentially expressed genes with a q value (FDR) < 0.05 using the open-source R software, v.3.0.1. and the LIMMA and QVALUE Bioconductor Packages. Genes with a log2 fold change ≥2 were taken as input to perform gene functional clustering analysis using the Database for Annotation, Visualization and Integrated Discovery (DAVID).
qRT-PCR
Gene expression from Tumor tissues was measured by qRT-PCR using the LightCycler 480 (Roche Diagnostics), the ready-to-use hot start reaction mix (LightCycler® 480 probe master), the specific hydrolysis probes (Universal Probe Library, UPL) and primers, designed through the Universal Probe Library Assay Designed Center (www.universalprobelibrary.com) (Table S5). Gene expression values were normalized to the housekeeping gene Hprt.
Statistical analysis
Statistically significant differences were calculated by using the nonparametric Mann–Whitney test (Prism software; GraphPad Software Inc.) with *p < 0.05, **p < 0.01, ***p < 0.001 considered significant.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Authors’ Contributions
Conception and design by RB, VGG, SBP; Development of methodology by RB, ISP, OD, DAG, MP, PGF; Acquisition of data by RB, ISP, JP, OD, DAG, MP, PGF; Analysis and interpretation of data by RB, ISP, DAG, HS, KE, PGF, VGG, SBP; Writing, review, and/or revision of the manuscript by RB, MC, ISP, DAG, VGG, SBP; Study supervision by VGG, SBP.
Supplemental Material
Supplemental data for this article can be accessed on the publisher's website.
1002721_Supplemental_Information.zip
Download Zip (33.5 MB)Acknowledgments
We thank Hanno Ewers, Katrin Westphal, Katrin Streek, Gesine Rode, Frauke Koops, Platonas N. Selemenakis, and Ioannis L. Aivaliotis for technical assistance, Zane Orinska for helpful discussion and Leo Zeef (University of Manchester) for the microarrays analysis.
Funding
This work was supported in part by European Commission FP7 funding (INFLACARE agreement number 223151 and INSPiRE agreement number 284460) and Aristeia II from GSRT, Greece.
References
- Jakobisiak M, Golab J, Lasek W. Interleukin 15 as a promising candidate for tumor immunotherapy. Cytokine Growth Factor Rev 2011; 22:99-108; PMID:21531164; http://dx.doi.org/10.1016/j.cytogfr.2011.04.001
- van Heel DA. Interleukin 15: its role in intestinal inflammation. Gut 2006; 55:444-5; PMID:16531523; http://dx.doi.org/10.1136/gut.2005.079335
- Doherty TM, Seder RA, Sher A. Induction and regulation of IL-15 expression in murine macrophages. J Immunol 1996; 156:735-41
- Schulthess J, Meresse B, Ramiro-Puig E, Montcuquet N, Darche S, Begue B, Ruemmele F, CombadiÈre C, Di Santo JP, Buzoni-Gatel D et al. Interleukin-15-dependent NKp46+ innate lymphoid cells control intestinal inflammation by recruiting inflammatory monocytes. Immunity 2012; 37:108-21; PMID:22705105; http://dx.doi.org/10.1016/j.immuni.2012.05.013
- Giri JG, Ahdieh M, Eisenman J, Shanebeck K, Grabstein K, Kumaki S, Namen A, Park LS, Cosman D, Anderson D. Utilization of the beta and gamma chains of the IL-2 receptor by the novel cytokine IL-15. EMBO J 1994; 13:2822-30; PMID:8026467
- Grabstein KH, Eisenman J, Shanebeck K, Rauch C, Srinivasan S, Fung V, Beers C, Richardson J, Schoenborn MA, Ahdieh M et al. Cloning of a T cell growth factor that interacts with the beta chain of the interleukin-2 receptor. Science 1994; 264:965-8; PMID:8178155; http://dx.doi.org/10.1126/science.8178155
- Bamford RN, Grant AJ, Burton JD, Peters C, Kurys G, Goldman CK, Brennan J, Roessler E, Waldmann TA. The interleukin (IL) 2 receptor beta chain is shared by IL-2 and a cytokine, provisionally designated IL-T, that stimulates T-cell proliferation and the induction of lymphokine-activated killer cells. Proc Natl Acad Sci U S A 1994; 91:4940-4; PMID:8197161; http://dx.doi.org/10.1073/pnas.91.11.4940
- Dubois S, Mariner J, Waldmann TA, Tagaya Y. IL-15Ralpha recycles and presents IL-15 In trans to neighboring cells. Immunity 2002; 17:537-47; PMID:12433361; http://dx.doi.org/10.1016/S1074-7613(02)00429-6
- Castillo EF, Schluns KS. Regulating the immune system via IL-15 transpresentation. Cytokine 2012; 59:479-90; PMID:22795955; http://dx.doi.org/10.1016/j.cyto.2012.06.017
- Kennedy MK, Glaccum M, Brown SN, Butz EA, Viney JL, Embers M, Matsuki N, Charrier K, Sedger L, Willis CR et al. Reversible defects in natural killer and memory CD8 T cell lineages in interleukin 15-deficient mice. J Exp Med 2000; 191:771-80; PMID:10704459; http://dx.doi.org/10.1084/jem.191.5.771
- Lodolce JP, Boone DL, Chai S, Swain RE, Dassopoulos T, Trettin S, Ma A. IL-15 receptor maintains lymphoid homeostasis by supporting lymphocyte homing and proliferation. Immunity 1998; 9:669-76; PMID:9846488; http://dx.doi.org/10.1016/S1074-7613(00)80664-0
- Hodge DL, Yang J, Buschman MD, Schaughency PM, Dang H, Bere W, Yang Y, Savan R, Subleski JJ, Yin XM et al. Interleukin-15 enhances proteasomal degradation of bid in normal lymphocytes: implications for large granular lymphocyte leukemias. Cancer Res 2009; 69:3986-94; PMID:19366803; http://dx.doi.org/10.1158/0008-5472.CAN-08-3735
- Shah MV, Zhang R, Irby R, Kothapalli R, Liu X, Arrington T, Frank B, Lee NH, Loughran TP Jr. Molecular profiling of LGL leukemia reveals role of sphingolipid signaling in survival of cytotoxic lymphocytes. Blood 2008; 112:770-81; PMID:18477771; http://dx.doi.org/10.1182/blood-2007-11-121871
- Kuniyasu H, Ohmori H, Sasaki T, Sasahira T, Yoshida K, Kitadai Y, Fidler IJ. Production of interleukin 15 by human colon cancer cells is associated with induction of mucosal hyperplasia, angiogenesis, and metastasis. Clin Cancer Res 2003; 9:4802-10; PMID:14581351
- Trentin L, Cerutti A, Zambello R, Sancretta R, Tassinari C, Facco M, Adami F, Rodeghiero F, Agostini C, Semenzato G. Interleukin-15 promotes the growth of leukemic cells of patients with B-cell chronic lymphoproliferative disorders. Blood 1996; 87:3327-35; PMID:8605349
- Kuniyasu H, Oue N, Nakae D, Tsutsumi M, Denda A, Tsujiuchi T, Yokozaki H, Yasui W. Interleukin-15 expression is associated with malignant potential in colon cancer cells. Pathobiology 2001; 69:86-95; PMID:11752902; http://dx.doi.org/10.1159/000048761
- Mishra A, Liu S, Sams GH, Curphey DP, Santhanam R, Rush LJ, Schaefer D, Falkenberg LG, Sullivan L, Jaroncyk L et al. Aberrant overexpression of IL-15 initiates large granular lymphocyte leukemia through chromosomal instability and DNA hypermethylation. Cancer Cell 2012; 22:645-55; PMID:23153537; http://dx.doi.org/10.1016/j.ccr.2012.09.009
- Grivennikov SI. Inflammation and colorectal cancer: colitis-associated neoplasia. Semin Immunopathol 2012; 35:229-44; PMID:23161445; http://dx.doi.org/10.1007/s00281-012-0352-6
- Wang K, Grivennikov SI, Karin M. Implications of anti-cytokine therapy in colorectal cancer and autoimmune diseases. Ann Rheum Dis 2013; 72 Suppl 2:ii100-3; PMID:23253923; http://dx.doi.org/10.1136/annrheumdis-2012-202201
- Meira LB, Bugni JM, Green SL, Lee CW, Pang B, Borenshtein D, Rickman BH, Rogers AB, Moroski-Erkul CA, McFaline JL et al. DNA damage induced by chronic inflammation contributes to colon carcinogenesis in mice. J Clin Invest 2008; 118:2516-25; PMID:18521188; http://dx.doi.org/10.1172/jci35073
- Mangerich A, Knutson CG, Parry NM, Muthupalani S, Ye W, Prestwich E, Cui L, McFaline JL, Mobley M, Ge Z et al. Infection-induced colitis in mice causes dynamic and tissue-specific changes in stress response and DNA damage leading to colon cancer. Proc Natl Acad Sci U S A 2012; 109:E1820-9; PMID:22689960; http://dx.doi.org/10.1073/pnas.1207829109
- Bondurant KL, Lundgreen A, Herrick JS, Kadlubar S, Wolff RK, Slattery ML. Interleukin genes and associations with colon and rectal cancer risk and overall survival. Inter J Cancer 2013; 132:905-15; PMID:22674296; http://dx.doi.org/10.1002/ijc.27660
- Graziano F, Ruzzo A, Canestrari E, Loupakis F, Santini D, Rulli E, Humar B, Galluccio N, Bisonni R, Floriani I et al. Variations in the interleukin-1 receptor antagonist gene impact on survival of patients with advanced colorectal cancer. Pharmacogenomics J 2009; 9:78-84; PMID:19104506; http://dx.doi.org/10.1038/tpj.2008.16
- Lurje G, Hendifar AE, Schultheis AM, Pohl A, Husain H, Yang D, Manegold PC, Ning Y, Zhang W, Lenz HJ et al. Polymorphisms in interleukin 1 beta and interleukin 1 receptor antagonist associated with tumor recurrence in stage II colon cancer. Pharmacogenetics Genomics 2009; 19:95-102; PMID:18987561; http://dx.doi.org/10.1097/FPC.0b013e32831a9ad1
- Nieminen U, Jussila A, Nordling S, Mustonen H, Farkkila MA. Inflammation and disease duration have a cumulative effect on the risk of dysplasia and carcinoma in IBD: a case-control observational study based on registry data. Int J Cancer 2014; 134:189-96; PMID:23797639; http://dx.doi.org/10.1002/ijc.28346
- Elinav E, Nowarski R, Thaiss CA, Hu B, Jin C, Flavell RA. Inflammation-induced cancer: crosstalk between tumours, immune cells and microorganisms. Nat Rev Cancer 2013; 13:759-71; PMID:24154716; http://dx.doi.org/10.1038/nrc3611
- Okayasu I, Ohkusa T, Kajiura K, Kanno J, Sakamoto S. Promotion of colorectal neoplasia in experimental murine ulcerative colitis. Gut 1996; 39:87-92; PMID:8881816; http://dx.doi.org/10.1136/gut.39.1.87
- Neufert C, Becker C, Neurath MF. An inducible mouse model of colon carcinogenesis for the analysis of sporadic and inflammation-driven tumor progression. Nat Protoc 2007; 2:1998-2004; PMID:17703211; http://dx.doi.org/10.1038/nprot.2007.279
- Obermeier F, Hausmann M, Kellermeier S, Kiessling S, Strauch UG, Duitman E, Bulfone-Paus S, Herfarth H, Bock J, Dunger N et al. IL-15 protects intestinal epithelial cells. Eur J Immunol 2006; 36:2691-9; PMID:16981178; http://dx.doi.org/10.1002/eji.200535173
- Steel JC, Waldmann TA, Morris JC. Interleukin-15 biology and its therapeutic implications in cancer. Trends Pharmacol Sci 2012; 33:35-41; PMID:22032984; http://dx.doi.org/10.1016/j.tips.2011.09.004
- Ochoa MC, Fioravanti J, Rodriguez I, Hervas-Stubbs S, Azpilikueta A, Mazzolini G, Gúrpide A, Prieto J, Pardo J, Berraondo P et al. Antitumor immunotherapeutic and toxic properties of an HDL-conjugated chimeric IL-15 fusion protein. Cancer Res 2013; 73:139-49; PMID:23149919; http://dx.doi.org/10.1158/0008-5472.CAN-12-2660
- Zhang M, Yao Z, Dubois S, Ju W, Muller JR, Waldmann TA. Interleukin-15 combined with an anti-CD40 antibody provides enhanced therapeutic efficacy for murine models of colon cancer. Proc Natl Acad Sci U S A 2009; 106:7513-8; PMID:19383782; http://dx.doi.org/10.1073/pnas.0902637106
- Marks-Konczalik J, Dubois S, Losi JM, Sabzevari H, Yamada N, Feigenbaum L, Waldmann TA, Tagaya Y. IL-2-induced activation-induced cell death is inhibited in IL-15 transgenic mice. Proc Natl Acad Sci U S A 2000; 97:11445-50; PMID:11016962; http://dx.doi.org/10.1073/pnas.200363097
- Reinecker HC, MacDermott RP, Mirau S, Dignass A, Podolsky DK. Intestinal epithelial cells both express and respond to interleukin 15. Gastroenterology 1996; 111:1706-13; PMID:8942753; http://dx.doi.org/10.1016/S0016-5085(96)70036-7
- Nishimura H, Fujimoto A, Tamura N, Yajima T, Wajjwalku W, Yoshikai Y. A novel autoregulatory mechanism for transcriptional activation of the IL-15 gene by a nonsecretable isoform of IL-15 generated by alternative splicing. FASEB J 2005; 19:19-28; PMID:15629891; http://dx.doi.org/10.1096/fj.04-2633com
- Chen J, Huang XF. The signal pathways in azoxymethane-induced colon cancer and preventive implications. Cancer Biol Ther 2009; 8:1313-7; PMID:19502780; http://dx.doi.org/10.4161/cbt.8.14.8983
- Gomez-Nicola D, Spagnolo A, Guaza C, Nieto-Sampedro M. Aggravated experimental autoimmune encephalomyelitis in IL-15 knockout mice. Exp Neurol 2010; 222:235-42; PMID:20070942; http://dx.doi.org/10.1016/j.expneurol.2009.12.034
- Rauch DA, Harding JC, Ratner L. IL-15 deficient tax mice reveal a role for IL-1alpha in tumor immunity. PloS One 2014; 9:e85028; PMID:24416335; http://dx.doi.org/10.1371/journal.pone.0085028
- Yoshihara K, Yajima T, Kubo C, Yoshikai Y. Role of interleukin 15 in colitis induced by dextran sulphate sodium in mice. Gut 2006; 55:334-41; PMID:16162679; http://dx.doi.org/10.1136/gut.2005.076000
- Yu P, Steel JC, Zhang M, Morris JC, Waldmann TA. Simultaneous blockade of multiple immune system inhibitory checkpoints enhances antitumor activity mediated by interleukin-15 in a murine metastatic colon carcinoma model. Clin Cancer Res 2010; 16:6019-28; PMID:20924130; http://dx.doi.org/10.1158/1078-0432.CCR-10-1966
- Nishiyama R, Sakaguchi T, Kinugasa T, Gu X, MacDermott RP, Podolsky DK, Reinecker HC. Interleukin-2 receptor beta subunit-dependent and -independent regulation of intestinal epithelial tight junctions. J Biol Chem 2001; 276:35571-80; PMID:11466322; http://dx.doi.org/10.1074/jbc.M106013200
- Epardaud M, Elpek KG, Rubinstein MP, Yonekura AR, Bellemare-Pelletier A, Bronson R, Hamerman JA, Goldrath AW, Turley SJ. Interleukin-15/interleukin-15R alpha complexes promote destruction of established tumors by reviving tumor-resident CD8+ T cells. Cancer Res 2008; 68:2972-83; PMID:18413767; http://dx.doi.org/10.1158/0008-5472.CAN-08-0045
- Davies E, Reid S, Medina MF, Lichty B, Ashkar AA. IL-15 has innate anti-tumor activity independent of NK and CD8 T cells. J Leukocyte Biol 2010; 88:529-36; PMID:20538758; http://dx.doi.org/10.1189/jlb.0909648
- Gillgrass A, Gill N, Babian A, Ashkar AA. The absence or overexpression of IL-15 drastically alters breast cancer metastasis via effects on NK cells, CD4 T cells, and macrophages. J Immunol 2014; 193:6184-91; PMID:25355926; http://dx.doi.org/10.4049/jimmunol.1303175
- Buonocore S, Ahern PP, Uhlig HH, Ivanov, II, Littman DR, Maloy KJ, Powrie F. Innate lymphoid cells drive interleukin-23-dependent innate intestinal pathology. Nature 2010; 464:1371-5; PMID:20393462; http://dx.doi.org/10.1038/nature08949
- Numasaki M, Fukushi J, Ono M, Narula SK, Zavodny PJ, Kudo T, Robbins PD, Tahara H, Lotze MT. Interleukin-17 promotes angiogenesis and tumor growth. Blood 2003; 101:2620-7; PMID:12411307; http://dx.doi.org/10.1182/blood-2002-05-1461
- Sugimoto K, Ogawa A, Mizoguchi E, Shimomura Y, Andoh A, Bhan AK, Blumberg RS, Xavier RJ, Mizoguchi A. IL-22 ameliorates intestinal inflammation in a mouse model of ulcerative colitis. J Clin Invest 2008; 118:534-44; PMID:18172556; http://dx.doi.org/10.1172/jci33194
- Andoh A, Zhang Z, Inatomi O, Fujino S, Deguchi Y, Araki Y, Tsujikawa T, Kitoh K, Kim-Mitsuyama S, Takayanagi A et al. Interleukin-22, a member of the IL-10 subfamily, induces inflammatory responses in colonic subepithelial myofibroblasts. Gastroenterology 2005; 129:969-84; PMID:16143135; http://dx.doi.org/10.1053/j.gastro.2005.06.071
- Kirchberger S, Royston DJ, Boulard O, Thornton E, Franchini F, Szabady RL, Harrison O, Powrie F. Innate lymphoid cells sustain colon cancer through production of interleukin-22 in a mouse model. J Exp Med 2013; 210:917-31; PMID:23589566; http://dx.doi.org/10.1084/jem.20122308
- Tosolini M, Kirilovsky A, Mlecnik B, Fredriksen T, Mauger S, Bindea G, Berger A, Bruneval P, Fridman WH, PagÈs F et al. Clinical impact of different classes of infiltrating T cytotoxic and helper cells (Th1, th2, treg, th17) in patients with colorectal cancer. Cancer Res 2011; 71:1263-71; PMID:21303976; http://dx.doi.org/10.1158/0008-5472.CAN-10-2907
- Wu S, Rhee KJ, Albesiano E, Rabizadeh S, Wu X, Yen HR, Huso DL, Brancati FL, Wick E, McAllister F et al. A human colonic commensal promotes colon tumorigenesis via activation of T helper type 17 T cell responses. Nat Med 2009; 15:1016-22; PMID:19701202; http://dx.doi.org/10.1038/nm.2015
- Perera LP, Goldman CK, Waldmann TA. IL-15 induces the expression of chemokines and their receptors in T lymphocytes. J Immunol 1999; 162:2606-12; PMID:10072502
- Chen JP, Liao NS, Lai SL, Hsu L, Mao WY, Ku MC, Liao F. Reduced 2,4-dinitro-1-fluorobenzene-induced contact hypersensitivity response in IL-15 receptor alpha-deficient mice correlates with diminished CCL5/RANTES and CXCL10/IP-10 expression. Eur J Immunol 2005; 35:690-8; PMID:15719370; http://dx.doi.org/10.1002/eji.200425577
- Chenoweth MJ, Mian MF, Barra NG, Alain T, Sonenberg N, Bramson J, Lichty BD, Richards CD, Ma A, Ashkar AA. IL-15 can signal via IL-15Ralpha, JNK, and NF-kappaB to drive RANTES production by myeloid cells. J Immunol 2012; 188:4149-57; PMID:22447977; http://dx.doi.org/10.4049/jimmunol.1101883
- Lavergne E, Combadiere C, Iga M, Boissonnas A, Bonduelle O, Maho M, DebrÉ P, Combadiere B. Intratumoral CC chemokine ligand 5 overexpression delays tumor growth and increases tumor cell infiltration. J Iimmunol 2004; 173:3755-62; PMID:15356122; http://dx.doi.org/10.4049/jimmunol.173.6.3755
- Nesbeth Y, Scarlett U, Cubillos-Ruiz J, Martinez D, Engle X, Turk MJ, Conejo-Garcia JR. CCL5-mediated endogenous antitumor immunity elicited by adoptively transferred lymphocytes and dendritic cell depletion. Cancer Res 2009; 69:6331-8; PMID:19602595; http://dx.doi.org/10.1158/0008-5472.CAN-08-4329
- DePaolo RW, Abadie V, Tang F, Fehlner-Peach H, Hall JA, Wang W, Marietta EV, Kasarda DD, Waldmann TA, Murray JA et al. Co-adjuvant effects of retinoic acid and IL-15 induce inflammatory immunity to dietary antigens. Nature 2011; 471:220-4; PMID:21307853; http://dx.doi.org/10.1038/nature09849
- Baier PK, Eggstein S, Wolff-Vorbeck G, Baumgartner U, Hopt UT. Chemokines in human colorectal carcinoma. Anticancer Res 2005; 25:3581-4; PMID:16101183
- Ullman TA, Itzkowitz SH. Intestinal inflammation and cancer. Gastroenterology 2011; 140:1807-16; PMID:21530747; http://dx.doi.org/10.1053/j.gastro.2011.01.057
- Gorgoulis VG, Vassiliou LV, Karakaidos P, Zacharatos P, Kotsinas A, Liloglou T, Venere M, Ditullio RA Jr, Kastrinakis NG, Levy B et al. Activation of the DNA damage checkpoint and genomic instability in human precancerous lesions. Nature 2005; 434:907-13; PMID:15829965; http://dx.doi.org/10.1038/nature03485
- Cooks T, Pateras IS, Tarcic O, Solomon H, Schetter AJ, Wilder S, Lozano G, Pikarsky E, Forshew T, Rosenfeld N et al. Mutant p53 prolongs NF-kappaB activation and promotes chronic inflammation and inflammation-associated colorectal cancer. Cancer Cell 2013; 23:634-46; PMID:23680148; http://dx.doi.org/10.1016/j.ccr.2013.03.022
- Schramek D, Kotsinas A, Meixner A, Wada T, Elling U, Pospisilik JA, Neely GG, Zwick RH, Sigl V, Forni G et al. The stress kinase MKK7 couples oncogenic stress to p53 stability and tumor suppression. Nat Genet 2011; 43:212-9; PMID:21317887; http://dx.doi.org/10.1038/ng.767
- Kotsinas A, Evangelou K, Zacharatos P, Kittas C, Gorgoulis VG. Proliferation, but not apoptosis, is associated with distinct beta-catenin expression patterns in non-small-cell lung carcinomas: relationship with adenomatous polyposis coli and G(1)-to S-phase cell-cycle regulators. Am J Pathol 2002; 161:1619-34; PMID:12414510; http://dx.doi.org/10.1016/S0002-9440(10)64440-9
- Gupta J, del Barco Barrantes I, Igea A, Sakellariou S, Pateras IS, Gorgoulis VG, Nebreda AR. Dual function of p38alpha MAPK in colon cancer: suppression of colitis-associated tumor initiation but requirement for cancer cell survival. Cancer Cell 2014; 25:484-500; PMID:24684847; http://dx.doi.org/10.1016/j.ccr.2014.02.019
