Abstract
Circulating fibrocytes were reported to represent a novel myeloid-derived suppressor cell (MDSC) subset and they were also proposed to be involved in the tumor immune escape. This novel fibrocyte subset had a surface phenotype resembling non-monocytic MDSCs (CD14−CD11chiCD123−) and exhibited immunomodulatory roles. Most effector functions of fibrocytes (circulating fibroblast-progenitors) are accomplished as tissue fibroblasts, likewise in the tumor microenvironment. Therefore, fibroblasts at tumor tissues should be evaluated whether they display similar molecular/gene expression patterns and functional roles to the blood-borne fibrocytes. A chemically induced rat breast carcinogenesis model was utilized to obtain cancer associated fibroblasts (CAFs). CAFs and normal tissue fibroblasts (NFs) were isolated from cancerous and healthy breast tissues, respectively, using a previously described enzymatic protocol. Both CAFs and NFs were analyzed for cell surface phenotypes by flow cytometry and for gene expression profiles by gene set enrichment analysis (GSEA). PBMCs were cocultured with either NFs or CAFs and proliferations of PBMCs were assessed by CFSE assays. Morphological analyses were performed by immunocytochemistry stainings with vimentin. CAFs were spindle shaped cells unlike their blood-borne counterparts. They did not express CD80 and their MHC-II expression was lower than NFs. Although CAFs expressed the myeloid marker CD11b/c, its expression was lower than that on the circulating fibrocytes. CAFs did not express granulocytic/neutrophilic markers and they seemed to have developed in an environment containing THELPER2-like cytokines. They also showed immunosuppressive effects similar to their blood-borne counterparts. In summary, CAFs showed similar phenotypic and functional characteristics to the circulating fibrocytes that were reported to represent a unique MDSC subset.
Abbreviations
| CAF | = | cancer associated fibroblast |
| CFSE | = | carboxyfluorescein succinimidyl ester |
| DAB | = | diaminobenzidine |
| DC | = | dendritic cell |
| FAP-α | = | fibroblast activation protein-α |
| FDR | = | false discovery rate |
| GEO | = | gene expression omnibus |
| GSEA | = | gene set enrichment analysis |
| IDO | = | indoleamine 2,3-dioxygenase |
| MDSC | = | myeloid-derived suppressor cell |
| MFI | = | median fluorescence intensity |
| NES | = | normalized enrichment score |
| NF | = | normal tissue fibroblast |
| NMU | = | N-Nitroso-N-methylurea |
| PBMC | = | peripheral blood mononuclear cell |
| PHA | = | phytohemagglutinin |
| SEM | = | standard error of mean |
| w/ | = | Coculture with the designated cells |
Introduction
MDSCs are activated immature cells of myeloid origin that increase in number in various pathological conditions, including cancer.Citation1,2 This heterogeneous group was shown to express Gr-1 and CD11b in murine models,Citation3 and was described as monocytic (Ly6C+) and neutrophilic subsets (Ly6G+).Citation4 Although rat MDSCs were first described as CD11b/c+ and HIS48+ cells,Citation5 there is an ongoing debate concerning the specific markers for rat MDSCs. On the other hand, human MDSCs also seem to be more complex than their murine counterparts, and are most commonly defined as CD33+CD14−CD11b+HLADR− cells.Citation6,7
The studies that have investigated the nature of the cells and molecules responsible for the functional insufficiency of the tumor infiltrating T cells usually focused on tumor cells themselves, MDSCs and/or regulatory T cells.Citation8-10 However, the contribution of stromal cellular elements has not yet been well established. Fibroblasts, one of the most abundant cell types found in the stroma, become activated and differentiate into myofibroblasts during wound healing and fibrosis; or into CAFs in the tumor microenvironment. CAFs are the most prominent cell type within the tumor microenvironment of various cancersCitation11,12 and approximately 80% of stromal fibroblasts are thought to show an activated phenotype in breast carcinomas.Citation13 As “tumors are wounds that do not heal”,Citation14 CAFs are similar to myofibroblasts. The paracrine crosstalk between the epithelium and the stroma results in the activation of fibroblasts.Citation15 CAF derived factors, in turn, are able to promote a tumor permissive microenvironment and contribute to the metastatic properties of cancer cells.Citation15-19
Recently, the expansion of a special population of HLA-DR+ cells that bear the morphological features of immature monocytes as well as the cell surface expression characteristics of neutrophilic MDSCs was reported in pediatric patients with metastatic sarcomas.Citation20 In contrast to a number of previous studies that focused on the monocytic or neutrophilic subsets of the MDSCs,Citation1,4,21,22 this study is of utmost importance as it redefines a player formerly known for its proinflammatory and profibrotic effects, the fibrocytes, that may also be involved in the tumor immune escape.Citation23-26 The results of Zhang et al. were obtained from a pediatric population and were not representative of other investigators' studies into MDSCs. They rather described a novel subset of cancer-induced MDSCs, which were CD11b+HLA-DR+.
Despite the discovery of the expansion of a novel circulating fibrocyte subset (CD45+CD34+HLA-DR+) which showed a surface molecular expression pattern similar to the non-monocytic MDSCs (CD14−CD11chiCD123−) and at the same time possessed immunosuppressive properties,Citation20 whether fibrocytes at the tumor site exhibit similar characteristics to the circulating fibrocytes has not yet been clarified. Fibrocytes are circulating progenitors of fibroblasts.Citation27,28 These surface expression findings concerning the circulating fibrocytesCitation20 call for further investigations to determine if those results are also relevant for tissue fibroblasts. Therefore, we investigated whether the CAFs exhibited the molecular or gene expression profiles and functional effects similar to that of the blood-borne fibrocytes with MDSC characteristics.Citation20 In this study, we report that fibroblasts at tumor sites display a similar expression profile and functional characteristics, with some modifications, to the circulating fibrocytes which were shown to represent a novel MDSC subset. Furthermore, these fibroblasts that are located in the tumor microenvironment may take part in immune modulation.
Results
CAFs and NFs were successfully isolated from cancerous and healthy tissues, respectively
The animals that were injected with N-Nitroso-N-methylurea (NMU) developed at least one palpable breast tumor after a period of 1–2 mo. Tumor excisions were performed after the tumors were ≥ 1 cm in diameter. NFs were obtained from healthy breast tissues of healthy control animals and CAFs were obtained from breast carcinomas, by enzymatic digestion with Collagenase I and Hyaluronidase.Citation29 These isolated fibroblasts were then cultured and stable primary cell cultures were achieved.
CAFs express MHC class II but do not provide costimulation through CD80
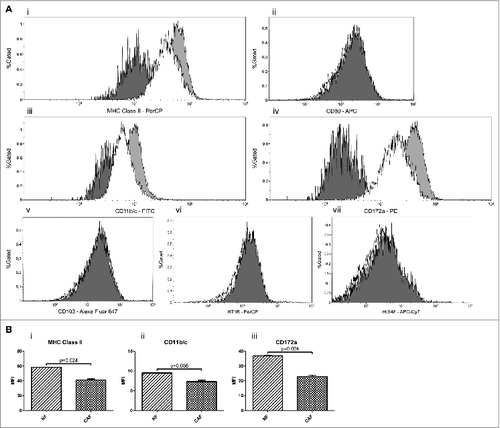
Fibrocytes were previously reported to express MHC class II (MHC-II) and CD80/86; thus functioning as antigen presenting cells.Citation30 Therefore, the expression of MHC-II on CAFs was analyzed and indeed, CAFs were found to express MHC-II (). In order to compare the MHC-II expressions of NFs and CAFs, NFs were obtained from the breast tissues of healthy control rats. NFs were also shown to express MHC-II, even more than CAFs did () (p value of MFI comparison was 0.024). Furthermore, the investigation of the costimulatory CD80 molecule expression on tumor-derived fibroblasts revealed that CAFs did not express CD80 ().
Figure 1. Fibrocytic cells at the tumor site exhibit similar –but not the same– surface expression characteristics to the circulating fibrocytes. (A) Expression profiles of CAF cells for MHC Class II (i), CD80 (ii), CD11b/c (iii), CD172a (iv), CD103 (v), RT1B (vi), HIS48 (vii) and expression profiles of NF cells for MHC Class II (i), CD11b/c (iii), CD172a (iv) are shown. Fibroblast cells in cultures were evaluated for morphological features,Citation76 immunocytochemical characteristics; and microscopic confirmation was achieved before the experiments. Fibroblast cells were previously reported to show a wide range of variation in terms of size and granularity in flow cytometric evaluations.Citation77,78 Our studies also demonstrated that forward and side scatter characteristics of NF and CAF cells do not represent a single distinct population based on size and granularity; thus, cannot be used as a sensitive and specific gating strategy as in the case of the lymphocyte gate (Fig. S1). As reported previously,Citation78 fibroblast cells were gated on scatterplot of side light scatter vs. forward light scatter, allowing for gating of only the viable cell population, excluding cellular debris after doublet discrimination. Heavily shaded (dark gray) areas represent background fluorescence on the designated population as indicated by suitable isotype controls. Unshaded (white) areas represent breast tumor-derived CAF cells and lightly shaded (light gray) areas represent NF cells acquired from healthy donors. CAFs were shown not to express CD80, CD103, RT1B or HIS48. On the other hand, CAFs were shown to express MHC class II, CD11b/c and CD172a and their expressions of these molecules were lower than that of NF cells. Representative flow cytometry plots of more than three experiments are shown. (B) Comparison of MFI values of surface expressions of CAF vs. NF cells. There were statistically significant differences between CAF and NF expressions of MHC Class II (p = 0.024) (i), CD11b/c (p = 0.036) (ii) and CD172a (p=0.024) (iii). Columns and error bars designate means and SEM, respectively. The results were derived from more than three experiments. CAF: Cancer Associated Fibroblast, NF: Normal Tissue Fibroblast, MFI: Median Fluorescence Intensity, SEM: Standard Error of Mean.
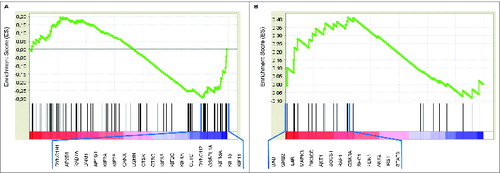
In order to further investigate the distinction of CAFs from the NFs, we analyzed a publicly available human microarray study to compare gene expression in these two cell types (GSE21440). The experiment was analyzed by GSEA, as described previously.Citation31 REACTOMEMHC_CLASS_II_ANTIGEN_PRESENTATION gene set, which comprises genes involved in MHC class II antigen presentation, was found to be differentially expressed. Furthermore, this gene set was under-expressed in CAFs compared with the NFs (NES: −1.27, FDR: 0.062). shows the enrichment plot with 20 of the genes which contribute most to the enrichment result. This GSEA result is consistent with our findings concerning MHC Class II surface expression characteristics of CAF cells () and confirms that CAFs represent a cell population with reduced antigen presenting capacity compared with NFs.
Figure 2. Fibrocytic cells mediate immune suppression. (A) Vimentin stained NF (i) and CAF (ii) cells from representative samples (original magnification x200 under light microscope and visualized by DAB). CAF and NF cells were immunostained to show their surface expressions of vimentin, in order to clearly visualize their morphologies and expression characters. Both of these spindle shaped cell types stained uniformly for the mesenchymal marker vimentin, as expected.Citation38 (B) Suppression of PHA mediated PBMC proliferation by fibrocytic cells. 20.26% of PBMCs cocultured with CAFs (white line) proliferated in contrast to the proliferation of 37.80% of PBMCs cocultured with NFs (gray line). Thus, CFSE proliferation assays showed the immunosuppressive effect of CAFs compared to NFs. A representative histogram of three experiments performed using PBMC from three separate rats is shown (see Fig. S2 for positive and negative controls and Fig. S4 for the effect of the number of CAF cells). CAF: Cancer Associated Fibroblast, NF: Normal Tissue Fibroblast, w/: Coculture with the designated cells, DAB: Diaminobenzidine, PHA: Phytohemagglutinin, PBMC: Peripheral Blood Mononuclear Cell, CFSE: Carboxyfluorescein Succinimidyl Ester.
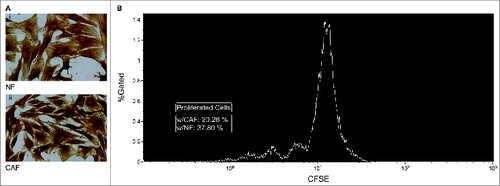
Figure 3. Genes involved in MHC class II antigen presentation were under-expressed and genes related to IL-4 signaling were upregulated in CAFs compared with NFs. (A) Enrichment plots of GSEA of CAFs vs. NFs for the REACTOMEMHC_CLASS_II_ANTIGEN_PRESENTATION (NES: –1.27, FDR: 0.062) and (B) SIG_IL4RECEPTOR_IN_B_LYPHOCYTES (NES: 1.21, FDR: 0.201) gene sets are shown. Gene expression data was acquired from NCBI Gene Expression Omnibus (GSE21440) and GSEA was performed as described previously.Citation31 Genes that contribute to the leading-edge subsets within each gene set (20 and 14 genes, respectively) are listed below the figures. These GSEA results are consistent with our flow cytomery findings concerning MHC Class II and CD172a expressions of CAF and NF cells. CAF: Cancer Associated Fibroblast, NF: Normal Tissue Fibroblast, GSEA: Gene Set Enrichment Analysis, NES: Normalized Enrichment Score, FDR: False Discovery Rate.
CAFs express the myeloid marker CD11b/c
CD11b/c is a common myeloid marker. Both murine and human MDSCs have been reported to express CD11b. It was demonstrated that a group of circulating fibrocytes also expressed CD11b.Citation20 Accordingly, CAFs were observed to be mildly positive for CD11b/c () in the current study. Moreover, NFs displayed slightly higher expressions of CD11b/c in comparison to CAFs () (p value of MFI comparison was 0.036). Furthermore, considering the results from Zhang et al.,Citation20 CD11b/c expression in CAFs appears to be lower than that in the circulating fibrocytes.
CAFs do not express granulocytic / neutrophilic markers despite having myeloid characteristics
CAFs were found to be mildly positive for CD11b/c (). Based on the finding that fibrocytes displayed cell surface expression characteristics of neutrophilic cells,Citation20 the expressions of granulocytic/neutrophilic markers that are recognized by such antibodies as RP-1Citation32 and HIS48,Citation33 were investigated in CAFs. Neither of these markers was present on CAFs (one representative marker, HIS48, is shown in ).
CAFs seem to have developed in an environment containing THELPER2-like cytokines
Pilling et al. previously proposed that CD172a can be used to demonstrate whether fibrocytes have been differentiated in a milieu that contains either profibrotic THELPER2-like cytokines (IL-4 and/or IL-13) or proinflammatory THELPER1-like cytokines (IL-12 and/or IFNγ).Citation34 Thus, CD172a (SIRPα) expression on CAFs was analyzed in comparison to NFs, in an attempt to indirectly investigate whether those findings suggesting an IL-4 rich milieu for the blood-borne fibrocytes, also applied to CAFs. Indeed, CD172a was found to be expressed on both CAFs and NFs, the former with lower surface expressions () (p value of MFI comparison was 0.024), likely indicating for higher levels of IL-4 in the milieu of CAFs (see also Fig. S3).
In order to more directly assess whether CAF cells were exposed to higher levels of IL-4 than NF cells, we investigated genes related to IL-4 mediated signaling by the “SIG_IL4RECEPTOR_IN_B_LYPHOCYTES” gene set with GSEA in a publicly available human microarray study (GSE21440), as described previously.Citation31 This IL-4 signaling gene set was found to be differentially expressed, and was upregulated in CAFs compared with the NFs (NES: 1.21, FDR: 0.201). shows the enrichment plot with 14 of the genes which contribute most to the enrichment result. The GSEA result suggests a higher IL-4 exposure for CAFs and thus is consistent with our findings of lower surface expressions of CD172a on CAFs than NFs (), implying higher IL-4 levels in the microenvironment of CAFs.
CAFs do not resemble the DCs that are present in the gut-associated lymphoid tissue since they are CD103−RT1B−
Abnormal expansions of CD11b+HLA-DR+ myeloid cells in peripheral blood of subjects with metastatic sarcomas were demonstrated.Citation20 It was also previously reported that a dendritic cell (DC) population is present at a high frequency in the gut-associated lymphoid tissue and non–T cell expression of a specific marker, CD103, is restricted primarily to these CD11chi MHC-IIhi DCs.Citation35 Given the resemblance of the phenotypic characteristics of those 2 cell types, CD103 expression of CAFs was analyzed. However, CAFs did not express CD103 (). In addition, as those DCs were also reported to express another marker, RT1B,Citation36 the expression of RT1B on CAFs was also investigated. Similar to CD103, RT1B was not expressed on CAFs, either ().
CAFs exhibit immunosuppressive characteristics
Blood-borne fibrocytes in cancer patients were reported to possess immunosuppressive properties.Citation20 Furthermore, Kraman et al. proposed a population of fibroblast activation protein-α (FAP-α) expressing cells to be an immunosuppressive component of the tumor microenvironment.Citation37 Accordingly, fibroblasts at the tumor site were analyzed to find out whether they exhibit similar immunosuppressive characteristics like their circulating counterparts. Similarly, carboxyfluorescein succinimidyl ester (CFSE) proliferation assays in cocultures of CAFs and NFs with peripheral blood mononuclear cells (PBMCs) were utilized for functional assessment and the results revealed the immunosuppressive effects of CAFs compared to NFs (), since PBMCs that were cocultured with CAF cells showed decreased proliferation.
CAFs are spindle shaped, mesenchymally-derived cells unlike their blood-borne counterparts
Finally, both CAF and NF cells were immunostained to show the expression of the mesenchymal tissue marker vimentin, in order to clearly visualize their morphologies and expression characteristics. Both of these cell types were uniformly positive for vimentin, as expectedCitation38 (). Although it was reported that CD11chiCD123−CD14− cells resembled immature monocytes,Citation20 their tissue counterparts (NFs and CAFs) were spindle shaped cells ex vivo.
Discussion
Fibroblasts are one of the most abundant cell types found in the stroma. They mostly differentiate into CAFs within the tumor microenvironment. The straightforward perspective about the sole structural role of these cells has been contradicted by the findings of several independent studies. At present, they are thought to be involved in a dynamic and dense crosstalk with other cells of the tumor microenvironment.Citation39 CAFs were demonstrated to directly promote tumor progression by taking active part in invasion and metastasis processes.Citation29,40,41 Fibrocytes are fibroblast progenitor cells of hematopoietic lineage. The expansion of a circulating fibrocyte subset which showed a surface molecular expression pattern similar to the non-monocytic MDSCs and also exhibited immunosuppressive properties was recently reported.Citation20
In the current study, we investigated whether these intriguing findings concerning the blood-borne fibrocytes which were proposed to represent an MDSC subset, were also relevant for the fibroblasts at tumor sites. We utilized a murine chemical breast carcinogenesis model in order to obtain CAFs and conducted functional as well as phenotypical and genotypic assessments, ex vivo. The findings of this study rely on a well-characterized rat chemical breast carcinogenesis model, since cell lines are not representative for the heterogeneity of tumors and xenograft / transplantation type tumor models do not sufficiently represent the human tumors.Citation42,43 NMU induced breast tumor models display similar histopathological lesions with their human counterparts.Citation44,45 Furthermore, the ability of this model to simulate human breast cancer histopathology is probably better than similar models utilized in mice.Citation46
In our study, we were able to demonstrate that CAFs expressed MHC-II. However, its expression was higher in NFs than in CAFs. Given the results stating that patients with metastatic cancer show an expansion of immunosuppressive fibrocytes,Citation20 the downregulation of both the MHC-II molecule and the genes involved in MHC class II antigen presentation on/in CAFs in comparison to NFs might constitute another mechanism for the immune escape of tumors, in addition to the pro-angiogenic and indoleamine 2,3-dioxygenase (IDO) mediated immune suppressive effects of fibrocytes.Citation20 Moreover, we demonstrated that CAFs did not express CD80. In contrast to the fibrocytes that were previously reported to express CD80,Citation30 no-to-low surface expression of CD80 on CAFs might be another immune escape mechanism, similar to what had been previously observed in colon carcinoma cells.Citation47
Even though most studies with human and/or mouse derived cells use antibodies specific to CD11b or CD11c, CD11b/c is a marker commonly used in rat studies.Citation48-53 The OX-42 antibody, which was used in the study, reacts with the CR3 complement (C3bi) receptor and recognizes a common epitope shared between CD11b and CD11c (integrin αM and αX chains).Citation54 This antibody immunoprecipitates three polypeptides with molecular weights of 160, 103 and 95 kD. OX-42 was also shown to inhibit complement mediated rosette formation.Citation54 The findings of the current study demonstrated that CAFs were mildly positive for CD11b/c. In addition, CD11b/c expression in CAFs was lower than that in the circulating fibrocytes. Such a decrease might be ascribed to the difference in tissue locations of the cells (blood vs. tumor). On the other hand, one must also bear in mind the fact that this might also be due to the biological differences between rats and humans. Furthermore, CAFs were previously shown not to express the granulocytic/neutrophilic markers (RP-1 and HIS48). Granulocytes, but not lymphocytes or monocytes, were reported to express the antigen recognized by RP-1 monoclonal antibodyCitation32,55 and HIS48 is also a commonly used phenotypic marker for rat granulocytes.Citation33,56,57 Even though rat MDSCs were first described to express the neutrophil marker that is recognized by HIS48 antibody,Citation5 our results demonstrated that CAFs did not show the exact same surface expression characteristics of neutrophilic MDSCs. In fact, the previous description of rat MDSCs,Citation5 might prove to be insufficient to address various MDSC subsets.
Zhang et al. demonstrated that monocytes from healthy donors cultured with IL-4, differentiated into fibrocytes with similar phenotypic and immunosuppressive properties as those observed in patients with cancer.Citation20 This finding is suggestive for a milieu rich in THELPER2-like cytokines (e.g., IL-4), in which those fibrocytes have differentiated.Citation20,34 It was also previously reported that fibrogenesis is linked with the development of a THELPER2 type response.Citation58 In another study, Pilling et al. reported that PBMCs cultured in serum-free medium containing IL-4 had increased numbers of fibrocytes and those fibrocytes had reduced expression of CD172a, in comparison to the fibrocytes cultured in serum-free medium alone.Citation34 They also suggested that CD172a can be used to determine whether fibrocytes have been differentiated in an environment containing profibrotic THELPER2-like cytokines (IL-4 and/or IL-13) or proinflammatory THELPER1-like cytokines (IL-12 and/or IFNγ). The direct assessment of IL-4 levels in the tumor microenvironment would only demonstrate whether mature fibroblasts reside in a milieu containing this cytokine and it would reveal limited information about the extent of fibroblast exposure to IL-4 during their differentiation. On the other hand, CD172a molecule and the genes related to IL-4 mediated signaling may give hints about the presence of IL-4 in the milieu where the fibrocytes have been differentiated. We found that CD172a was expressed on both CAFs and NFs, the latter displaying higher surface expressions. Furthermore, IL-4 signaling gene set was shown to be upregulated in CAFs compared with the NFs. Our results are consistent with the findings of Zhang et al.,Citation20 suggesting a role for IL-4 in the immunosuppressive effects of fibrocytes/fibroblasts in cancer bearing hosts. Furthermore, Haniffa et al. previously discovered a marked induction of THELPER2 cytokines in T cells exposed to stromal cells, but no increase in THELPER1 cytokines.Citation59 Taken together, all of these findings might suggest a positive feedback loop between stromal cells and the immune system, resulting in a more tumor permissive milieu. In another study, Dugast et al. identified plastic-adherent cells that expressed CD172a, as well as other myeloid markers.Citation60 These cells were able to inhibit proliferation of effector T cells and their suppressive action was under the control of inducible nitric oxide synthase. They proposed that these cells can be defined as MDSCs.Citation60 Our findings demonstrated that CAFs, too, expressed CD172a.
Blood-borne fibrocytes in cancer patients have immunosuppressive properties.Citation20 Accordingly, in our ex vivo model, CAFs also exhibited similar inhibitory effects since PBMC proliferation was significantly decreased in CAF cocultures. NFs were previously reported to have immunoinhibitory effects on T cells.Citation61,62 Pinchuk et al. reported that human colonic myofibroblasts also suppressed the proliferation of peripheral blood lymphocytes.Citation63 However, co-implantation tumor xenograft model experiments revealed that CAFs were able to promote the growth of breast carcinoma cells significantly more than mammary NFs did.Citation29 In accordance with those findings, our results also demonstrated that the negative impact of CAFs on PBMC proliferation was prominent in comparison to NFs.
Although their blood-borne counterparts resembled immature monocytes,Citation20 CAFs were spindle shaped cells that were strongly positive for vimentin. In addition, the phenotypic resemblance between the DCs that are present in the gut-associated lymphoid tissue and CAFs, led us to investigate their similarities further. However, CAFs did not express CD103 and RT1B, which were reported to be expressed by those DCs.
Even though the murine chemical carcinogenesis models are widely used in cancer studies and they were shown to display histopathological similarities with their human counterparts,Citation44,45 differences between rat and human biologies should also be taken into consideration while interpreting the findings of the current study. Hence, we believe it would be useful to confirm the results of the current study by further studies in humans. Moreover, the relationship of fibrocytes and MDSCs, which was pointed out by our findings, might be assessed in more detail by labeling the blood-borne fibrocytes and tracking their localizations in the tumor microenvironment in vivo. CAFs may also be labeled in a co-implantation model in order to investigate whether tissue fibroblasts enter the bloodstream, thus allowing for better understanding of their association with MDSCs.
In summary, this study investigated the phenotype, genotype and functional characteristics of CAFs in contrast to NFs. Although circulating fibrocytes were not directly studied in this project, our findings suggest that fibrocytes at tumor sites (CAFs) show similar expression and functional characteristics to the circulating fibrocytes which represent an MDSC subset, with some modifications; based on the findings of Zhang et al.Citation20 CAFs expressed MHC-II, but the expression was lower than that in NFs. The expression of lower levels of MHC-II molecule and the genes involved in MHC class II antigen presentation, and also the lack of the expression of CD80 together may decrease the antigen presenting capacity and contribute to the mechanisms of tumor immune escape. Although CAFs were positive for CD11b/c, its expression was lower compared to their circulating counterparts. CAFs also showed immunosuppressive effects similar to the blood-borne fibrocytes that represent an MDSC subset. Our findings support a role for IL-4 in the immunosuppressive effects of fibrocytes, too. However, tissue fibrocytes did not express granulocytic/neutrophilic markers and were spindle shaped, contrary to their circulating counterparts.
Methods
Animals and cell isolations
In order to acquire tumor tissue associated fibroblasts, NMU induced experimental breast carcinogenesis model in Sprague Dawley rats was utilized, as previously described.Citation44,64 21 d old female Sprague Dawley rats were injected (n = 20 ) once a week for 4 weeks with i.p. 50 mg/kg NMU (Sigma–Aldrich, St. Louis, MO, USA). When the animals were about 3 mo old, developed breast tumors were surgically harvested under sterile conditions for CAF isolation. The tissues were selected to be minimally necrotic regions of the tumor mass. CAFs were isolated from breast tumor tissues using a previously described protocol that utilizes Collagenase I (1 mg/mL) and Hyaluronidase (125 U/mL) enzymes (Sigma–Aldrich, St. Louis, MO, USA) followed by differential sedimentation and plating.Citation29 Enzymatically digested tissues were then cultured in high serum media conditions which select for fibroblast growth (DMEM supplemented with 10% fetal calf serum at 37°C, 5% CO2).Citation29 The same protocol was also used to isolate NFs from healthy breast tissues (n = 7 ). We used fibroblasts passaged for up to five population doublings for the experiments, in order to minimize culture stress and clonal selection. All animal studies were performed in compliance with the US Department of Health and Human Services Guide for the Care and Use of Laboratory Animals and in accordance with the Helsinki Declaration of 1975. Animal studies were reviewed and approved by “Hacettepe University Institutional Animal Care and Use Committee, Ankara” before the commencement of the experiments (Approval number: 2011/28–3).
Flow cytometry analyses
CAF and NF cells (which were acquired enzymatically from breast tumors and healthy donors, respectively) were analyzed for cell surface phenotypes. Cultures were incubated at most two weeks before the flow cytometry analyses. Fibroblast cells were stained with appropriate monoclonal antibodies in FACS staining solution and were analyzed by FACS Aria II (equipped with 488 nm and 635 nm lasers) using FACS Diva software. Doublets were gated out by plotting voltage pulse width vs. area. Mouse anti-rat MHC Class II (PerCP, OX-6), mouse anti-rat CD11b/c (FITC, OX-42), mouse anti-rat RT1B (PerCP, OX-6) and mouse anti-rat granulocytes (PE, RP-1) were obtained from BD Biosciences, San Jose, CA, USA. Mouse anti-rat CD172a (PE, OX-41), mouse anti-rat CD103 (Alexa Fluor 647, OX-62) and APC-Cy7 labeled streptavidin were obtained from BioLegend, San Diego, CA, USA. Mouse anti-rat CD80 (APC, 3H5) was obtained from Invitrogen, Camarillo, CA, USA. Mouse anti-rat granulocyte (Biotinylated, HIS48) was obtained from eBioscience, San Diego, CA, USA. Non-specific isotype-matched antibodies were used as controls.
PBMCs, cocultures and CFSE proliferation assay
PBMCs were obtained from healthy adult Sprague Dawley rats' peripheral blood (by venipuncture) using density gradient separation with Histopaque-1077 (Sigma–Aldrich, St. Louis, MO, USA), as previously described.Citation65 PBMCs were cultured in RPMI 1640 medium supplemented with 10% fetal calf serum, 2.1 mM L-glutamine, 100 U/mL penicillin and 100 µg/mL streptomycin at 37°C, 5% CO2. Cell counts and viability were assessed using the trypan blue dye exclusion method with a hemocytometer. Proliferation of fresh PBMCs was evaluated by CFSE (Life Technologies, Carlsbad, CA, USA) proliferation assay,Citation66 according to the manufacturer's instructions. 1 × 106 PBMCs were cocultured with 5 × 104 of either NF or CAF cells in a 24-well plate with the presence of 1 µg/mL of phytohemagglutinin (PHA) (Biochrom, Berlin, Germany). CFSE (5 µM) proliferation assay was performed for four d and the percentage of proliferated PBMCs was evaluated with FACS Aria II according to CFSE fluorescent signal intensities.
Gene expression profiling by gene set enrichment analysis
We performed analyses on a publicly available gene expression study. The raw data was extracted from a major public repository of microarray experiments, NCBI Gene Expression Omnibus (GEO).Citation67 Walter et al., 2010, performed microarray experiments in pancreatic CAFs and control fibroblasts investigating signaling pathways important in tumor-stromal cell interactions (data accessible at NCBI GEO database,Citation68 accession GSE21440). This microarray study, titled “Gene expression analysis of pancreatic cancer associated fibroblasts,” included both CAF and NF samples (platform: Affymetrix Human Exon 1.0 ST).
In addition to manual curation and customized scripts, we used methods available from GEOquery,Citation69 oligo,Citation70 limma,Citation71 packages for R/BioconductorCitation72,73 to retrieve the data and get the “ExpressionSet.” After preparing the expression set; we performed GSEA as described previously,Citation31 in order to investigate pathways and biological processes associated with antigen presentation and IL-4 receptor signaling. Gene lists were obtained from Molecular Signatures Database (MSigDB).Citation31 REACTOMEMHC_CLASS_II_ANTIGEN_PRESENTATION” gene set includes genes involved in MHC class II antigen presentation (Reactome DOI: 10.3180/REACT_121399.1). “SIG_IL4RECEPTOR_IN_B_LYPHOCYTES” gene set comprises genes related to IL-4 mediated signalingCitation74 (Signaling Gateway – UCSD Molecule PagesCitation75).
Morphological analyses - immunocytochemistry
Morphological analyses and characterizations of CAF and NF cells were performed by immunocytochemistry stainings with vimentin (Clone V9; ScyTek Laboratories, West Logan, UT, USA), according to the manufacturer's instructions. Cells (6 × 104 cells / 400 µL in each well) were cultured in eight well chamber slides (BD Biosciences, San Jose, CA, USA) and immunocytochemical/morphological assessments were performed when the cells reached a surface confluency of >70 %. These CAFs and NFs were immunostained to show their surface expressions of vimentin, in order to clearly visualize their morphologies and expression characters. A biotin/streptavidin/horseradish peroxidase detection system was utilized and binding of the antibody was demonstrated with diaminobenzidine (DAB) substrate. The images were captured by Olympus BX50 microscope (Tokyo, Japan).
Statistical analyses
Statistical analyses (except for GSEA) were performed with Mann–Whitney U-test using IBM SPSS Statistics 22, with p < 0.05 taken as statistically significant. Gene set enrichment analyses were performed by the software provided by the Broad Institute.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Supplemental Material
Supplemental data for this article can be accessed on the publisher's website.
Supplemental_Material.zip
Download Zip (511.2 KB)Acknowledgments
The authors wish to thank Nilgun Gunaydin for technical assistance during the microarray analyses; Ihsan Gursel and Burcu Beksac for their support; and N Sahin Gunaydin for critical review of the manuscript.
Funding
This study was supported in part by the Scientific and Technological Research Council of Turkey (111S271).
References
- Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol 2009; 9:162-74; PMID:19197294; http://dx.doi.org/10.1038/nri2506
- Senovilla L, Aranda F, Galluzzi L, Kroemer G. Impact of myeloid cells on the efficacy of anticancer chemotherapy. Curr Opin Immunol 2014; 30C:24-31; PMID:24950501; http://dx.doi.org/10.1016/j.coi.2014.05.009
- Bronte V, Wang M, Overwijk WW, Surman DR, Pericle F, Rosenberg SA, Restifo NP. Apoptotic death of CD8+ T lymphocytes after immunization: induction of a suppressive population of Mac-1+/Gr-1+ cells. J Immunol 1998; 161:5313-20; PMID:9820504
- Youn JI, Nagaraj S, Collazo M, Gabrilovich DI. Subsets of myeloid-derived suppressor cells in tumor-bearing mice. J Immunol 2008; 181:5791-802; PMID:18832739; http://dx.doi.org/10.4049/jimmunol.181.8.5791
- Prins RM, Scott GP, Merchant RE, Graf MR. Irradiated tumor cell vaccine for treatment of an established glioma. II. Expansion of myeloid suppressor cells that promote tumor progression. Cancer Immunol, Immunother 2002; 51:190-9; PMID:12012106; http://dx.doi.org/10.1007/s00262-002-0270-x
- Almand B, Clark JI, Nikitina E, van Beynen J, English NR, Knight SC, Carbone DP, Gabrilovich DI. Increased production of immature myeloid cells in cancer patients: a mechanism of immunosuppression in cancer. J Immunol 2001; 166:678-89; PMID:11123353; http://dx.doi.org/10.4049/jimmunol.166.1.678
- Ochoa AC, Zea AH, Hernandez C, Rodriguez PC. Arginase, prostaglandins, and myeloid-derived suppressor cells in renal cell carcinoma. Clin Cancer Res 2007; 13:721s-6s; PMID:17255300; http://dx.doi.org/10.1158/1078-0432.CCR-06-2197
- Schreiber RD, Old LJ, Smyth MJ. Cancer immunoediting: integrating immunity's roles in cancer suppression and promotion. Science 2011; 331:1565-70; PMID:21436444; http://dx.doi.org/10.1126/science.1203486
- Zitvogel L, Tesniere A, Kroemer G. Cancer despite immunosurveillance: immunoselection and immunosubversion. Nat Rev Immunol 2006; 6:715-27; PMID:16977338; http://dx.doi.org/10.1038/nri1936
- Zitvogel L, Tanchot C, Granier C, Tartour E. Following up tumor-specific regulatory T cells in cancer patients. Oncoimmunology 2013; 2:e25444; PMID:24073383; http://dx.doi.org/10.4161/onci.25444
- Pietras K, Ostman A. Hallmarks of cancer: interactions with the tumor stroma. Exp Cell Res 2010; 316:1324-31; PMID:20211171; http://dx.doi.org/10.1016/j.yexcr.2010.02.045
- Kalluri R, Zeisberg M. Fibroblasts in cancer. Nat Rev Cancer 2006; 6:392-401; PMID:16572188; http://dx.doi.org/10.1038/nrc1877
- Sappino AP, Skalli O, Jackson B, Schurch W, Gabbiani G. Smooth-muscle differentiation in stromal cells of malignant and non-malignant breast tissues. Int J Cancer 1988; 41:707-12; PMID:2835323; http://dx.doi.org/10.1002/ijc.2910410512
- Dvorak HF. Tumors: wounds that do not heal. Similarities between tumor stroma generation and wound healing. N Engl J Med 1986; 315:1650-9; PMID:3537791; http://dx.doi.org/10.1056/NEJM198612253152606
- Schauer IG, Sood AK, Mok S, Liu J. Cancer-associated fibroblasts and their putative role in potentiating the initiation and development of epithelial ovarian cancer. Neoplasia 2011; 13:393-405; PMID:21532880; http://dx.doi.org/10.1593/neo.101720
- Kharaziha P, Rodriguez P, Li Q, Rundqvist H, Bjorklund AC, Augsten M, Ullen A, Egevad L, Wiklund P, Nilsson S et al. Targeting of distinct signaling cascades and cancer-associated fibroblasts define the efficacy of Sorafenib against prostate cancer cells. Cell Death Dis 2012; 3:e262; PMID:22278289; http://dx.doi.org/10.1038/cddis.2012.1
- Ostman A, Augsten M. Cancer-associated fibroblasts and tumor growth–bystanders turning into key players. Curr Opin Genet Dev 2009; 19:67-73; PMID:19211240; http://dx.doi.org/10.1016/j.gde.2009.01.003
- Joyce JA, Pollard JW. Microenvironmental regulation of metastasis. Nat Rev Cancer 2009; 9:239-52; PMID:19279573; http://dx.doi.org/10.1038/nrc2618
- Giannoni E, Bianchini F, Masieri L, Serni S, Torre E, Calorini L, Chiarugi P. Reciprocal activation of prostate cancer cells and cancer-associated fibroblasts stimulates epithelial-mesenchymal transition and cancer stemness. Cancer Res 2010; 70:6945-56; PMID:20699369; http://dx.doi.org/10.1158/0008-5472.CAN-10-0785
- Zhang H, Maric I, DiPrima MJ, Khan J, Orentas RJ, Kaplan RN, Mackall CL. Fibrocytes represent a novel MDSC subset circulating in patients with metastatic cancer. Blood 2013; 122:1105-13; PMID:23757729; http://dx.doi.org/10.1182/blood-2012-08-449413
- Talmadge JE, Gabrilovich DI. History of myeloid-derived suppressor cells. Nat Rev Cancer 2013; 13:739-52; PMID:24060865; http://dx.doi.org/10.1038/nrc3581
- Jitschin R, Braun M, Buttner M, Dettmer-Wilde K, Bricks J, Berger J, Eckart MJ, Krause SW, Oefner PJ, Le Blanc K et al. CLL-cells induce IDOhi CD14+HLA-DRlo myeloid derived suppressor cells that inhibit T-cell responses and promote TRegs. Blood 2014; 124(5):750–760; PMID:24850760; http://dx.doi.org/10.1182/blood-2013-12-546416
- Schirmer C, Klein C, von Bergen M, Simon JC, Saalbach A. Human fibroblasts support the expansion of IL-17-producing T cells via up-regulation of IL-23 production by dendritic cells. Blood 2010; 116:1715-25; PMID:20538798; http://dx.doi.org/10.1182/blood-2010-01-263509
- Quan TE, Cowper SE, Bucala R. The role of circulating fibrocytes in fibrosis. Curr Rheumatol Rep 2006; 8:145-50; PMID:16569374; http://dx.doi.org/10.1007/s11926-006-0055-x
- Reilkoff RA, Bucala R, Herzog EL. Fibrocytes: emerging effector cells in chronic inflammation. Nat Rev Immunol 2011; 11:427-35; PMID:21597472; http://dx.doi.org/10.1038/nri2990
- Shinde AV, Frangogiannis NG. Fibroblasts in myocardial infarction: a role in inflammation and repair. J Mol Cell Cardiol 2014; 70:74-82; PMID:24321195; http://dx.doi.org/10.1016/j.yjmcc.2013.11.015
- Andersson-Sjoland A, de Alba CG, Nihlberg K, Becerril C, Ramirez R, Pardo A, Westergren-Thorsson G, Selman M. Fibrocytes are a potential source of lung fibroblasts in idiopathic pulmonary fibrosis. Int J Biochem Cell Biol 2008; 40:2129-40; PMID:18374622; http://dx.doi.org/10.1016/j.biocel.2008.02.012
- Ogawa M, LaRue AC, Drake CJ. Hematopoietic origin of fibroblasts/myofibroblasts: Its pathophysiologic implications. Blood 2006; 108:2893-6; PMID:16840726; http://dx.doi.org/10.1182/blood-2006-04-016600
- Orimo A, Gupta PB, Sgroi DC, Arenzana-Seisdedos F, Delaunay T, Naeem R, Carey VJ, Richardson AL, Weinberg RA. Stromal fibroblasts present in invasive human breast carcinomas promote tumor growth and angiogenesis through elevated SDF-1/CXCL12 secretion. Cell 2005; 121:335-48; PMID:15882617; http://dx.doi.org/10.1016/j.cell.2005.02.034
- Chesney J, Bacher M, Bender A, Bucala R. The peripheral blood fibrocyte is a potent antigen-presenting cell capable of priming naive T cells in situ. Proc Natl Acad Sci U S A 1997; 94:6307-12; PMID:9177213; http://dx.doi.org/10.1073/pnas.94.12.6307
- Subramanian A, Tamayo P, Mootha VK, Mukherjee S, Ebert BL, Gillette MA, Paulovich A, Pomeroy SL, Golub TR, Lander ES et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci U S A 2005; 102:15545-50; PMID:16199517; http://dx.doi.org/10.1073/pnas.0506580102
- Gotoh S, Itoh M, Fujii Y, Arai S, Sendo F. Enhancement of the expression of a rat neutrophil-specific cell surface antigen by activation with phorbol myristate acetate and concanavalin A. J Immunol 1986; 137:643-50; PMID:2424975
- Rhodes NP, Hunt JA, Williams DF. Macrophage subpopulation differentiation by stimulation with biomaterials. J Biomed Mater Res 1997; 37:481-8; PMID:9407296; http://dx.doi.org/10.1002/(SICI)1097-4636(19971215)37:4%3c481::AID-JBM6%3e3.0.CO;2-H
- Pilling D, Fan T, Huang D, Kaul B, Gomer RH. Identification of markers that distinguish monocyte-derived fibrocytes from monocytes, macrophages, and fibroblasts. PloS One 2009; 4:e7475; PMID:19834619; http://dx.doi.org/10.1371/journal.pone.0007475
- Annacker O, Coombes JL, Malmstrom V, Uhlig HH, Bourne T, Johansson-Lindbom B, Agace WW, Parker CM, Powrie F. Essential role for CD103 in the T cell-mediated regulation of experimental colitis. J Exp Med 2005; 202:1051-61; PMID:16216886; http://dx.doi.org/10.1084/jem.20040662
- Munoz L, Jose Borrero M, Ubeda M, Lario M, Diaz D, Frances R, Monserrat J, Pastor O, Aguado-Fraile E, Such J et al. Interaction between intestinal dendritic cells and bacteria translocated from the gut in rats with cirrhosis. Hepatology 2012; 56:1861-9; PMID:22611024; http://dx.doi.org/10.1002/hep.25854
- Kraman M, Bambrough PJ, Arnold JN, Roberts EW, Magiera L, Jones JO, Gopinathan A, Tuveson DA, Fearon DT. Suppression of antitumor immunity by stromal cells expressing fibroblast activation protein-α. Science 2010; 330:827-30; PMID:21051638; http://dx.doi.org/10.1126/science.1195300
- Chang HY, Chi JT, Dudoit S, Bondre C, van de Rijn M, Botstein D, Brown PO. Diversity, topographic differentiation, and positional memory in human fibroblasts. Proc Natl Acad Sci U S A 2002; 99:12877-82; PMID:12297622; http://dx.doi.org/10.1073/pnas.162488599
- Baglole CJ, Ray DM, Bernstein SH, Feldon SE, Smith TJ, Sime PJ, Phipps RP. More than structural cells, fibroblasts create and orchestrate the tumor microenvironment. Immunol Invest 2006; 35:297-325; PMID:16916756; http://dx.doi.org/10.1080/08820130600754960
- Dimanche-Boitrel MT, Vakaet L Jr., Pujuguet P, Chauffert B, Martin MS, Hammann A, Van Roy F, Mareel M, Martin F. In vivo and in vitro invasiveness of a rat colon-cancer cell line maintaining E-cadherin expression: an enhancing role of tumor-associated myofibroblasts. Int J Cancer 1994; 56:512-21; PMID:8112888; http://dx.doi.org/10.1002/ijc.2910560410
- Gaggioli C, Hooper S, Hidalgo-Carcedo C, Grosse R, Marshall JF, Harrington K, Sahai E. Fibroblast-led collective invasion of carcinoma cells with differing roles for RhoGTPases in leading and following cells. Nat Cell Biol 2007; 9:1392-400; PMID:18037882; http://dx.doi.org/10.1038/ncb1658
- Ostrand-Rosenberg S. Animal models of tumor immunity, immunotherapy and cancer vaccines. Curr Opin Immunol 2004; 16:143-50; PMID:15023405; http://dx.doi.org/10.1016/j.coi.2004.01.003
- Vargo-Gogola T, Rosen JM. Modelling breast cancer: one size does not fit all. Nat Rev Cancer 2007; 7:659-72; PMID:17721431; http://dx.doi.org/10.1038/nrc2193
- Singh M, McGinley JN, Thompson HJ. A comparison of the histopathology of premalignant and malignant mammary gland lesions induced in sexually immature rats with those occurring in the human. Lab Invest 2000; 80:221-31; PMID:10701691; http://dx.doi.org/10.1038/labinvest.3780025
- Thompson HJ, Singh M. Rat models of premalignant breast disease. J Mammary Gland Biol Neoplasia 2000; 5:409-20; PMID:14973385; http://dx.doi.org/10.1023/A:1009582012493
- Iannaccone PM, Jacob HJ. Rats! Dis Models Mech 2009; 2:206-10; PMID:19407324
- Tirapu I, Huarte E, Guiducci C, Arina A, Zaratiegui M, Murillo O, Gonzalez A, Berasain C, Berraondo P, Fortes P et al. Low surface expression of B7-1 (CD80) is an immunoescape mechanism of colon carcinoma. Cancer Res 2006; 66:2442-50; PMID:16489051; http://dx.doi.org/10.1158/0008-5472.CAN-05-1681
- Cheng HY, Ghetu N, Huang WC, Wang YL, Wallace CG, Wen CJ, Chen HC, Shih LY, Lin CF, Hwang SM et al. Syngeneic adipose-derived stem cells with short-term immunosuppression induce vascularized composite allotransplantation tolerance in rats. Cytotherapy 2014; 16:369-80; PMID:24119648; http://dx.doi.org/10.1016/j.jcyt.2013.06.020
- Furuhashi K, Tsuboi N, Shimizu A, Katsuno T, Kim H, Saka Y, Ozaki T, Sado Y, Imai E, Matsuo S et al. Serum-starved adipose-derived stromal cells ameliorate crescentic GN by promoting immunoregulatory macrophages. J Am Soc Nephrol 2013; 24:587-603; PMID:23471196; http://dx.doi.org/10.1681/ASN.2012030264
- Takeda Y, Marumo M, Wakabayashi I. Attenuated phagocytic activity of monocytes in type 2 diabetic Goto-Kakizaki rats. Immunobiology 2011; 216:1094-102; PMID:21652107; http://dx.doi.org/10.1016/j.imbio.2011.05.003
- Thomas RA, Pietrzak DC, Scicchitano MS, Thomas HC, McFarland DC, Frazier KS. Detection and characterization of circulating endothelial progenitor cells in normal rat blood. J Pharmacol Toxicol Methods 2009; 60:263-74; PMID:19577656; http://dx.doi.org/10.1016/j.vascn.2009.06.002
- Muehlbauer SM, Lima H Jr., Goldman DL, Jacobson LS, Rivera J, Goldberg MF, Palladino MA, Casadevall A, Brojatsch J. Proteasome inhibitors prevent caspase-1-mediated disease in rodents challenged with anthrax lethal toxin. Am J Pathol 2010; 177:735-43; PMID:20595632; http://dx.doi.org/10.2353/ajpath.2010.090828
- Mayer AM, Zhang P, Spitzer JA. Increased surface expression of CD11b/c and CD18 appears to be dissociated from anti-CD11b/c monoclonal antibody stimulated O2- anion generation in in vivo Escherichia coli lipopolysaccharide and tumor necrosis factor-α-treated rat neutrophils. Shock 1994; 2:289-95; PMID:7757523; http://dx.doi.org/10.1097/00024382-199410000-00010
- Robinson AP, White TM, Mason DW. Macrophage heterogeneity in the rat as delineated by two monoclonal antibodies MRC OX-41 and MRC OX-42, the latter recognizing complement receptor type 3. Immunology 1986; 57:239-47; PMID:3512425
- Yrlid U, Jenkins CD, MacPherson GG. Relationships between distinct blood monocyte subsets and migrating intestinal lymph dendritic cells in vivo under steady-state conditions. J Immunol 2006; 176:4155-62; PMID:16547252; http://dx.doi.org/10.4049/jimmunol.176.7.4155
- Badger DA, Sauer JM, Hoglen NC, Jolley CS, Sipes IG. The role of inflammatory cells and cytochrome P450 in the potentiation of CCl4-induced liver injury by a single dose of retinol. Toxicol Appl Pharmacol 1996; 141:507-19; PMID:8975775; http://dx.doi.org/10.1006/taap.1996.0316
- Reckless J, Tatalick LM, Grainger DJ. The pan-chemokine inhibitor NR58-3.14.3 abolishes tumour necrosis factor-α accumulation and leucocyte recruitment induced by lipopolysaccharide in vivo. Immunology 2001; 103:244-54; PMID:11412312; http://dx.doi.org/10.1046/j.1365-2567.2001.01228.x
- Wynn TA. Fibrotic disease and the T(H)1/T(H)2 paradigm. Nat Rev Immunol 2004; 4:583-94; PMID:15286725; http://dx.doi.org/10.1038/nri1412
- Haniffa MA, Wang XN, Holtick U, Rae M, Isaacs JD, Dickinson AM, Hilkens CM, Collin MP. Adult human fibroblasts are potent immunoregulatory cells and functionally equivalent to mesenchymal stem cells. J Immunol 2007; 179:1595-604; PMID:17641026; http://dx.doi.org/10.4049/jimmunol.179.3.1595
- Dugast AS, Haudebourg T, Coulon F, Heslan M, Haspot F, Poirier N, Vuillefroy de Silly R, Usal C, Smit H, Martinet B et al. Myeloid-derived suppressor cells accumulate in kidney allograft tolerance and specifically suppress effector T cell expansion. J Immunol 2008; 180:7898-906; PMID:18523253; http://dx.doi.org/10.4049/jimmunol.180.12.7898
- Davis LS, Oppenheimer-Marks N, Bednarczyk JL, McIntyre BW, Lipsky PE. Fibronectin promotes proliferation of naive and memory T cells by signaling through both the VLA-4 and VLA-5 integrin molecules. J Immunol 1990; 145:785-93; PMID:1695644
- Jones S, Horwood N, Cope A, Dazzi F. The antiproliferative effect of mesenchymal stem cells is a fundamental property shared by all stromal cells. J Immunol 2007; 179:2824-31; PMID:17709496; http://dx.doi.org/10.4049/jimmunol.179.5.2824
- Pinchuk IV, Saada JI, Beswick EJ, Boya G, Qiu SM, Mifflin RC, Raju GS, Reyes VE, Powell DW. PD-1 ligand expression by human colonic myofibroblasts/fibroblasts regulates CD4+ T-cell activity. Gastroenterology 2008; 135:1228-37, 37 e1-2; PMID:18760278; http://dx.doi.org/10.1053/j.gastro.2008.07.016
- Macejova D, Brtko J. Chemically induced carcinogenesis: a comparison of 1-methyl-1-nitrosourea, 7,12-dimethylbenzanthracene,diethylnitroso-amine and azoxymethan models (minireview). Endocr Regul 2001; 35:53-9; PMID:11308997
- Fazzino F, Obregon F, Lima L. Taurine and proliferation of lymphocytes in physically restrained rats. J Biomed Sci 2010; 17 Suppl 1:S24; PMID:20804599; http://dx.doi.org/10.1186/1423-0127-17-S1-S24
- Lyons AB. Analysing cell division in vivo and in vitro using flow cytometric measurement of CFSE dye dilution. J Immunol Methods 2000; 243:147-54; PMID:10986412; http://dx.doi.org/10.1016/S0022-1759(00)00231-3
- Barrett T, Wilhite SE, Ledoux P, Evangelista C, Kim IF, Tomashevsky M, Marshall KA, Phillippy KH, Sherman PM, Holko M et al. NCBI GEO: archive for functional genomics data sets–update. Nucleic Acids Res 2013; 41:D991-5; PMID:23193258; http://dx.doi.org/10.1093/nar/gks1193
- Walter K, Omura N, Hong SM, Griffith M, Vincent A, Borges M, Goggins M. Overexpression of smoothened activates the sonic hedgehog signaling pathway in pancreatic cancer-associated fibroblasts. Clin Cancer Res 2010; 16:1781-9; PMID:20215540; http://dx.doi.org/10.1158/1078-0432.CCR-09-1913
- Davis S, Meltzer PS. GEOquery: a bridge between the Gene Expression Omnibus (GEO) and BioConductor. Bioinformatics 2007; 23:1846-7; PMID:17496320; http://dx.doi.org/10.1093/bioinformatics/btm254
- Carvalho BS, Irizarry RA. A framework for oligonucleotide microarray preprocessing. Bioinformatics 2010; 26:2363-7; PMID:20688976; http://dx.doi.org/10.1093/bioinformatics/btq431
- Smyth GK. Limma: linear models for microarray data. In: Gentleman R, Carey V, Dudoit S, Irizarry R, Huber W, eds. Bioinformatics and Computational Biology Solutions Using R and Bioconductor. New York: Springer, 2005:397-420; http://dx.doi.org/10.1007/0-387-29362-0
- Ihaka R, Gentleman R. R: a language for data analysis and graphics. J Comput Graphic Stat 1996; 5:299-314; http://dx.doi.org/10.1080/10618600.1996.10474713
- Gentleman RC, Carey VJ, Bates DM, Bolstad B, Dettling M, Dudoit S, Ellis B, Gautier L, Ge Y, Gentry J et al. Bioconductor: open software development for computational biology and bioinformatics. Genome Biol 2004; 5:R80; PMID:15461798; http://dx.doi.org/10.1186/gb-2004-5-10-r80
- Kelly-Welch AE, Hanson EM, Boothby MR, Keegan AD. Interleukin-4 and interleukin-13 signaling connections maps. Science 2003; 300:1527-8; PMID:12791978; http://dx.doi.org/10.1126/science.1085458
- Saunders B, Lyon S, Day M, Riley B, Chenette E, Subramaniam S, Vadivelu I. The molecule pages database. Nucleic Acids Res 2008; 36:D700-6; PMID:17965093; http://dx.doi.org/10.1093/nar/gkm907
- Miller CC, Godeau G, Lebreton-DeCoster C, Desmouliere A, Pellat B, Dubertret L, Coulomb B. Validation of a morphometric method for evaluating fibroblast numbers in normal and pathologic tissues. Exp Dermatol 2003; 12:403-11; PMID:12930296; http://dx.doi.org/10.1034/j.1600-0625.2003.00023.x
- De LKT, Whiting CV, Bland PW. Proinflammatory cytokine synthesis by mucosal fibroblasts from mouse colitis is enhanced by interferon-gamma-mediated up-regulation of CD40 signalling. Clin Exp Immunol 2007; 147:313-23; PMID:17223973
- Boquest AC, Day BN, Prather RS. Flow cytometric cell cycle analysis of cultured porcine fetal fibroblast cells. Biol Reprod 1999; 60:1013-9; PMID:10084979; http://dx.doi.org/10.1095/biolreprod60.4.1013
- Aslakson CJ, Miller FR. Selective events in the metastatic process defined by analysis of the sequential dissemination of subpopulations of a mouse mammary tumor. Cancer Res 1992; 52:1399-405; PMID:1540948
