Abstract
Pancreatic ductal adenocarcinoma (PDAC) represents one of the deadliest cancers in the world. PDAC cells activate tumor-specific immune responses but simultaneously trigger a strong immunosuppression. We showed that PDAC cells produce high amount of chronic inflammatory mediators and PDAC tumors build an immunosuppressive cytokine milieu, which correlates with tumor progression. We observed a low frequency of dendritic cells (DC) and a pronounced accumulation of macrophages and myeloid-derived suppressor cells (MDSC) in murine PDAC tumors. A strong accumulation of MDSC has also been demonstrated in the peripheral blood of resected PDAC patients. While DC and macrophages seem not to play a significant role in this PDAC model in the context of immunosuppression, MDSC are highly suppressive, and their accumulation is associated with an increase in intratumoral VEGF concentration during the PDAC progression. Application of the phosphodiesterase-5 inhibitor sildenafil led to a prolonged survival of PDAC-bearing female mice, which was due to the decrease in MDSC frequencies and in the systemic VEGF level. This led to a restoration of anticancer immune responses, manifested in the recovery of T lymphocyte functions and in an increase in the frequency of conventional CD4+ T cells in tumors and IFNγ level in serum of PDAC-bearing mice. Thus, MDSC are strongly involved in the PDAC-associated immunosuppression and that their depletion could create new approaches for therapy of PDAC.
Introduction
PDAC is one of the deadliest cancers in the world.Citation1 Patients with PDAC have an especially poor prognosis, with 5-y survival rates of only ∼1% and median survival of 4–6 mo. Upon tumor resection, 5-y survival rates increase to approximately 15%, and a 25% survival is attained in the context of adjuvant chemotherapy.Citation2 The reasons for such a poor prognosis are multiple, including rapid tumor dissemination, and latent nonspecific symptoms, which are associated with a delayed diagnosis.Citation3 Moreover, PDAC is highly resistant to both conventional chemotherapies and targeted therapies. Currently, regimens based on gemcitabine or 5-fluorouracil constitute the standard therapeutic approach to advanced PDAC.Citation4 Therefore, the clinical impact of these treatments is very limited and new therapeutic options are urgently needed to improve the survival of PDAC patients. In this context, immunotherapy might be considered as an attractive approach.
One of the major obstacles to the development of beneficial immunotherapeutic approaches is the tumor-induced immunosuppression; this is also a case for PDAC.Citation5 PDAC cells activate tumor-specific immune responses, manifesting by the expression of tumor-associated antigens, and by the association between intratumoral CD8+ T cell accumulation and increased patient survival.Citation5,6 However, simultaneously to the activation of antitumor immune response, PDAC triggers a strong immunosuppression,Citation5,6 which has a molecular or cellular background. Firstly, the immunosuppressive molecule CD274 (also known as B7-H1) has been shown to be upregulated in human PDAC, strongly correlating with a poor disease outcome.Citation7 On the level of adaptive immune response, the immunosuppression in PDAC is mainly triggered by regulatory T cells (Tregs),Citation6 which exhibit the effector memory phenotype, suggesting their enhanced suppressive activity and higher proliferation capacity.Citation8
Innate immune response should also have an impact on the immunosuppression in PDAC. For example, tolerogenic DC can be induced by PDAC Citation9 and promote tumor growth via inhibition of CD8+ T cells.Citation10 Tumor-infiltrated macrophages exhibiting pro- and anti-inflammatory features can also be involved in PDAC progression.Citation11 MDSC represent another group of innate immune cells with suppressive functions. In tumor-bearing hosts, MDSC comprise a heterogeneous population of immature myeloid cells that are precursors of DC, macrophages, and granulocytes.Citation12 This cell population could also be involved in the immunosuppression induced by PDAC.Citation13–15 However, a thorough immunologic characterization of these cell populations in PDAC models is not completed yet. There is an evidence indicating that in PDAC, a lack of tumor-infiltrating effector T cells strongly correlates with the enrichment of intratumoral MDSC.Citation16 Furthermore, an increase in MDSC frequencies in the peripheral blood of PDAC patients has been found to be an independent prognostic factor for their survival.Citation17 Taking these data into account, it has been proposed that the elimination of MDSC could be a promising strategy to enhance the effectiveness of cancer immunotherapy,Citation15 and in particular, PDAC immunotherapy.Citation6 However, a selective depletion of MDSC with antibodies to achieve a therapeutic efficacy remains elusive because of the high MDSC heterogeneity.
The aim of this work was to characterize potential immunosuppressive myeloid lineage within the tumor-infiltrating leukocytes (TIL) in an orthotopic mouse model of PDAC. The results of our work present evidence that MDSC are mostly involved in PDAC-associated immunosuppression and the decrease in MDSC frequencies prolongs the survival of female PDAC-bearing mice.
Results
Cytokine milieu in PDAC
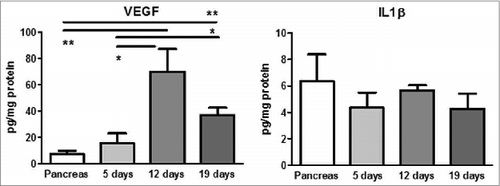
Cytokine levels were analyzed in supernatants of the cultivated Panc02 murine cells and in PDAC tumors from the Panc02 orthotopic model. We found a high amount of IL-13, TGF-β, and VEGF. IL-6, IL-10, and KC have been also produced by the cells (Table S1). Low amount of INFγ, IL-1β, IL-2, and IL-17 was found in supernatant and/or in tumors of Panc02 (Table S1. In our recent work, we observed a steady increase in TGF-β during tumor progression with the concomitant enhancement of INFγ production.Citation8 In this study, we additionally showed an increase in the VEGF concentration during the tumor progression and lack of changing in the IL-1β amount (). These data point out the importance of chronic inflammatory mediators for PDAC, which can build an innate immunosuppressive milieu.
DC in tumor-infiltrated leukocytes from PDAC-bearing mice
In tumor of PDAC-bearing mice, a little amount of cDC (1.75 ± 1.00% of TIL) and pDC (0.15 ± 0.05% of TIL) cells have been found in the leukocyte gate (Fig. S2). While practically all cDC cells were maturated (93 ± 4% cells were I-Ab positive), only 38 ± 2% of pDC were found to have a maturated phenotype (Fig. S2, and data not shown).
A co-cultivation of isolated CD11c+ cells with splenocytes from tumor-bearing mice induced a decrease in frequencies of CD69+ and CD25+ CD4+ T lymphocytes (Fig. S3A). No effects were found on the activation markers on CD8+ T lymphocytes (data not shown). However, while no pronounced effect on CD8+ T cell proliferation was detected (data not shown), an increase in proliferation of CD4+ T cell was uncovered (Fig. S3A). A profile of chronic inflammatory factors was determined in the supernatant of co-cultures after co-cultivation. An increase in the INFγ, IL-1β, IL-6, IL-10, and VEGF level was found after incubation of splenocytes with CD11c+ cells even without stimulation with CD3/CD28 antibody. A co-cultivation of splenocytes with CD11c+ cells after the stimulation led to a pronounced increase in a concentration of these mediators (Fig. S4). Interestingly, the IL-17 level was highly increased after the co-cultivation only in combination with the activation (Fig. S4).
CD11b+Gr1−F4/80+ cells in TIL from PDAC-bearing mice
Analyzing CD11b+Gr1−F4/80+ leukocytes in the mouse tumors, we found 50 ± 6% of such cells within TIL (Fig. S2, and data not shown). In addition, such accumulation correlated positively with the tumor volume (r = 0.77, p < 0.0001). However, only 6 ± 3% of these tumor-infiltrated cells showed a phagocytic activity in vitro (data not shown). It should be stressed that only about 4.1 ± 3.0% of CD11b+Gr1−F4/80+ leukocytes were CD206 positive (data not shown). A co-cultivation of isolated CD11b+Gr1− with splenocytes from tumor-bearing mice had a trend to increase the frequency of CD25+ cells within CD4+ T lymphocytes without affecting of the frequency of CD69+ cells (Fig. S3B). Expression of neither CD25 nor CD69 was changed on CD8+ T lymphocytes (data not shown). In addition, no effect on proliferation of T lymphocytes was registered (data not shown). In these experiments, the profile of chronic inflammatory factors has been investigated in the supernatant of splenocyte co-cultures. An increase in the TNF-α, IL-10, IL-17, KC, IL-6, and VEGF level was found after incubation of splenocytes with CD11b+Gr1− cells even without stimulation with CD3/CD28 antibody, and an additional stimulation highly increased the IL-17 and IL-6 production (Fig. S4). However, IFNγ level was strongly elevated only in combination with the CD3/CD28 activation (Fig. S4).
CD11b+Gr1+ cells in TIL from PDAC-bearing mice
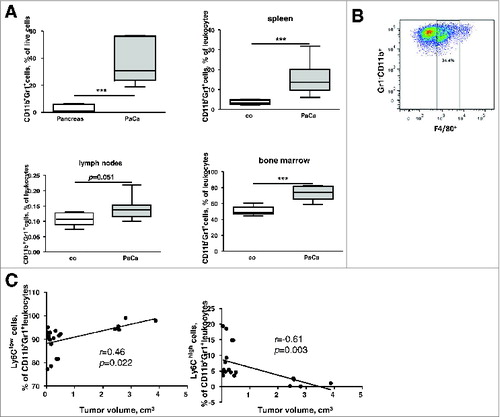
A strong tumoral accumulation of CD11b+Gr1+ myeloid cells (), both of granulocytic (CD11b+Gr1+Ly6C−/low) and monocytic origin (CD11b+Gr1+Ly6Chigh,) was found in the murine PDAC. Of note, 44 ± 8% of all CD11b+Gr1+ expressed the F4/80 marker (). It should also be mentioned that, while the percentage of the whole CD11b+Gr1+ population did not reveal any correlation with the tumor volume, the frequency of the granulocytic cells correlated positively and that of the monocytic cells negatively with the tumor volume ().
Figure 2. Phenotypic analysis of MDSC in PDAC. (A), MDSC frequencies in tumors and lymphatic organs of PDAC-bearing mice expressed as the percentage among live leukocytes. Data from two independent experiments are presented as box-and-whiskers plots (n = 5–9), ***p < 0.001. (B), a representative FACS analysis of F4/80+ MDSC (CD11b+Gr1+) subpopulation. (C), correlation of tumor volume (cm3) with the frequencies of intratumoral granulocytic and monocytic MDSC, r – Pearson correlation coefficient.
CD11b+Gr1+ myeloid cells from tumor bearing hosts – MDSC can exert their immunosuppressive functions via the production of nitric oxide (NO) and the upregulation of arginase-1 (ARG-1) expression.Citation12 We found that 88.0 ± 8.7% of MDSC from tumors of the Panc02 model express ARG-1 and 66 ± 30% MDSC were positive for an inducible nitric oxide synthase (iNOS), catalyzing the production of NO (). In ex vivo co-cultures, MDSC isolated from tumors inhibited proliferation of CD4+ and CD8+ T cells isolated from spleens of PDAC-bearing mice ().
Figure 3. PDAC-infiltrating MDSC display immunosuppressive functions. (A), representative histograms of expression of arginase-1 and iNOS in MDSC. (B), data for the inhibition of CD4+ and CD8+ T cell proliferation by MDSCs are presented as the percentage of divided T cells. T cell:MDSC ratio was 1:1. Data are presented as a mean of two independent experiments (4–6 mice for each experiment), *p < 0.05 and **p < 0.01. (C), a representative FACS picture of the CD8+ cell proliferation assay with or without addition of MDSC.
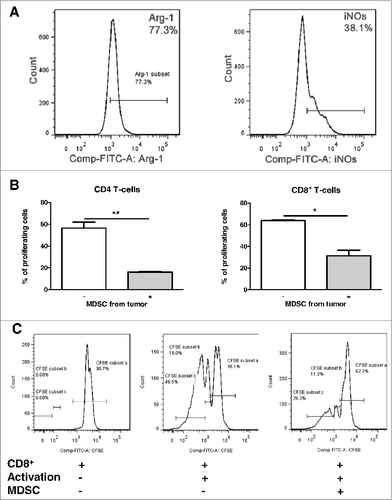
Analysis of chronic inflammatory factors in co-culture supernatants revealed an increase in the TNF-α, IL-6, IL-13, VEGF, TGF-β, and KC level after incubation of splenocytes with MDSC even without stimulation with CD3/CD28 antibodies. A co-cultivation of splenocytes with MDSC after the stimulation led to a further elevation in a concentration of VEGF, IL-13, and KC (Fig. S4). IL-1β and IL-17 concentrations were highly increased after the co-cultivation only in combination with the CD3/CD28 activation (Fig. S4).
Prolonged survival of female PDAC-bearing mice treated with sildenafil
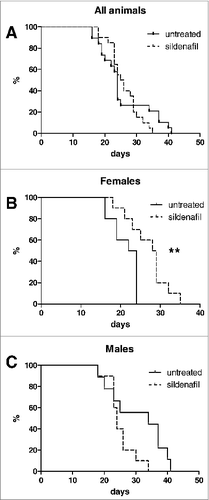
Considering our earlier data on the therapeutic properties of sildenafil in ret transgenic melanoma-bearing mice,Citation18 and suppressive potential of MDSC showed by us in PDAC, we applied sildenafil for treatment of PDAC-bearing mice beginning at day 5 after the Panc02 cell implantation. In the whole population of our experimental mice, comprising equal proportion of male and female animals, sildenafil failed to improve the survival of tumor-bearing mice ( and Table S2). However, the analysis based on the mouse gender revealed that sildenafil significantly improved the survival of female mice, but not of male ones ( and Table S2). Thus, sildenafil has indeed a positive effect on survival of PDAC-bearing mice, but this effect is gender specific.
Sildenafil-mediated MDSC decrease in female PDAC mice leads to the activation of tumor-infiltrating T lymphocytes
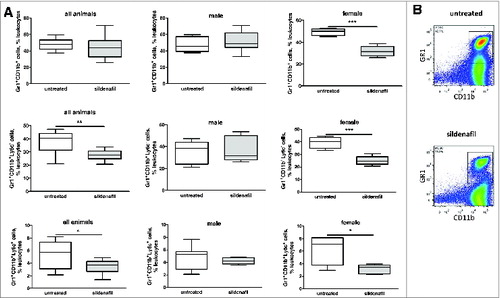
To address the mechanisms of such therapeutic effect of sildenafil we first analyzed the frequency of MDSC in tumors of sildenafil-treated mice (). Whereas the sildenafil treatment did not show any effect on the frequency of MDSC in the whole group of mice, a decrease in this cell population was observed in the tumors of female but not of male animals after the treatment.
Figure 5. Sildenafil decreases the frequency of MDSC in tumors from PDAC-bearing mice. (A), the frequency of tumor-infiltrating total MDSC and their subpopulations upon the treatment with sildenafil is shown as the percentage within live leukocytes. Data from two independent experiments are presented (n = 12–14), *p < 0.05, **p < 0.01, ***p < 0.001. (B), representative FACS pictures for a whole MDSC population from tumor-bearing mice without and with sildenafil treatment.
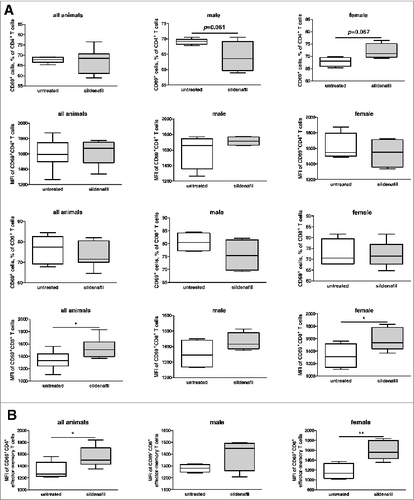
Such MDSC reduction led to a trend toward an increase in tumor infiltrating CD4+ T lymphocytes in the tumor-bearing female animals, but not in CD8+ T lymphocytes (Fig. S5). Importantly, sildenafil treatment increased the frequency of CD69+CD4+ T lymphocytes in female mice and intensity of CD69 expression on the surface of CD8+ T lymphocytes both in the whole mouse group and in the female population (). Sildenafil did not affect the percentage of different T lymphocyte subpopulations, such as naïve, effector, effector-memory, or central-memory cells (data not shown). However, this treatment increased the CD69 expression on the surface of effector-memory CD8+ T cells (). Moreover, an increase in the frequencies of activated conventional T cells (CD4+FoxP3-CD25+) was observed (Fig. S6). We found also a trend toward the reduction of regulatory T cell (CD4+FoxP3+CD25+ lymphocytes) frequencies in tumors of female mice treated with sildenafil (Fig. S6).
Figure 6. Effect of sildenafil on the amount of CD69+ T lymphocytes and CD69 expression. (A) FACS analysis of CD69 expression on CD4+ and CD8+ T lymphocytes in tumors of animals treated with sildenafil. (B) FACS analysis of CD69 expression on the surface of effector-memory CD8+ T lymphocytes in tumors of mice treated with sildenafil. Data from two independent experiments are presented (n = 5–6), *p < 0.05, control vs. sildenafil-treated tumor-bearing mice.
Analysis of the DC compartment of the mice revealed no influence of sildenafil either on the frequency of conventional and plasmocytoid DC or on their maturation state, or on the expression of co-stimulatory receptors CD80 and CD86 in both genders (data not shown). No sildenafil effects on macrophage population were observed (data not shown). B cells have been shown to be important for maturation of DC.Citation19 Therefore, we also examined the CD3−CD19+ cells in the CD45+ cell compartment. However, no effects of sildenafil on B cells have been registered.
The measurement of inflammatory factors in serum of tumor-bearing mice treated with sildenafil revealed a reduction in the VEGF level in all mice irrespective of their gender, a trend toward a decrease in concentration of IL-6 and IL-2 in all mice and in the group of female animals. In addition, significant elevation in the INFγ level in sildenafil treated female tumor-bearing mice was found (Fig. S7).
Sildenafil induces an increase in amount of the B7H1 positive immune cells but does not affect PD-1
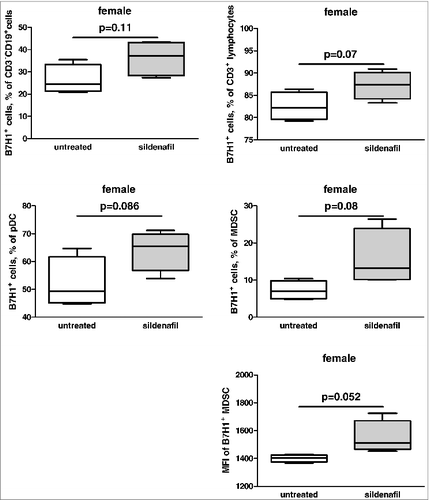
Analysis of B7H1/ PD1 pathway before and after sildenafil treatment revealed an increase in the amount of B7H1 positive B and T cells, MDSC and pDC but not cDC (). Moreover, the B7H1 expression (reflected by MFI) was higher on the MDSC surface after sildenafil treatment (). At the same time no effects have been found in respect of PD-1 expression (data not shown).
Direct effects of sildenafil on tumor cells and MDSC in vitro
To exclude the direct effects of sildenafil on the viability, proliferation, migration, and invasion of tumor cells, we performed appropriate in vitro assays using the Panc02 cell line. No effect of sildenafil was observed on the above-mentioned cell features (Fig. S8), implying the important influence of sildenafil on the activation of anticancer immune response in vivo.
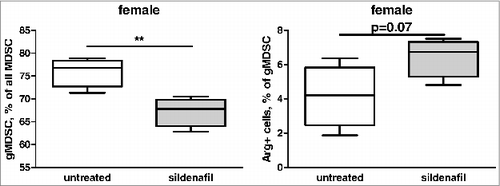
Next, we asked whether sildenafil can directly modulate the frequency of MDSC in vitro. To this end, splenocytes from tumor-bearing mice were isolated and cultured with or without sildenafil for 48 h. Afterwards, the frequency of CD11b+Gr1+ and CD11b+Gr1+Ly6low was assessed in the CD45+ cell gate. Interestingly, although the whole MDSC population showed only a trend toward reduction after the sildenafil treatment, the granulocytic subset was significantly diminished in treated animals (). In vitro cultures generally contained low frequency of Arg-1 positive cells (Fig.8). Nevertheless, the proportion of the Arg-1-expressing MDSC showed a trend toward an increase after incubation with sildenafil in vitro ().
Figure 8. In vitro direct effect of sildenafil on MDSC. FACS analysis of CD11b+Gr1+Ly6Clow cells and their Arg expression. The cells were obtained from PDAC-bearing mice and cultivated for 48 h treated with sildenafil. Data from three independent experiments are presented, **p < 0.01, control vs. sildenafil-treated splenocytes.
MDSC accumulation in peripheral blood of PDAC patients
Finally, we examined the frequency of MDSC in our cohort of resected PDAC patients. In the peripheral blood of PDAC patients, even after tumor resection, we found an accumulation of MDSC (CD11b+HLA-DR−/low) as compared to healthy donors (Fig. S9). This cell population has been further characterized by the presence of mostly granulocytic MDSC (CD15+CD14−) and, to a lesser extent, of monocytic MDSC (CD15–CD14+). Both MDSC subpopulations were enriched in PDAC patients as compared to healthy donors (Fig. S9).
Discussion
The fundamental role of the innate immune system is to protect the organisms from pathogens. In the pathological state (i.e., cancer development), components of the innate immune system can be recruited by tumor and promote tumor progression.
In this and our recent works,Citation8 we presented evidence that PDAC tumors can build a highly immunosuppressive microenvironment. Moreover, we observed an increase in the levels of IFNγ, VEGF, and TGF-β and a stable concentration of IL-2 and IL-1β during tumor progression in the Panc02 model of PDAC. Such chronic inflammatory tumor milieu has an opportunity to recruit not only the immunosuppressive cells of adaptive immune system (i.e., Treg Citation8) but also cells of the innate immunity like macrophages Citation10 and MDSC Citation20 or promote a differentiation of DC toward the tolerogenic lineage.Citation9
From these three prominent cellular populations macrophages and MDSC were strongly accumulated in PDAC tumors, though DC represented only a very small population in PDAC TIL. It has been shown in vitro that IL-6, IL-10, and TGF-β produced by human PDAC cell lines trigger immature monocyte-derived DC to be tolerogenic.Citation9 In our murine model, DC did not show a tolerogenic phenotype, were mature and could improve the proliferation capacity of the co-cultured splenocytes. On the other hand, in vitro co-cultivation of DC with splenocytes revealed a high production of chronic inflammatory factors in the supernatants, suggesting a heterogeneous DC effect on splenocytes. However, it cannot be excluded, that these factors were produced by DC during the co-cultivation with splenocytes from PDAC-bearing mice. Thus, we conclude that DC are not a major player in the context of immunosuppression in this model of PDAC.
Based on the production of soluble factors and marker profile, tumor-infiltrated macrophages can be divided in two groups: M1-polarized macrophages involved in tumor combat, and M2-polarized macrophages promoting the tumor growth.Citation21 In our model, only about 4% of all macrophages could be described as M2-macrophages based on the CD206 expression. In vitro data of co-cultivation of macrophages with splenocytes did not reveal an inhibition of the T cell proliferation. Furthermore, a slightly positive effect on the CD4+ T cell activation has been found in these experiments. In addition an enrichment of chronic inflammatory factors has been observed in the supernatant of co-cultured splenocytes and macrophages. Thus, in this model, macrophages seem to be not actively involved in immunosuppression trigged by PDAC. These results are consistent with recent data showing that PDAC tumor-infiltrated macrophages exhibit complex properties.Citation11
Whereas DC and macrophages seem not to play a significant role in this PDAC model in context of immunosuppression, MDSC are highly suppressive and their accumulation is associated with an increase in an intratumoral VEGF concentration during the tumor progression. This is in line with the fact that VEGF was shown to influence MDSC development and its production can be affected by MDSC itself.Citation22 On the other hand, our data could indicate that VEGF produced by MDSC may promote tumor progression as a strong pro-angiogenic factor.Citation23
The fundamental role of MDSC in suppressing T cell-mediated antitumor immune responses and promoting tumor progression is well documented.Citation12,24 PDAC is characterized by accumulation of MDSC, which correlates with the survival of PDAC patients.Citation14 In this study, using our cohort of PDAC patients with resected tumors and an orthotopic mouse model of PDAC, we demonstrated the enrichment of MDSC in tumor-bearing hosts even after resection of the tumor in case of PDAC patients. Furthermore, we found an improved survival of female mice bearing orthotopic PDAC upon the treatment with sildenafil, confirming an important role of MDSC in the progression of this cancer.
Previous studies in animals concerning MDSC in PDAC have been performed using the subcutaneous model or, rarely, in transgenic mice.Citation13,25,26 Using an orthotopic Panc02 model of murine PDAC, we revealed a strong accumulation of both immunosuppressive monocytic and granulocytic subpopulations of MDSC in the tumor microenvironment and lymphatic organs. These data are in accordance with earlier works performed in the subcutaneous model of PDAC.Citation25,26 Interestingly, we found for the first time that tumor progression was accompanied by an enrichment of granulocytic MDSC but simultaneously by a decrease in the frequency of monocytic MDSC. In different tumor models, the proportion of granulocytic and monocytic MDSC subsets is highly variable and depends on factors that have not yet been well investigated.Citation27 The question whether our findings are common to all tumors or represent a PDAC-specific feature should be addressed in other tumor models.
In PDAC patients, we also detected an increased MDSC frequency in the peripheral blood as compared to healthy donors, which corroborates earlier publications.Citation17,28 However, in this work we analyzed patients after a complete tumor resection. This means that increased frequency of MDSC can be found even in the absence of primary tumor, which could be explained by a presence of micrometastases maintaining further the immunosuppressive milieu. Taken together, these data underscore the important cancer-promoting role of MDSC in the PDAC-bearing hosts.
Therefore, it is tempting to speculate that the inhibition of MDSC in PDAC-bearing hosts could result in the restoration of antitumor immune responses and finally lead to better survival. However, because of heterogeneity of MDSC population, the depletion and inhibiting strategies for this cell type are still very limited. Presently, two approaches have been tested to inhibit MDSC in PDAC-bearing animals. The first attempt has been carried out with zoledronic acid using the subcutaneous murine Panc02 model of PDAC.Citation25 The authors were successful in depleting intratumoral MDSC that resulted in the restoration of antitumor immune responses and prolonged mouse survival.Citation25 Another approach represents the usage of the chemotherapeutic drug gemcitabine alone or in combination with other chemotherapeutics or with immunotherapy. This approach has also been tested in mice with subcutaneous PDAC, showing a decrease in intratumoral MDSC amount and associated with a restoration of anticancer immune responses and prolonged survival of tumor-bearing mice.Citation15,26
It has been shown that phosphodiesterase (PDE)-5 inhibitors sildenafil and tadalafil, which are used for the treatment of erectile dysfunction, pulmonary hypertension, and cardiac hypertrophy,Citation29 can exert antitumor effects in various implantation tumor mouse models by abrogating MDSC functions associated with the T cell accumulation and activation.Citation30,31 We have recently reported that sildenafil reduced the amount of chronic inflammatory factors (IL-1β, VEGF, and GM-CSF) and inhibited immunosuppressive MDSC functions, which led to the restoration of the T cell antitumor reactivity and to a significantly prolonged survival of treated mice.Citation18 In the present work, sildenafil also prolonged survival of PDAC-bearing female mice, but surprisingly had no effect in males. This effect cannot be ascribed to the direct cytotoxicity of sildenafil, but to the stimulation of anticancer immune responses. In female tumor-bearing mice, we detected a decrease in the frequency of MDSC at the tumor site and a decrease in the concentration of VEGF and IL-6 in serum. Such abrogation of immunosuppression led to the activation of CD8+ and CD4+ T cells in tumors of PDAC-bearing female mice after the treatment with sildenafil. However, an increase in INFγ level could reflect the activation of T cells, but might be contra-productive in the case of INFγ stimulation of MDSC.
It is important to note that only female mice responded to the sildenafil therapy, which was manifesting in the immunomodulation and prolonged survival. We have recently shown in healthy mice that sildenafil displayed gender-specific immunomodulatory properties.Citation32 In the same study, we also found a reduction of CD11b+Gr1+ cells in female animals after sildenafil treatment. We concluded that the mechanisms underlining gender-specific immunological effects of sildenafil can be attributed to distinct pharmacokinetics and pharmacodynamics of the drug in males and females.Citation32 In general, the gender-dependent differences in immunological parameters have been already described Citation33 and could be explained by endocrine and genetic differences between males and females.Citation34 From the genetic point of view, some X chromosome- linked genes play an important role in functions of the immune system (for review see Citation35) that could be manifested in different gender-mediated effects of some drugs on immune cells. It has been shown that melatonin possesses gender-dependent effects on the development of systemic lupus erythematosus.Citation36 Thus, our gender-specific effects of sildenafil are in the line with the above-mentioned publications.
It should be stressed that sildenafil influenced not only the cellular level of immunosuppression but also the molecular one. While sildenafil had no effects on PD1 expression, B7H1 expression was, surprisingly, increased after the sildenafil treatment on all immune cells investigated. Because of multiple roles of B7H1 in immune regulation especially in cancer and inflammation,Citation37 we cannot know for sure whether this upregulation of B7H1 expression upon sildenafil is beneficial or not in context of PDAC treatment. A detailed investigation of this sildenafil effect on B7H1 should be addressed in further studies.
Taking into account the MDSC suppressive role in PDAC and the prolonged survival of PDAC-bearing female mice after sildenafil treatment, our data suggest that targeting of MDSC with sildenafil could be an attractive option for developing novel approaches for the therapy of PDAC. Therefore, clinical studies in women suffering from PDAC should be performed to investigate the therapeutic potential of sildenafil in combination with the current standard chemotherapy.
Materials and Methods
Materials
The following anti-mouse directly conjugated monoclonal antibodies (mAbs) were used for the flow cytometry analyses (FACS): rat or hamster anti-mouse antibodies F4/80-FITC (eBioscience), CD45-Amcyan, CD4-Pacific Blue, CD3e- APC-Cy7,CD3e-V500, CD44-FITC, CD45RB-PE, CD8a-PerCP-Cy5.5, CD62L-APC, CD45R-Pacific Blue, CD80-FITC, I-A[b]-PE, CD11b-PerCP-Cy5.5, CD11c-APC, CD86-PE-Cy7, Gr-1-APC-Cy7, Ly6C-V450, Ly6G-PE, NK1.1-PE, CD69-PerCP-Cy5.5, CD25-APC, PD1-PE, Purified-Arginase-1, FITC-goat-Ig, (BD Bioscience, Germany). Fc receptor block (anti-mouse CD16/CD32), and Foxp3-FITC were purchased from eBioscience (Germany). B7H1-FITC was provided from LSBio (Seattle, USA). For the examination of human samples following directly conjugated mAbs were used: CD11b-FITC, HLA-DR-APC-Cy7, CD14-Pacific blue, CD15-PE-Cy7 (all BD Bioscience, Germany). A NOS (Nitric Oxide Synthase) detection kit was purchased from Cell Technology and used according to manufacturer's instructions. Sildenafil citrate was obtained from Sigma–Aldrich (Germany). Revatio® 20 mg tablets (containing sildenafil) were purchased from Pfizer (Germany).
Patients
Twenty to thirty mL of venous blood was collected from 9 PDAC patients after tumor resection and from 12 healthy individuals as the control. Cancer patients were recruited from the Department of General Surgery at the University Hospital Heidelberg in 2012. All patients had histological confirmed PDAC. All patients provided written informed consent. The study was approved by the ethics committee of the Medical Faculty of the University of Heidelberg. The clinical data of the patients are summarized in the Table S3.
Mice
C57BL/6 mice (6–8 weeks) were purchased from Charles River (Germany) and kept under specific pathogen-free conditions in the animal facility of the University of Heidelberg (IBF, Heidelberg). Animal experiments were carried out after approval by the authorities (Regierungspraesidium Karlsruhe).
Cell culture
The murine pancreatic adenocarcinoma cell line Panc02 was originally from Corbett et al.Citation38 Cells were cultivated in RPMI-1640 medium with 10% fetal calf serum (FCS), 100 U/mL penicillin and 100 μg/mL streptomycin, PAA Laboratories (Germany). Cells were cultivated at 37°C and 5% CO2 and routinely checked for mycoplasma contamination.
Orthotopic mouse model of pancreatic carcinoma
Syngeneic pancreatic carcinoma cells were injected orthotopically in C57BL/6 mice as described elsewhere.Citation8 Mice were narcotized with isoflurane/O2 inhalation. After achieving surgical tolerance (stage III2), mice received laparotomy with an abdominal incision. 5 μL of mycoplasma-free Panc02 cells in a concentration of 5 × 107 cells/mL in PBS were injected in the pancreatic head with a 25 μL gastight syringe (Hamilton, Reno, USA). The injection site was clamped for about 30 sec after removal of the syringe to avoid leakage. The tissue was carefully returned to its original position and all layers of the wound (peritoneum, muscles, and skin) were sutured with synthetic absorbable suture material (polysorb 6–0, Tyco Healthcare). Tumors and spleens were harvested 3 weeks after Panc02 cells implantations and used for the flow cytometry analysis.
Sildenafil treatment of mice
Drinking water for C57BL/6 mice was supplemented with sildenafil (compounded from pulverized Revatio® tablets) to yield a dose of 20 mg/kg body weight per day.Citation30 The treatment was started at day 5 after Panc02 cell implantation. The mice were sacrificed 21 d after the cell implantation and their tumors were removed to prepare single cell suspensions as described elsewhere.Citation39
Flow cytometry analysis of MDSC in peripheral blood of PDAC patients
Peripheral blood mononuclear cells (PBMC) were obtained from the blood of resected PDAC patients by gradient centrifugation using Biocoll separating solution (Biochrom AG, Germany), for 25 min, as described elsewhere.Citation40 50 μL cell suspension was incubated with 50 μL FACS buffer (supplemented with human serum albumin or FCS) containing various mAbs at 4°C for 20 min. Human MDSC were characterized as CD11b+HLA-DR−/low cells and further divided into granulocytic (CD15+CD14−) and monocytic (CD15−CD14+) MDSC subpopulations (Fig. S1). The samples were measured (at least 300,000 events) by FACS Canto II with FACSDiva Software (BD Biosciences) and the results analyzed with FlowJo Software (Tree Star, Inc., USA). All gates were set according to the corresponding FMO control.
Flow cytometry analysis of murine samples
Flow cytometry of murine leukocytes was performed as described elsewhere.Citation8 Briefly, single cell suspensions of tumors and spleens were prepared in the stain buffer (PBS supplemented with 1% mouse serum and 1 mM EDTA). The unspecific binding caused by the Fc receptors was blocked by incubating the cells with anti-mouse CD16/CD32 antibody at 4°C in the dark for 20 min. Then, 50 μL cell suspension was incubated with 50 μL of the stain buffer, containing various mAbs at 4°C for 20 min. A FoxP3 staining buffer set (eBioscience) was used for intracellular staining according to the manufacturer's instructions. The samples were measured using FACS Canto II with FACSDiva Software (BD Biosciences). Different cell subsets of DC, CD8+ T cells, CD4+ T cells, Tcon (CD4+FoxP3-), and Treg (CD4+CD25+FoxP3+) cells were characterized. Murine DC were characterized as CD11c+CD11bint/+ (cDC, conventional DC) and CD11cintCD45R+ (pDC, plasmacytoid DC). Macrophages in mice were characterized as CD11b+Gr1-F4/80+. MDSC in mice were characterized as CD11b+Gr1+ and additionally divided into monocytic (Ly6G+Ly6Chigh) and granulocytic (Ly6G+Ly6Clow/-) subsets (Fig. S2). FlowJo software (Tree Star) was used to analyze at least 300,000 events. All gates were set according to the corresponding fluorescence minus one (FMO) control.
Isolation of DC, CD11b+Gr1–, and CD11b+Gr1+ cells
MDSC, DC, and CD11b+Gr1− macrophages were isolated from freshly prepared tumor single cell suspension. DC Isolation Kit and GR1/CD11b isolation beads (all Miltenyi, Germany) according to the manufacturer's protocol. The purified cells represented the MDSC, DC, and CD11b+Gr1− macrophage populations as confirmed by FACS.
Proliferation assay
Freshly purified DC, macrophages and MDSC were tested for their suppression/activation capacity in a proliferation assay. Freshly isolated splenocytes from tumor-bearing mice were labeled with 0.5 μM CFSE proliferation dye. Afterwards, 2 × 105 cells/well splenocytes were cultured in the presence or absence of activating CD3/CD28 antibodies either alone or together with 2 × 105 DC or MDSC, or macrophages in round bottom 96-well plates for 72 h. Then, the cultures were harvested, stained with fluorescence-coupled mAbs and examined by flow cytometry. According to the expression of specific T cell markers proliferation of CD4+ and CD8+ T cells was quantified using FlowJo software by determination of CFSE fluorescence intensity.
Analysis of chronic inflammatory mediators with the Luminex assay
Analysis of IL-1β and VEGF in tumor lysates and IL-1β, VEGF, IL-6, IL-10, IL-2, IL-17, IL-13, TNF-α, TGF-β, KC (CXCL1), and INFγ in serum was performed as described elsewhere Citation8 using a MILLIPLEX® MAP Kit (Millipore GmbH, Schwalbach/TS, Germany) according to the manufacturer's instructions. The measurement was performed using a Luminex® 100/200 System.
In vitro assays
Panc02 cells were treated 48 h with different concentrations of sildenafil. After that viability, proliferation, migration and invasion assays were performed as described elsewhere.Citation41
Freshly isolated splenocytes from PDAC-bearing mice were cultivated for 48 h in presence or absence of sildenafil (750 nM) as described previously.Citation32 Afterword cells were investigated by flow cytometry.
Statistical analysis
GraphPad Prism Version 5.01 software was used for statistical analyses. Distributions of continuous variables were described by means, SE, median, 25% and 75% percentiles, and were presented as dot plots, box-and-whiskers plots or as column bar graphs. D’Agostino and Pearson omnibus normality tests were conducted to estimate the distribution of data. The null hypothesis (mean values were equal) vs. the alternative hypothesis (mean values were not equal) was tested by T-test or repeated measures ANOVA with the Bonferroni's multiple comparison post-hoc test for the normal distributed variants and by Mann–Whitney test for the data which did not pass the normality test. Survival of tumor-bearing mice was analyzed with Kaplan–Meier curves with subsequent Log-rank and Wilcoxon–Gehan tests. Correlation analyses of nonparametric data were conducted using the Spearman correlation coefficient (r). All statistical tests were two-tailed. The significance level was α = 5%.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Supplemental Material
Supplemental data for this article can be accessed on htp://dx.doi.org/10.1080/2162402X.2014.998519 the publisher's website.
998519_Figure_captions_S1-S9.docx
Download MS Word (15.4 KB)998519_Supplementary_Materials.zip
Download Zip (888.6 KB)Acknowledgments
We thank Mr. Markus Herbst, Ms. Tina Maxelon and Ms. Inna Schwarting for their excellent technical assistance. We acknowledge Prof. M. Schnurr for a fruitful discussion and a critical reading of the manuscript.
Funding
This work was supported in part by a grant from B. Braun Stiftung to AVB, a grant from Else Kröner-Fresenius-Stiftung (A2010.A124) to AVB and VU, and a grant from National Natural Science Foundation of China (No. 81402553) to YY.
References
- Warshaw AL, Fernandez-del Castillo C. Pancreatic carcinoma. N Engl J Med 1992; 326:455-65; PMID:1732772; http://dx.doi.org/10.1056/NEJM199202133260706
- Cress RD, Yin D, Clarke L, Bold R, Holly EA. Survival among patients with adenocarcinoma of the pancreas: a population-based study (United States). Cancer Causes Control 2006; 17:403-9; PMID:16596292; http://dx.doi.org/10.1007/s10552-005-0539-4
- Rosty C, Goggins M. Early detection of pancreatic carcinoma. Hematol Oncol Clin North Am 2002; 16:37-52; PMID:12063828; http://dx.doi.org/10.1016/S0889-8588(01)00007-7
- Michl P, Gress TM. Current concepts and novel targets in advanced pancreatic cancer. Gut 2013; 62:317-26; PMID:23112132; http://dx.doi.org/10.1136/gutjnl-2012-303588
- Bazhin AV, Shevchenko I, Umansky V, Werner J, Karakhanova S. Two immune faces of pancreatic adenocarcinoma: possible implication for immunotherapy. Cancer Immunol Immunother 2014; 63:59-65; PMID:24129765; http://dx.doi.org/10.1007/s00262-013-1485-8
- Bazhin AV, Bayry J, Umansky V, Werner J, Karakhanova S. Overcoming immunosuppression as a new immunotherapeutic approach against pancreatic cancer. Oncoimmunology 2013; 2:e25736; PMID:24327934; http://dx.doi.org/10.4161/onci.25736
- Loos M, Giese NA, Kleeff J, Giese T, Gaida MM, Bergmann F, Laschinger M, W Büchler M, Friess H. Clinical significance and regulation of the costimulatory molecule B7-H1 in pancreatic cancer. Cancer Lett 2008; 268:98-109; PMID:18486325; http://dx.doi.org/10.1016/j.canlet.2008.03.056
- Shevchenko I, Karakhanova S, Soltek S, Link J, Bayry J, Werner J, Umansky V, Bazhin AV. Low-dose gemcitabine depletes regulatory T cells and improves survival in the orthotopic Panc02 model of pancreatic cancer. Int J Cancer 2013; 133:98-107; PMID:23233419; http://dx.doi.org/10.1002/ijc.27990
- Bellone G, Carbone A, Smirne C, Scirelli T, Buffolino A, Novarino A, Stacchini A, Bertetto O, Palestro G, Sorio C et al. Cooperative induction of a tolerogenic dendritic cell phenotype by cytokines secreted by pancreatic carcinoma cells. J Immunol 2006; 177:3448-60; PMID:16920987; http://dx.doi.org/10.4049/jimmunol.177.5.3448
- Wormann SM, Diakopoulos KN, Lesina M, Algul H. The immune network in pancreatic cancer development and progression. Oncogene 2014; 33:2956-67; PMID:23851493; http://dx.doi.org/10.1038/onc.2013.257
- Helm O, Held-Feindt J, Grage-Griebenow E, Reiling N, Ungefroren H, Vogel I, Krüger U, Becker T, Ebsen M, Röcken C et al. Tumor-associated macrophages exhibit pro- and anti-inflammatory properties by which they impact on pancreatic tumorigenesis. Int J Cancer 2014; 135:843-61; PMID:24458546; http://dx.doi.org/10.1002/ijc.28736
- Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol 2009; 9:162-74; PMID:19197294; http://dx.doi.org/10.1038/nri2506
- Zhao F, Obermann S, von Wasielewski R, Haile L, Manns MP, Korangy F, Greten TF. Increase in frequency of myeloid-derived suppressor cells in mice with spontaneous pancreatic carcinoma. Immunology 2009; 128:141-9; PMID:19689743; http://dx.doi.org/10.1111/j.1365-2567.2009.03105.x
- Goedegebuure P, Mitchem JB, Porembka MR, Tan MC, Belt BA, Wang-Gillam A, Gillanders WE, Hawkins WG, Linehan DC. Myeloid-derived suppressor cells: general characteristics and relevance to clinical management of pancreatic cancer. Curr Cancer Drug Targets 2011; 11:734-51; PMID:21599634; http://dx.doi.org/10.2174/156800911796191024
- Ghansah T. A novel strategy for modulation of MDSC to enhance cancer immunotherapy. Oncoimmunology 2012; 1:984-5; PMID:23162780; http://dx.doi.org/10.4161/onci.20201
- Clark CE, Hingorani SR, Mick R, Combs C, Tuveson DA, Vonderheide RH. Dynamics of the immune reaction to pancreatic cancer from inception to invasion. Cancer Res 2007; 67:9518-27; PMID:17909062; http://dx.doi.org/10.1158/0008-5472.CAN-07-0175
- Gabitass RF, Annels NE, Stocken DD, Pandha HA, Middleton GW. Elevated myeloid-derived suppressor cells in pancreatic, esophageal and gastric cancer are an independent prognostic factor and are associated with significant elevation of the Th2 cytokine interleukin-13. Cancer Immunol Immunother 2011; 60:1419-30; PMID:21644036; http://dx.doi.org/10.1007/s00262-011-1028-0
- Meyer C, Sevko A, Ramacher M, Bazhin AV, Falk CS, Osen W, Borrello I, Kato M, Schadendorf D, Baniyash M et al. Chronic inflammation promotes myeloid-derived suppressor cell activation blocking antitumor immunity in transgenic mouse melanoma model. Proc Natl Acad Sci U S A 2011; 108:17111-6; PMID:21969559; http://dx.doi.org/10.1073/pnas.1108121108
- Maddur MS, Sharma M, Hegde P, Stephen-Victor E, Pulendran B, Kaveri SV, Bayry J. Human B cells induce dendritic cell maturation and favour Th2 polarization by inducing OX-40 ligand. Nat Commun 2014; 5:4092; PMID:24910129; http://dx.doi.org/10.1038/ncomms5092
- Almand B, Resser JR, Lindman B, Nadaf S, Clark JI, Kwon ED, Carbone DP, Gabrilovich DI. Clinical significance of defective dendritic cell differentiation in cancer. Clin Cancer Res 2000; 6:1755-66; PMID:10815894
- Mantovani A, Germano G, Marchesi F, Locatelli M, Biswas SK. Cancer-promoting tumor-associated macrophages: new vistas and open questions. Eur J Immunol 2011; 41:2522-5; PMID:21952810; http://dx.doi.org/10.1002/eji.201141894
- Gabrilovich D, Ishida T, Oyama T, Ran S, Kravtsov V, Nadaf S, Carbone DP. Vascular endothelial growth factor inhibits the development of dendritic cells and dramatically affects the differentiation of multiple hematopoietic lineages in vivo. Blood 1998; 92:4150-66; PMID:9834220
- Ferrara N. Vascular endothelial growth factor and the regulation of angiogenesis. Recent Prog Horm Res 2000; 55:15-35; discussion -6; PMID:11036931
- Ostrand-Rosenberg S, Sinha P. Myeloid-derived suppressor cells: linking inflammation and cancer. J Immunol 2009; 182:4499-506; PMID:19342621; http://dx.doi.org/10.4049/jimmunol.0802740
- Porembka MR, Mitchem JB, Belt BA, Hsieh CS, Lee HM, Herndon J, Gillanders WE, Linehan DC, Goedegebuure P. Pancreatic adenocarcinoma induces bone marrow mobilization of myeloid-derived suppressor cells which promote primary tumor growth. Cancer Immunol Immunother 2012; 61:1373-85; PMID:22215137; http://dx.doi.org/10.1007/s00262-011-1178-0
- Bunt SK, Mohr AM, Bailey JM, Grandgenett PM, Hollingsworth MA. Rosiglitazone and Gemcitabine in combination reduces immune suppression and modulates T cell populations in pancreatic cancer. Cancer Immunol Immunother 2013; 62:225-36; PMID:22864396; http://dx.doi.org/10.1007/s00262-012-1324-3
- Youn JI, Nagaraj S, Collazo M, Gabrilovich DI. Subsets of myeloid-derived suppressor cells in tumor-bearing mice. J Immunol 2008; 181:5791-802; PMID:18832739; http://dx.doi.org/10.4049/jimmunol.181.8.5791
- Ohki S, Shibata M, Gonda K, Machida T, Shimura T, Nakamura I, Ohtake T, Koyama Y, Suzuki S, Ohto H et al. Circulating myeloid-derived suppressor cells are increased and correlate to immune suppression, inflammation and hypoproteinemia in patients with cancer. Oncol Rep 2012; 28:453-8; PMID:22614133; http://dx.doi.org/10.3892/or.2012.1812
- Ghofrani HA, Osterloh IH, Grimminger F. Sildenafil: from angina to erectile dysfunction to pulmonary hypertension and beyond. Nat Rev Drug Discov 2006; 5:689-702; PMID:16883306; http://dx.doi.org/10.1038/nrd2030
- Serafini P, Meckel K, Kelso M, Noonan K, Califano J, Koch W, Dolcetti L, Bronte V, Borrello I. Phosphodiesterase-5 inhibition augments endogenous antitumor immunity by reducing myeloid-derived suppressor cell function. J Exp Med 2006; 203:2691-702; PMID:17101732; http://dx.doi.org/10.1084/jem.20061104
- Serafini P, Mgebroff S, Noonan K, Borrello I. Myeloid-derived suppressor cells promote cross-tolerance in B-cell lymphoma by expanding regulatory T cells. Cancer Res 2008; 68:5439-49; PMID:18593947; http://dx.doi.org/10.1158/0008-5472.CAN-07-6621
- Karakhanova S, Yang Y, Link J, Soltek S, von Ahn K, Umansky V, Werner J, Bazhin AV. Gender-specific immunological effects of the phosphodiesterase 5 inhibitor sildenafil in healthy mice. Mol Immunol 2013; 56:649-59; PMID:23911424; http://dx.doi.org/10.1016/j.molimm.2013.06.021
- Fish EN. The X-files in immunity: sex-based differences predispose immune responses. Nat Rev Immunol 2008; 8:737-44; PMID:18728636; http://dx.doi.org/10.1038/nri2394
- Klein SL. Immune cells have sex and so should journal articles. Endocrinology 2012; 153:2544-50; PMID:22434079; http://dx.doi.org/10.1210/en.2011-2120
- Libert C, Dejager L, Pinheiro I. The X chromosome in immune functions: when a chromosome makes the difference. Nat Rev Immunol 2010; 10:594-604; PMID:20651746; http://dx.doi.org/10.1038/nri2815
- Jimenez-Caliani AJ, Jimenez-Jorge S, Molinero P, Fernandez-Santos JM, Martin-Lacave I, Rubio A, Guerrero JM, Osuna C. Sex-dependent effect of melatonin on systemic erythematosus lupus developed in Mrl/Mpj-Faslpr mice: it ameliorates the disease course in females, whereas it exacerbates it in males. Endocrinology 2006; 147:1717-24; PMID:16373423; http://dx.doi.org/10.1210/en.2005-0648
- Greenwald RJ, Freeman GJ, Sharpe AH. The B7 family revisited. Annu Rev Immunol 2005; 23:515-48; PMID:15771580; http://dx.doi.org/10.1146/annurev.immunol.23.021704.115611
- Corbett TH, Roberts BJ, Leopold WR, Peckham JC, Wilkoff LJ, Griswold DP Jr, Schabel FM Jr. Induction and chemotherapeutic response of two transplantable ductal adenocarcinomas of the pancreas in C57BL/6 mice. Cancer Res 1984; 44:717-26; PMID:6692374
- Yang Y, Karakhanova S, Soltek S, Werner J, Philippov PP, Bazhin AV. In vivo immunoregulatory properties of the novel mitochondria-targeted antioxidant SkQ1. Mol Immunol 2012; 52:19-29; PMID:22591624; http://dx.doi.org/10.1016/j.molimm.2012.04.010
- Karakhanova S, Meisel S, Ring S, Mahnke K, Enk AH. ERK/p38 MAP-kinases and PI3K are involved in the differential regulation of B7-H1 expression in DC subsets. Eur J Immunol 2010; 40:254-66; PMID:19830728; http://dx.doi.org/10.1002/eji.200939289
- Karakhanova S, Golovastova M, Philippov PP, Werner J, Bazhin AV. Interlude of cGMP and cGMP/protein kinase G type 1 in pancreatic adenocarcinoma cells. Pancreas 2014; 43:784-94; PMID:24826884; http://dx.doi.org/10.1097/MPA.0000000000000104