Abstract
Cutaneous T-cell lymphomas (CTCLs) represent a group of rarely occurring and clinically and pathologically heterogeneous diseases that are considered incurable at advanced stages. Current treatments provide limited clinical benefit and are thus largely amenable to improvement. An antibody-based CTCL-specific immunotherapy targeting the KIR3DL2 receptor expressed by the tumor cells in CTCL is currently under development and has shown encouraging results in pre-clinical studies.
The denomination cutaneous T-cell lymphoma (CTCL) encompasses a group of T-cell derived, non-Hodgkin's lymphomas which, by definition, initially develop in the skin. The World Health Organization-European Organization for the Research and Treatment of Cancer (WHO-EORTC) classification distinguishes several subtypes of CTCL that vary considerably in terms of clinical presentation and prognosis.Citation1 Therefore, patients with CTCL display diverse risks for disease progression or death. Furthermore, CTCL can be debilitating because of the elicitation of specific symptoms (e.g., pruritus, fatigue, sleep disturbance) and the social stigmata of displaying obvious skin lesions.
There is no current standard of care for CTCL. Therapeutic algorithm is guided by the disease stage according to the revised tumor-node-metastasis-blood (TNMB) classification,Citation2 another key prognostic factor. The treatment of CTCL aims at clearing skin and extra cutaneous disease, minimizing recurrence, preventing progression and preserving quality of life. While early stage patients are treated with topical therapies and have normal life expectancy, patients with more advanced disease receive systemic therapies that provide only limited clinical benefit with frequent relapse. Five-year survival rate in late stage patients does not exceed 15%. This population of advanced relapsed/refractory CTCL patients is thus in dire need of more efficient therapeutic options.
KIR3DL2 belongs to the killer-cell immunoglobulin (Ig)-like receptor (KIR) family, receptors that share significant structure and sequence homology. Similarly to the other inhibitory KIR, KIR3DL2 negatively modulates immune effector cell functions through binding to its cognate HLA-Class I ligands.Citation3 In healthy individuals, KIR3DL2 expression is restricted to minor subpopulations of peripheral blood natural killer (NK) lymphocytes, and a few CD4+ and CD8+ T cells,Citation4 and was more recently reported at the surface of cutaneous CD4+ T cells.Citation5 In transformed Mycosis Fungoides (tMF) and Sézary Syndrome (SS), the CTCL subtypes exhibiting the poorest prognosis, KIR3DL2 was identified over a decade ago as a reliable marker of the malignant T-cell clone invading the skin and blood stream.Citation6 More recently using more specific and sensitive detection reagents, KIR3DL2 expression was extended to additional CTCL subtypes, ranging from the clinically indolent to the most aggressive forms (unpublished data). Of note, while KIR3DL2 expression has been evinced on other T- or NK-cell derived malignancies, it has not been detected in diseases involving other immune cell types, such as B-cell or myeloid hematologic malignancies, or in solid tumors (unpublished data). In fact, KIR3DL2 pattern of expression in cancer quite faithfully mirrors its pattern of expression on healthy cells. Therefore, KIR3DL2 offers a unique opportunity to develop a long-awaited CTCL-targeted therapy.
We recently reported the development of IPH4102, the first-in-class anti-KIR3DL2 monoclonal antibody (mAb) selected to bind and deplete KIR3DL2-expressing tumor cells.Citation7 IPH4102 is a humanized IgG1 the selection of which as lead therapeutic candidate was based on its intrinsic preclinical features. IPH4102 has shown compelling efficacy in several non-clinical models in vitro and in vivo. In cell culture assays, IPH4102 was found capable of recruiting human NK cells as well as human macrophages to kill KIR3DL2+ tumor cells in vitro via antibody-dependent cell cytotoxicity (ADCC) and antibody-dependent cell phagocytosis (ADCP), respectively. In addition, in KIR3DL2+ xenograft mouse models, IPH4102 treatment significantly delayed tumor growth and improved the overall survival of the animals in a dose-dependent fashion.
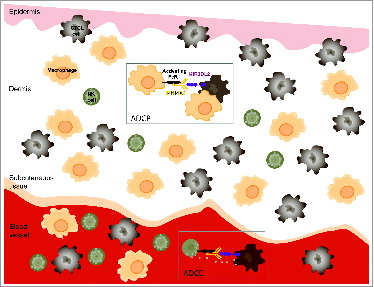
More importantly, IPH4102 has demonstrated a highly favorable antitumor efficacy profile against CTCL patients' cells. Ex vivo autologous ADCC assays were set up using primary tumor and NK cells purified from the same blood sample of patients suffering from Sézary syndrome, the leukemic form of CTCL.Citation7 Remarkably, while Sézary patients' NK cells displayed no spontaneous cytotoxic activity toward malignant cells in these experimental conditions, addition of IPH4102 induced a dose-dependent killing of the malignant T-cell clone by the effector NK cells. Of note, the level of tumor cell lysis observed in the presence of IPH4102 can reach that obtained when using the anti-CD52 antibody alemtuzumab. Additionally, NK cells survival appeared compromised when using alemtuzumab,Citation7 reflecting its potent yet non-selective cytotoxic activity known to profoundly elicit immune suppression in patients.Citation8 In sharp contrast, IPH4102 did not elicit significant lysis of the effector NK cells, suggesting that IPH4102 lacks this potentially detrimental activity. Considering the above data and the tissue distribution of NK cells and macrophages, we anticipate that the tumor cell elimination will mainly involve ADCC in the blood and ADCP in the skin, both mechanisms, however, not being necessarily mutually exclusive in a given tissue ().
Figure 1. Potential immune antitumor modes of action of IPH4102 in CTCL. Binding of IPH4102 to Fc receptor-bearing natural killer (NK) cells or macrophages leads to the targeting and elimination of KIR3DL2+ cutaneous T-cell lymphoma (CTCL) tumor cells by antibody-dependent cell cytotoxicity (ADCC) or/and antibody-dependent cell phagocytosis (ADCP). Because of the reciprocal relative ratios of NK cells to macrophages in the blood stream and skin, one can predominantly expect ADCC in the blood and ADCP in the dermis as immunologic mechanisms of tumor target elimination.
Finally, IPH4102 has also demonstrated a favorable pre-clinical safety profile in regulatory pharmaco-toxicology experiments in non-human primates where it induced no clinically relevant safety-related findings (unpublished results). Furthermore, as expected from the ability of IPH4102 to recruit and activate various types of immune effector cells, a moderate and transient cytokine release has been observed in cell supernatants recovered from ADCC assays, suggesting that, in addition to its antitumor targeted effects, IPH4102 could promote a cytokine profile that may counteract the detrimental Th2 pattern characteristic of CTCL microenvironment.Citation9 Overall, it is acknowledged that preserving a fully active immune system while eliminating tumor cells is a key element for a successful therapeutic strategy in CTCL patients.Citation10 IPH4102 bears the potential to activate and recruit immune effector cells to all tumor sites, to spare useful immune components and to positively modify the tumor microenvironment to favor the elimination of CTCL cells and therefore fulfills the criteria expected for an efficient and safe clinical activity.
Disclosure of Potential Conflicts of Interest
HS, CB and AM are employees of Innate Pharma.
References
- Willemze R, Jaffe ES, Burg G, Cerroni L, Berti E, Swerdlow SH, Ralfkiaer E, Chimenti S, Diaz-Perez JL, Duncan LM, et al. WHO-EORTC classification for cutaneous lymphomas. Blood 2005; 105:3768–85; PMID:15692063; http://dx.doi.org/10.1182/blood-2004-09-3502
- Olsen E, Vonderheid E, Pimpinelli N, Willemze R, Kim Y, Knobler R, Zackheim H, Duvic M, Estrach T, Lamberg S, et al. Revisions to the staging and classification of mycosis fungoides and sezary syndrome: a proposal of the international society for cutaneous lymphomas (ISCL) and the cutaneous lymphoma task force of the european organization of research and treatment of cancer (EORTC). Blood 2007; 110:1713–22; PMID:17540844; http://dx.doi.org/10.1182/blood-2007-03-055749
- Thielens A, Vivier E, Romagne F. NK cell MHC class I specific receptors (KIR): from biology to clinical intervention. Curr Opinion Immunol 2012; 24:239–45; PMID:22264929; http://dx.doi.org/10.1016/j.coi.2012.01.001
- Chan AT, Kollnberger SD, Wedderburn LR, Bowness P. Expansion and enhanced survival of natural killer cells expressing the killer immunoglobulin-like receptor KIR3DL2 in spondylarthritis. Arthritis Rheumatism 2005; 52:3586–95; PMID:16255049; http://dx.doi.org/10.1002/art.21395
- Sako N, Schiavon V, Bounfour T, Dessirier V, Ortonne N, Olive D, Ram-Wolff C, Michel L, Sicard H, Marie-Cardine A, et al. Membrane expression of NK receptors CD160 and CD158k contributes to delineate a unique CD4 T-lymphocyte subset in normal and mycosis fungoides skin. Cytometry Part A 2014; 85:869-82; PMID:25044837; http://dx.doi.org/10.1002/cyto.a.22512
- Bagot M, Moretta A, Sivori S, Biassoni R, Cantoni C, Bottino C, Boumsell L, Bensussan A. CD4(+) cutaneous T-cell lymphoma cells express the p140-killer cell immunoglobulin-like receptor. Blood 2001; 97:1388–91; PMID:11222384; http://dx.doi.org/10.1182/blood.V97.5.1388
- Marie-Cardine A, Viaud N, Thonnart N, Joly R, Chanteux S, Gauthier L, Bonnafous C, Rossi B, Blery M, Paturel C, et al. IPH4102, a humanized KIR3DL2 antibody with potent activity against cutaneous T-cell lymphoma. Cancer Res 2014; 74:6060–70; PMID:25361998; http://dx.doi.org/10.1158/0008-5472.CAN-14-1456
- Alinari L, Lapalombella R, Andritsos L, Baiocchi RA, Lin TS, Byrd JC. Alemtuzumab (Campath-1H) in the treatment of chronic lymphocytic leukemia. Oncogene 2007; 26:3644–53; PMID:17530018; http://dx.doi.org/10.1038/sj.onc.1210380
- Kim EJ, Hess S, Richardson SK, Newton S, Showe LC, Benoit BM, Ubriani R, Vittorio CC, Junkins-Hopkins JM, Wysocka M, et al. Immunopathogenesis and therapy of cutaneous T cell lymphoma. J Clin Invest 2005; 115:798–812; PMID:15841167; http://dx.doi.org/10.1172/JCI200524826
- Guenova E, Watanabe R, Teague JE, Desimone JA, Jiang Y, Dowlatshahi M, Schlapbach C, Schaekel K, Rook AH, Tawa M, et al. TH2 cytokines from malignant cells suppress TH1 responses and enforce a global TH2 bias in leukemic cutaneous T-cell lymphoma. Clin Cancer Res 2013; 19:3755–63; PMID:23785046; http://dx.doi.org/10.1158/1078-0432.CCR-12-3488
