Abstract
The maturation of a specific subset of CD4+ T lymphocytes in the thymus is dependent on cortical thymic epithelial cells expressing the protease thymus-specific serine protease (TSSP, also known as PRSS16). Recently, we unveiled the involvement of TSSP in tumor suppression through its effect on the CD4+ T compartment.
During cancer development, immune cells restrict cancer initiation by preventing the emergence of neoplastic cells and inhibit cancer progression by a process called immunosurveillance. However, immunity (both innate and adaptive) can also promote tumor development during inflammation: chronic inflammatory diseases are known to favor the growth of many human cancers. Thus, the role of adaptive immune cells is dual, depending on the context and cell type.Citation1 In particular, CD4+ (T helper) lymphocytes can either impair or favor carcinogenesis. A better understanding of the respective role of each immune population in either context is required in order to develop efficient immunotherapy strategies emerging as promising alternatives for cancer treatment.
We have previously reported the importance of the thymus-specific serine protease (TSSP) in CD4+ T cell maturation in the thymus. TSSP is encoded by the PRSS16 gene, which is highly conserved between human and mouse.Citation2-4 It is a serine protease predominantly found in endosomes of thymic cortical epithelial cells (cTECs). TSSP/PRSS16 is the third member of the S28 group of serine proteases, and shows homologies to the lysosomal prolylcarboxypeptidase (PRCP; S28.001) that cleaves C-terminal amino acids linked to proline, and to the dipeptidyl peptidase-2/7 (DPP2/7; S28.002) that cleaves X-proline dipeptides from the N-terminus of proteins. The enzymatic function of TSSP has not yet been deciphered and requires further investigation.
TSSP was first reported to be linked to a type 1 (autoimmune) diabetes (T1D) susceptibility locus in the extended Class I region of the Major Histocompatibility Complex (MHC) on human chromosome 6. To address the function of TSSP, we generated TSSP-deficient mice via a genetically inactivated Prss16−/− mouse model. These mice present a normal number of CD4+ T cells but decreased antigen-recognition repertoire of T-cell receptor (TCR) diversity.Citation5,6 Moreover, we have reported that the absence of TSSP on a non-obese diabetes (NOD) background completely prevents the development of T1D, through impaired thymic selection of CD4+ T cells specific for islet antigens.Citation7 These data demonstrated the direct involvement of TSSP in T1D disease development. Taken together, our previous studies highlight a crucial role of TSSP in the production of a functional CD4+ T lymphocytes compartment.
The role of TSSP in cancer progression has been poorly understood until recently. In thymic tumors, TSSP was reported to be either over- or under-expressed depending on the cell type (stromal cells or thymocytes) affected by cancerous proliferation in cortex thymoma or thymic lymphoma respectively.Citation8,9 Over routine breeding of our TSSP-deficient mice, we serendipitously observed that they developed spontaneous tumors upon aging.Citation10 We thus sought to assess the underlying mechanism. For that purpose, we used an experimental model of induced tumorigenesis in the colon, i.e., experimental colitis-associated colorectal (CAC) tumorigenesis. Inflammatory bowel disease (IBD) patients are at increased risk of developing colorectal cancer (CRC). In CRC, T cells are protective against cancer progression by eliminating cancerous cells. Nevertheless, T cells also participate in the protumorigenic inflammatory reaction during IBD, which can stimulate the uncontrolled growth of epithelial cancer cells. We observed an increased rate of induced CAC tumor formation in TSSP-deficient mice compared to TSSP-proficient, thus confirming their enhanced susceptibility to cancer development. Induced colitis experiments showed that exacerbated colon inflammation in the absence of TSSP likely accounts for their cancer susceptibility.
We then addressed the question whether the increased CAC development in TSSP-deficient mice results from a defect in epithelial cells (which give rise to colorectal tumors) or from a function of the immune system (which is involved in epithelial barrier homeostasis). In support of the latter, we observed that TSSP is highly expressed in the thymus (in the stromal compartment) and very poorly in all other tissues including the colon. Adoptive transfer of T cells in various combinations (CD4+ and CD8+ from TSSP-proficient and/or -deficient mice) into T cell-deficient mice showed that tumor development is favored by the CD4+ T-cell compartment of TSSP-deficient mice. The presence of TSSP-deficient CD4+ T cells is also associated with elevated levels of the cytokine IL-17A. These data suggest that promotion of CAC development likely results from extrinsic signals from the inflammatory microenvironment containing CD4+ T cells shaped in the TSSP-deficient thymus. In addition, our study clearly points out a prominent over-expression of the pro-tumoral IL-17A cytokine in a TSSP-deficient immune context. Interestingly, although the role of Th17 cytokines in cancer is potentially dualistic (i.e., tumor-inhibiting or promoting) depending on the context, evidence previously reported in the literature of decreased CAC in IL-17A-deficient mice strongly supports its protumor role in the colon. We thus inhibited IL-17A with specific antibodies during CAC tumor formation, convincingly showing that reducing the level of IL-17A prevents the increased carcinogenesis and colic immune disequilibrium observed in TSSP-deficient mice.
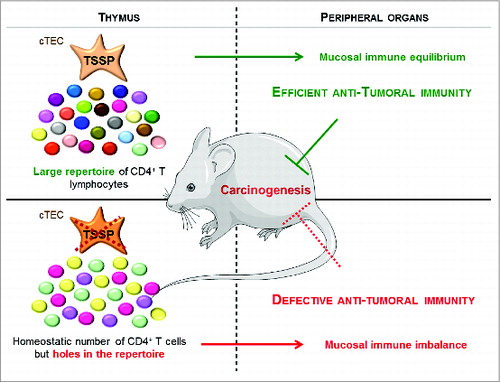
In conclusion, our study demonstrates that antitumor immune surveillance requires TSSP-driven production of a subset of CD4+ T cells in the thymus (Fig. 1). These T cells that have matured in a TSSP-proficient context contribute to the inflammatory equilibrium, and their absence favors tumor development. In the thymus, TSSP contributes to the presentation of self-peptides that are bound to MHC Class II molecules expressed at the cTEC surface and are thus involved in the selection of CD4+ thymocytes. However, the molecular activity of TSSP in cTEC remains elusive and requires further elucidation. Finally, in humans, whether TSSP activity is decreased in the thymus of cancer patients warrants investigation, which will provide insight into the mechanism of immunodeficiency observed during aging (immunosenescence) and in pathological contexts such as cancer.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Figure 1. TSSP mediates tumor suppression by impacting the development of fully efficient antitumor immunity. The TSSP protease is highly expressed in cortical thymic epithelial cells (cTEC) involved in T-lymphocyte maturation through cross-talk with thymocytes. TSSP activity is required for the maturation of a subset of CD4+ T lymphocytes. Thus, TSSP is necessary for the intra-thymic selection of a diverse repertoire of T lymphocytes (shown by multi-colored dots). In the absence of TSSP, the CD4+ T-cell numbers are not decreased, but the diversity of the T-lymphocyte repertoire is reduced (shown by a reduction of dot color diversity). Considering that T lymphocytes play an essential role in antitumor immunity, this defect underlies the decrease in antitumor immunity efficiency observed in the absence of TSSP.
References
- Fridman WH, Pages F, Sautes-Fridman C, Galon J. The immune contexture in human tumours: impact on clinical outcome. Nat Rev Cancer 2012; 12:298-306; PMIsD:22419253; http://dx.doi.org/10.1038/nrc3245
- Bowlus CL, Ahn J, Chu T, Gruen JR. Cloning of a novel MHC-encoded serine peptidase highly expressed by cortical epithelial cells of the thymus. Cell Immunol 1999; 196:80-6; PMID:10527559; http://dx.doi.org/10.1006/cimm.1999.1543
- Carrier A, Nguyen C, Victorero G, Granjeaud S, Rocha D, Bernard K, Miazek A, Ferrier P, Malissen M, Naquet P, et al. Differential gene expression in CD3epsilon- and RAG1-deficient thymuses: definition of a set of genes potentially involved in thymocyte maturation. Immunogenetics 1999; 50:255-70; PMID:10630289; http://dx.doi.org/10.1007/s002510050601
- Carrier A, Wurbel MA, Mattei MG, Kissenpfennig A, Malissen M, Malissen B. Chromosomal localization of two mouse genes encoding thymus-specific serine peptidase and thymus-expressed acidic protein. Immunogenetics 2000; 51:984-6; PMID:11003393; http://dx.doi.org/10.1007/s002510000230
- Gommeaux J, Gregoire C, Nguessan P, Richelme M, Malissen M, Guerder S, Malissen B, Carrier A. Thymus-specific serine protease regulates positive selection of a subset of CD4+ thymocytes. Eur J Immunol 2009; 39:956-64; PMID:19283781; http://dx.doi.org/10.1002/eji.200839175
- Viret C, Lamare C, Guiraud M, Fazilleau N, Bour A, Malissen B, Carrier A, Guerder S. Thymus-specific serine protease contributes to the diversification of the functional endogenous CD4 T cell receptor repertoire. J Exp Med 2011; 208:3-11; PMID:21173102; http://dx.doi.org/10.1084/jem.20100027
- Viret C, Leung-Theung-Long S, Serre L, Lamare C, Vignali DA, Malissen B, Carrier A, Guerder S. Thymus-specific serine protease controls autoreactive CD4 T cell development and autoimmune diabetes in mice. J Clin Invest 2011; 121:1810-21; PMID:21505262; http://dx.doi.org/10.1172/JCI43314
- Strobel P, Hartmann E, Rosenwald A, Kalla J, Ott G, Friedel G, Schalke B, Kasahara M, Tomaru U, Marx A. Corticomedullary differentiation and maturational arrest in thymomas. Histopathology 2014; 64:557-66
- Lin YW, Aplan PD. Gene expression profiling of precursor T-cell lymphoblastic leukemia/lymphoma identifies oncogenic pathways that are potential therapeutic targets. Leukemia 2007; 21:1276-84; PMID:17429429; http://dx.doi.org/10.1038/sj.leu.2404685
- Brisson L, Pouyet L, N'Guessan P, Garcia S, Lopes N, Warcollier G, Iovanna JL, Carrier A. The Thymus-Specific Serine Protease TSSP/PRSS16 Is Crucial for the Antitumoral Role of CD4(+) T Cells. Cell Rep 2015; 10:39-46; PMID:25543139; http://dx.doi.org/10.1016/j.celrep.2014.12.009
