Abstract
We propose here that cigarette smoke (CS), in addition to its established genotoxic effects, elicits chronic albeit sub-clinical immune suppression, which is a major contributor to cancer progression. This hypothesis, presented here primarily in the context of bladder cancers (BCs), is applicable to other cancers, including those without a confirmed link to smoking.
Abbreviations
| 1BBN | = | N-butyl-N-(4-hydroxybutyl) nitrosamine |
| BC | = | bladder cancer |
| CS | = | cigarette smoke |
| DC | = | dendritic cell |
| MCS | = | mainstream cigarette smoke |
| NK | = | natural killer |
| SCS | = | sidestream cigarette smoke |
| TIL | = | tumor infiltrating lymphocyte |
| T-reg | = | regulatory T cells |
Introduction
Smoking is strongly associated with BC, also referred to as transitional cell carcinoma or urothelial carcinoma. Indeed, cigarette smoking is the single biggest risk factor for BC with an estimated 40–60% causally related.Citation1 Using data from >450,000 participants in the NIH-AARP Diet & Health Study (1995–2007), Freedman et al.Citation2 concluded, “former smokers were twice as likely to develop BC as those who never smoked, and current smokers were four times more likely…smoking cessation was associated with reduced BC risk.” The current hypothesis for this epidemiological association is that arylamines from CS enter the bloodstream, and thence proceed to the kidneys where they are concentrated in the urine which is subsequently sequestered in the bladder until micturition. This prolonged exposure of the urothelium to the arylamines causes mutagenesis (formation of DNA adducts) leading to transformation.Citation3 This explanation, which posits transformation by a direct effect of the components of smoke on the target cells, is supported by ample evidence. It explains the primary event in the genesis of BC, i.e., the malignant transformation of the urothelium. In terms of the direct genotoxic effect of carcinogens in CS on the target tissue epithelium, it is also consistent with the mechanism of carcinogenesis in lung cancer (seeCitation4): lungs of smokers harbor thousands of mutations, as described recently in several studies utilizing high throughput DNA sequencing (Citation5 and subsequent studies).
Interestingly, exposure of mice to CS reveals a complex pattern of tumorigenesis, perhaps partly because mice and rats do not inhale CS, but actually avoid it.Citation6 Broadly speaking, “it is difficult to reproduce the carcinogenicity of CS in animal models”.Citation7 There are significant differences between the effects of sidestream CS (SCS) and mainstream CS (MCS), and between mice exposed to CS neonatally vs. adult mice. Specifically, with respect to BC, the results of testing of tumorigenicity of CS as a complex mixture in rodents are inconsistent. Two recent studies, among many others, make that point clearly. Ohnishi et al.Citation8 who exposed C57BL/6 mice to MCS and SCS for several months, failed to observe neoplastic or even pre-neoplastic bladder lesions, even as they did observe a transient increase in urothelial proliferation by Ki67 labeling index at 3 mo but not at any later time point. The proliferation was attributed to regenerative proliferation secondary to urothelial cytotoxicity. Kato et al.Citation9 used an initiation-promotion model instead. “After initiation of carcinogenesis with N-butyl-N-(4-hydroxybutyl) nitrosamine (BBN) followed by 22 weeks of exposure to CS, there were no significant increases in bladder tumors compared with the BBN-initiated clean air controls. There was, however, a non-significant increase in bladder tumors in the CS-exposed group, suggesting that longer exposure to CS may enhance bladder carcinogenesis in this model.” These inconsistent results in CS-elicited bladder carcinogenesis in mice are in sharp contrast to consistent bladder carcinogenesis in transgenic mouse models where SV40 T antigen expression is driven by the urothelium-specific uroplakin II promoter.
Hypothesis
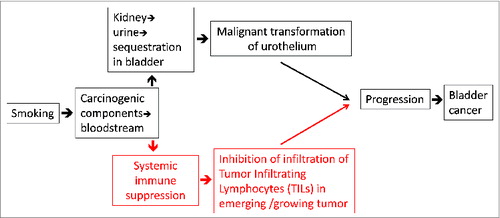
Difficulties in reproducing the effects of CS in animal models, and the many reasons for inconsistencies, have been discussed elsewhere,Citation6,9 and in the primary papers documenting the inconsistent results. Here, we contribute to that discussion by suggesting that in addition to exerting its undisputed genotoxic effects, smoking acts by mediating immune suppression, which promotes progression of the transformed urothelium into a full-grown cancer. Some previous investigators have occasionally referred to immune suppression as a consequence of exposure to CS (discussed later); however, the explicit suggestion that smoking-mediated immune suppression is an essential contributor to progression of BC, is novel. In this reasoning, small animal models such as mice and rats are inherently limited by their life spans to provide the long-term chronic immune-suppressed milieu in which transformed cells grow into malignant tumors and progress to full-blown BCs (Figure 1). Our hypothesis thus augments the mechanisms through which smoking may cause and exacerbate BC.
The role of the immune system in counteracting tumorigenesis/tumor progression is well established. As an example, patients whose immune systems are suppressed because they have received a transplant of some organ (and take immunosuppressant medicines to avoid rejection of the transplant) have a 5 to 6 fold higher incidence of BC (and of other malignancies) than individuals with normal immune systems. Studies from experimental work with mice have largely substantiated the role of immune surveillance,Citation10 although evidence to the contrary also exists.Citation11
There is a considerable albeit, fragmented literature documenting the effects of CS on some components of the immune system. A considerable amount of work has focused on CS-associated inflammation.Citation12 A smaller number of studies have described how CS inhibits innate immunity. Concisely, Mehta et al.Citation13 review the evidence that CS impairs phagocytic functions of macrophage, alters the ratio of helper to suppressor cells, lowers the natural killer (NK) activity, and reduces the production of interferon γ by lymphocytes. In a study focused on human monocyte-derived dendritic cells (DCs),Citation14 such DCs differentiated in the presence of nicotine showed suppressed secretion of IL-12 and TNFα by lipopolysaccharide (LPS)-stimulation as compared to that seen in DCs grown without nicotine. DCs cultured in presence of nicotine also “displayed a diminished capacity to induce allogeneic T cell proliferation with a reduced production of IFNγ, and maintained/enhanced LPS-mediated expression of co-inhibitory molecules.” In a study on the effects of CS extracts on T cell function in vitro, Hernandez et al.Citation15 observed, “activated T cells cultured in the presence of CS extract displayed a dose-dependent decrease in cell proliferation, which associated with the induction of cellular apoptosis. T cell apoptosis by CS extract was independent of caspases and mediated through reactive oxygen and nitrogen species endogenously contained within CS extract.”
Only rarely have studies shown a functional and mechanistic connection between CS and immunological impairm-ent and a disease outcome. In one such notable study, Feng et al.Citation16 exposed mice to CS and infected them with Mycobacterium tuberculosis or influenza A. They observed that CS “inhibited the lung T-cell production of IFNγ, compared with controls, during stimulation in vitro with anti-CD3, after vaccination with a construct expressing an immunogenic mycobacterial protein, and during infection with M. tuberculosis and influenza A virus in vivo. Reduced IFNγ production was mediated through the decreased phosphorylation of transcription factors that positively regulate IFNγ expression. CS exposure increased the bacterial burden in mice infected with M. tuberculosis and increased weight loss and mortality in mice infected with influenza virus.” This recent study was the first demonstration of CS-mediated inhibition of T cell function in vivo leading to increased morbidity and mortality in excellent mouse models of animal disease.
The aforementioned study by Feng et al. provides a particularly relevant vantage point to frame the discussion of our hypothesis, since it demonstrates immunosuppressive effects of CS on disease progression in a disease where T cells play an important role. In contrast to the acute infection with a bacterium or a virus where disease progression is swift, genesis and progression of BC (as in other cancers) is a long-term process that involves one or more primary events followed by considerable evolution of disease. It is our premise that the immune system applies brakes to this long-term process of cancer evolution at multiple points, such that in the short life span of the mouse, the immune system prevails, and no consistent CS-induced bladder carcinogenesis is seen, even though CS-induced primary genotoxic events do occur. In contrast, in the human situation, the relatively long human life span allows the evolutionary mechanisms of oncogenesis to play out the battle with the immune system, and eventually prevail.
A small number of small epidemiological studies also point to the association of CS and immune suppression. In a cross-sectional study of 75 healthy women, who participated in the Data Bank and Bio Repository program at Roswell Park Cancer Institute, higher peripheral blood levels of CD4(+) CD25(+) FOXP3(+) regulatory T (T-reg) cells were significantly associated with smoking, among other parameters.Citation17 Such T-reg cells play a major role in down-regulating immune response; hence these observations are consistent with the thesis that smoking suppresses immune response. In a study of 27 patients under the age of 40 treated for invasive vulvar cancer at the Women's Cancer Center, University of Minnesota, smoking and a history of an immunosuppressive medical illness were the most common parameters in this patient population.Citation18 In a study of 441 patients consecutively diagnosed with Herpes Zoster, smoking was observed to be an independent predictor of post-herpetic neuralgia.Citation19
The proposed contribution of CS-induced immune suppression is particularly relevant for BC, because of the compelling and multi-faceted evidence of the role of immune response in BC (see Citation20 for an excellent review). BC was the first cancer formally shown to be immune-responsive. Specifically, high-grade superficial BC is responsive to intra-vesicular instillation of Bacillus Calmette-Guérin in a significant number of patients with multi-focal carcinoma in situ (CIS) or unifocal pathologic T1 lesions.Citation21 Based on extensive data on cancer incidence, immune-suppressed transplant patients are 5.5 fold more likely to develop BC than an age-matched control population.Citation22 Although the urothelium shows a good expression of HLA A, B, and C molecules (which are essential for immune recognition of cancer cells), these molecules can be lost in BC cells, suggesting the tumor's response to immunological attack is suppressed (discussed in 28). In BC patients, there is a statistically significant correlation of the levels of NK cell activity with clinical evolution and pathological stage of BC.Citation23,24
The evidence linking the numbers and types of tumor infiltrating lymphocytes (TILs) to clinical outcomes is the clearest in the case of colonCitation25 and ovarian cancersCitation26 and melanomas.Citation27 However, evidence is now emerging for BC. In a multivariate analysis of 514 BC patients and a follow-up of over >9 years,Citation28 TILs were a highly significant indicator of a favorable prognosis (p = 0.007). More recently, Sharma et al.Citation29 “analyzed the presence of intratumoral CD8+ T cells… in BC samples. Immunohistochemical staining for intratumoral CD8+ T cells in tissue samples from 69 patients with BC showed that patients with advanced urothelial carcinoma (UC) (pT2, pT3, or pT4) and higher numbers of CD8+ TILs within the tumor (8 or more) had better disease-free survival (p < 0.001) and overall survival (p = 0.018) than did patients with similar-staged UC and fewer intratumoral CD8+ TILs” (39).
Altogether, our hypothesis is based on the compelling evidence of the role of the immune response in modulating the course of disease in BC, and the considerable anecdotal, yet largely non-mechanistic studies of the effects of CS exposure on immune responses. It may be logically argued that if smoking leads to systemic immune suppression (and since immune response seems to play a vital role in controlling cancers in general), smoking should be associated with almost all cancers. However, smoking seems to be a risk factor specifically for cancers of the lung, head and neck, urinary tract (including bladder), and pancreas. In this regard, it is our thinking that smoking acts at two distinct levels (see Figure 1), one at the level of direct effects of the components of smoke in causing malignant transformation of the exposed cells, (i.e., at the initiation of malignancy), and two, at the level of immune suppression leading to progression of malignancy. The effects of smoking at these two levels are synergistic, which is why smoking is an obvious risk factor only for those cancers where components of the smoke directly interact with the target cells, i.e., those of the lung, head and neck, urinary tract (including bladder), and pancreas. We suggest that smoking shall turn out be a weaker and less obvious risk factor for other cancers as well. Indeed, the Surgeon General's reportCitation30 on the Health Consequences of Smoking, opines that “evidence is sufficient to infer a causal relationship” between smoking and hepatocellular carcinoma, as well as colorectal adenomas and colorectal cancer. It also says “evidence is suggestive but not sufficient to infer a causal relationship” between tobacco smoke and breast cancer.
Testing the Hypothesis
We suggest three methods to interrogate the hypothesis. A longitudinal study of detailed immune status of early smokers stratified by intensity of CS exposure, including flow cytometric analyses of well-known biomarkers, as well as assays of T cell function (allo-reactivity or T cell response to influenza as examples), can provide a strong test of the idea. Secondly, conducting a detailed retrospective analysis of a sufficiently large cohort of BC patients with respect to smoking status, TIL content, and overall survival shall be instructive. Correlation between TIL content and survival has already been sought and obtained to a degree; analysis of similar data with the superimposition of smoking status will be informative with respect to our hypothesis. Finally, if the proposed hypothesis is correct, the use of immunological depletion (through NOD scid, and NSG mice, or CD8+ depletion of immunocompetent mice) in mice exposed to CS, should promote a consistent incidence of BC as compared with previous models. Each of these approaches is completely feasible in the short and medium term. Needless to say, this hypothesis has far reaching implications for prevention and control of solid cancers beyond BC.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- Zeegers MP, Tan FE, Dorant E, Van Den Brandt PA. The impact of characteristics of cigarette smoking on urinary tract cancer risk: a meta-analysis of epidemiologic studies. Cancer 2000; 89(3):630-9; PMID:10931463; http://dx.doi.org/10.1002/1097-0142(20000801)89:3%3c630::AID-CNCR19%3e3.0.CO;2-Q
- Freedman ND, Silverman DT, Hollenbeck AR, Schatzkin A, Abnet CC. Association between smoking and risk of bladder cancer among men and women. J Am Med Assoc 2011; 306:737-45; http://dx.doi.org/10.1001/jama.2011.1142
- Vineis P, Talaska G, Malaveille C, Bartsch H, Martone T, Sithisarankul P, Strickland P. DNA adducts in urothelial cells: relationship with biomarkers of exposure to arylamines and polycyclic aromatic hydrocarbons from tobacco smoke. Int J Cancer 1996 65(3):314-6; PMID:8575850; http://dx.doi.org/10.1002/(SICI)1097-0215(19960126)65:3%3c314::AID-IJC6%3e3.0.CO;2-2
- Hecht SS. Lung carcinogenesis by tobacco smoke. Int J Cancer 2012; 131(12):2724-32; PMID:22945513; http://dx.doi.org/10.1002/ijc.27816
- Greenman C, Stephens P, Smith R, Dalgliesh GL, Hunter C, Bignell G, Davies H, Teague J, Butler A, Stevens C et al. Patterns of somatic mutations in human cancer genomes. Nature 2007; 446:153-8; PMID:17344846; http://dx.doi.org/10.1038/nature05610
- Hecht SS. Carcinogenicity studies of inhaled cigarette smoke in laboratory animals: old and new. Carcinogenesis 2005; 26:1499-92; PMID:15930027; http://dx.doi.org/10.1093/carcin/bgi148
- D'Agostini F, Balansky R, Steele VE, Ganchev G, Pesce C, De Flora S. Preneoplastic and neoplastic lesions in the lung, liver and urinary tract of mice exposed to environmental cigarette smoke and UV light since birth. Int J Cancer 2008; 123(11):2497-502; PMID:18770867; http://dx.doi.org/10.1002/ijc.23836
- Ohnishi T, Arnold LL, He J, Clark NM, Kawasaki S, Rennard SI, Boyer CW, Cohen SM. Inhalation of tobacco smoke induces increased proliferation of urinary bladder epithelium and endothelium in female C57BL/6 mice. Toxicology 2007; 241(1-2):58-65; PMID:17897767; http://dx.doi.org/10.1016/j.tox.2007.08.088
- Kato M, Wei M, Yamano S, Fujioka M, Kakehashi A, Wanibuchi H. Evaluation of the modifying effect of inhalation of mainstream cigarette smoke on mouse bladder carcinogenesis. J Toxicol Pathol 2013; 26(4):447-51; PMID:24526820; http://dx.doi.org/10.1293/tox.2013-0039
- Swann JB, Smyth MJ. Immune surveillance of tumors. J Clin Invest 2007; 117(5):1137-46; PMID:17476343; http://dx.doi.org/10.1172/JCI31405
- Rogers LM, Olivier AK, Meyerholz DK, Dupuy AJ. Adaptive immunity does not strongly suppress spontaneous tumors in a sleeping beauty model of cancer. J. Immunol 2013; 190:4393-9; PMID:23475219; http://dx.doi.org/10.4049/jimmunol.1203227
- Rom O, Avezov K, Aizenbud D, Reznick AZ. Cigarette smoking and inflammation revisited. Respir Physiol Neurobiol 2013; 187:5-10; PMID:23376061; http://dx.doi.org/10.1016/j.resp.2013.01.013
- Mehta H, Nazzal K, Sadikot RT. Cigarette smoking and innate immunity. Inflamm Res 2008; 57:497-503; PMID:19109742; http://dx.doi.org/10.1007/s00011-008-8078-6
- Yanagita M, Kobayashi R, Kojima Y, Mori K, Murakami S. Nicotine modulates the immunological function of dendritic cells through peroxisome proliferator-activated receptor-γ upregulation. Cell Immunol 2012; 274(1-2):26-33; PMID:22425227; http://dx.doi.org/10.1016/j.cellimm.2012.02.007
- Hernandez CP, Morrow K, Velasco C, Wyczechowska DD, Naura AS, Rodriguez PC. Effects of cigarette smoke extract on primary activated T cells. Cell Immunol 2013 282(1):38-43; PMID:23665673; http://dx.doi.org/10.1016/j.cellimm.2013.04.005
- Feng Y, Kong Y, Barnes PF, Huang FF, Klucar P, Wang X, Samten B, Sengupta M, Machona B, Donis R et al. Exposure to cigarette smoke inhibits the pulmonary T-cell response to influenza virus and Mycobacterium tuberculosis. Infect Immun 2011; 79:229-37; PMID:20974820; http://dx.doi.org/10.1128/IAI.00709-10
- Hampras SS, Nesline M, Wallace PK, Odunsi K, Furlani N, Davis W, Moysich KB. Predictors of immunosuppressive regulatory T lymphocytes in healthy women. J Cancer Epidemiol 2012:2012:191090; PMID:22969801; http://dx.doi.org/10.1155/2012/191090
- Carter J, Carlson J, Fowler J, Hartenbach E, Adcock L, Carson L, Twiggs LB. Invasive vulvar tumors in young women-a disease of the immunosuppressed? Gynecol Oncol 1993; 51(3):307-10; PMID:8112637; http://dx.doi.org/10.1006/gyno.1993.1295
- Parruti G, Tontodonati M, Rebuzzi C, Polilli E, Sozio F, Consorte A, Agostinone A, Di Masi F, Congedo G, D'Antonio D et al. VZV pain study group. Predictors of pain intensity and persistence in a prospective Italian cohort of patients with herpes zoster: relevance of smoking, trauma and antiviral therapy. BMC Med 2010; 8:58; PMID:20937086; http://dx.doi.org/10.1186/1741-7015-8-58
- Liakou CI, Narayanan S, Tang DN, Logothedis CJ, Sharma P. Focus on TILs: prognostic significance of tumor infiltrating lymphocytes in human bladder cancer. Cancer Immun 2007; 7:10; PMID:17591743
- Alexandroff AB, Jackson AM, O'Donnell MA, James K. BCG immunotherapy of bladder cancer: 20 years on. Lancet 1999; 353:1689-94; PMID:10335805; http://dx.doi.org/10.1016/S0140-6736(98)07422-4
- Birkeland SA, Storm HH, Lamm LU, Barlow L, Blohmé I, Forsberg B, Eklund B, Fjeldborg O, Friedberg M, Frödin L et al. Cancer risk after renal transplantation in the Nordic countries, 1964-1986. Int J Cancer 1995; 60:183-9; PMID:7829213; http://dx.doi.org/10.1002/ijc.2910600209
- Morita T, Tokue A, Minato N. Analysis of natural killer activity and natural killer cell subsets in patients with bladder cancer. Cancer Immunol Immunother 1990; 32:191-4; PMID:1705177; http://dx.doi.org/10.1007/BF01771456
- Carballido J, Alvarez-Mon M, Solovera OJ, Menéndez-Ondina L, Durántez A. Clinical significance of natural killer activity in patients with transitional cell carcinoma of the bladder. J Urol 1990; 143:29-33; PMID:2294256
- Naito Y, Saito K, Shiiba K, Ohuchi A, Saigenji K, Nagura H, Ohtani H. CD8+ T cells infiltrated within cancer cell nests as a prognostic factor in human colorectal cancer. Cancer Res 1998; 58:3491-4; PMID:9721846
- Zhang L, Conejo-Garcia JR, Katsaros D, Gimotty PA, Massobrio M, Regnani G, Makrigiannakis A, Gray H, Schlienger K, Liebman MN et al. Intratumoral T cells, recurrence, and survival in epithelial ovarian cancer. N Engl J Med 2003; 348:203-13; PMID:12529460; http://dx.doi.org/10.1056/NEJMoa020177
- Clemente CG, Mihm MC Jr, Bufalino R, Zurrida S, Collini P, Cascinelli N. Prognostic value of tumor infiltrating lymphocytes in the vertical growth phase of primary cutaneous melanoma. Cancer 1996; 77:1303-10; PMID:8608507; http://dx.doi.org/10.1002/(SICI)1097-0142(19960401)77:7%3c1303::AID-CNCR12%3e3.0.CO;2-5
- Lipponen PK, Eskelinen MJ, Jauhiainen K, Harju E, Terho R. Tumour infiltrating lymphocytes as an independent prognostic factor in transitional cell bladder cancer. Eur J Cancer 1993; 29:69-75; PMID:1445749; http://dx.doi.org/10.1016/0959-8049(93)90579-5
- Sharma P, Shen Y, Wen S, Yamada S, Jungbluth AA, Gnjatic S, Bajorin DF, Reuter VE, Herr H, Old LJ et al. CD8 tumor-infiltrating lymphocytes are predictive of survival in muscle-invasive urothelial carcinoma. Proc Natl Acad Sci U S A 2007; 104:3967-72. 26; PMID:17360461; http://dx.doi.org/10.1073/pnas.0611618104
- The Health Consequences of Smoking-50 years of progress. A Report of the Surgeon G, Executive summary. Department of Health and Human Services, US Govt