 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
9 kDa granulysin is a protein present in the granules of human CTL and NK cells, with cytolytic activity against microbes and tumors. Previous work from our group demonstrated that this granulysin isoform induced apoptosis in vitro on hematological tumor cells and on primary tumor cells from B-CLL patients. In the present work, recombinant 9 kDa granulysin was used as an anti-tumoral agent to study its in vivo effect on tumor development in athymic “nude” mice models bearing human breast adenocarcinoma MDA-MB-231 or multiple myeloma NCI-H929–derived xenografts. Granulysin prevented the in vivo development of detectable MDA-MB-231-derived tumors. In addition, recombinant granulysin was able to completely eradicate NCI-H929-derived tumors. All granulysin-treated tumors exhibited signs of apoptosis induction and an increased NK cell infiltration inside the tumor tissue comparing to control ones. Moreover, no in vivo deleterious effects of the recombinant 9 kDa granulysin doses used in this study were observed on the skin or on the internal organs of the animals. In conclusion, granulysin was able to inhibit the progression of MDA-MB-231-derived xenografts and also to eradicate multiple myeloma NCI-H929-derived xenografts. This work opens the door to the initiation of preclinical and possibly clinical studies for the use of 9 kDa granulysin as a new anti-tumoral treatment.
Abbreviations:
- GNLY, granulysin
- CTL, cytotoxic T lymphocyte
- NK, natural killer
- PBS, phosphate buffered saline
- FCS, fetal calf serum
- DNA, deoxy-ribonucleic acid
- mAb, monoclonal antibody
- B-CLL, B cell chronic lymphocytic leukemia
- LPS, lipopolysaccharide
- ROS, reactive oxygen species
- AIF, apoptosis inducing factor
- PBMC, peripheral blood mononuclear cells
- LAL, Limulus amoebocyte lysate
- FITC, fluorescein isothiocianate
- PE, phycoerythrin
- 7-AAD, 7-amine-actinomycin D
- H&E, hematoxylin/eosin
- EpCAM, epithelial cell adhesion molecule
- ALT, alanine aminotransferase
Introduction
Granulysin is a protein with two isoforms, of 9 and 15 kDa molecular weight respectively.Citation1 The 9 kDa isoform is a cytolytic protein present in the granules of activated human cytotoxic T lymphocytes (CTL) and natural killer (NK) cells.Citation1 The 15 kDa isoform is rather constitutively secreted from these cells, it is not cytotoxic and induces monocytes to differentiate to dendritic cells.Citation2 It has been demonstrated that the main role of the 9 kDa cytotoxic isoform is the killing of intracellular bacteria such as M. Tuberculosis in concert with perforin.Citation3 Granulysin is also able to kill other bacterial types,Citation4 fungi such as Cryptococcus Neoformans,Citation5 viruses such as Varicella Zoster,Citation6 playing also a main role in immunity against leprosy.Citation7 Recently, it has been also suggested that, making pores on the surface of bacteria, granulysin facilitates bacterial lysis exerted by granzyme B.Citation8
In addition, at least in vitro, 9 kDa granulysin is able to directly kill tumor cells.Citation1,9-11 On the other hand, it has been shown that the 15 kDa form, through its action on antigen-presenting cells, acts rather as an immune alarmin.Citation12
Our group demonstrated in pioneering works that recombinant 9 kDa granulysin was able to induce apoptotic cell death in acute T-cell leukemia Jurkat cells.Citation10,11,13 In a more recent and detailed work, our group has demonstrated that granulysin induced apoptotic cell death on Jurkat cells, with PS exposure at the plasma membrane preceding membrane rupture, caspase-3 activation and apoptotic nuclear morphology.Citation9 It was also demonstrated that the first biochemical signal in recombinant granulysin-induced apoptosis is the increase in intracellular [Ca2+], which induces the generation of mitochondrial reactive oxygen species (ROS) preceding the loss of mitochondrial membrane potential and release of cytochrome c and apoptosis-inducing factor (AIF, Citation9,11,14). In addition, Jurkat cells over-expressing Bcl-2 or Bcl-XL or lacking Bax and Bak or Bim expression were protected from granulysin-induced apoptosis.Citation9 On the other hand, the mechanism of cell death induced by granulysin delivered by perforin in cytotoxic cells seems to be different to that reported for recombinant granulysin alone, and mainly dependent in this last case on ER stress signals.Citation15
It is well characterized that CTL and NK cells, the two cell types that express granulysin, are the main mediators of immune surveillance against cancer.Citation16 In this connection, granulysin expression has been correlated with good outcomes in a variety of cancers. Kishi et al. first showed that low intracellular expression of granulysin in NK cells correlated with progression of cancer.Citation17 Pages et al. found in a complete transcriptome study in colorectal carcinoma that low granulysin expression in effector memory T cells in tumor infiltrates correlated with early metastasis and poor survival rates, while high levels correlated with good outcomes.Citation18 Other authors have suggested that granulysin expression is a useful biomarker for outcomes in gastric carcinoma Citation19 and neuroblastoma.Citation20 In addition, transgenic mice expressing human granulysin are partially protected against tumor development as compared to wild-type mice.Citation21 Of note, granulysin has no homolog in mice, so its anti-tumoral role cannot be studied in knockout mice models.
Independently of its possible physiological anti-tumoral role, recombinant granulysin is a good candidate to be used directly in tumor therapy. Peptides derived from granulysin and NK-lysin have shown their anti-tumor potential in vivo.Citation22 However, peptides derived from granulysin, but not granulysin itself, are hemolytic on erythrocytes.Citation23 Accordingly, it should be desirable to use recombinant granulysin as a whole molecule in future cancer therapies. In this line, in our previous study granulysin was tested on several human multiple myeloma cell lines showing different degrees of sensitivity. In that study, it was also demonstrated that granulysin was not cytotoxic against PBMC from healthy donors. Moreover, granulysin was tested on cells from B-cell chronic lymphocytic leukemia (B-CLL) patients in ex vivo cultures, showing that it was cytotoxic against these primary tumor cells.Citation9
As the following step in this research, we have tested in the present work the use of recombinant granulysin as an anti-tumoral treatment in two in vivo models of tumor development: breast adenocarcinoma, the tumor with higher incidence in women, and multiple myeloma, an hematological malignancy with bad prognosis, where new treatments are needed.
Results
In vitro analysis of the cytotoxic capacity of recombinant granulysin
In our previous studies, we have demonstrated that Jurkat T-cell leukemia is highly sensitive to granulysin cytotoxicity.Citation9,10 Hence, before beginning the in vivo experiments, we tested in parallel the in vitro toxicity of recombinant granulysin batches on MDA-MB-231 and NCI-H929 cells and on Jurkat cells, used as standard.
As indicated above, we chose breast adenocarcinoma and multiple myeloma models to study the in vivo effect of granulysin, specifically tumors induced in athymic mice by MDA-MB-231 and NCI-H929 cell lines, respectively.
Contrary to that observed for Jurkat cells, MDA-MB-231 cells showed no sensitivity to 50 µM granulysin after 4 h of incubation (data not shown). Extending the treatment to 24 h, and augmenting the granulysin concentration to 75 µM, a slight but detectable increase of granulysin-induced cell death was observed on MDA-MB-231 cells (around 20%), while granulysin-induced cell death on Jurkat cells was around 70% ().
Figure 1. In vitro granulysin-induced death of Jurkat, MDA-MB-231 and NCI-H929 cells. Jurkat (A, B), MDA-MB-231 (A) and NCI-H929 cells (B) were incubated or not (CRTL) with 75 (A) or 50 µM (B) recombinant granulysin (GNLY) during 24 (A) or 18 h (B), as indicated. Apoptotic cell death was determined by phosphatidylserine exposure by staining with Annexin-V-FITC and plasma membrane integrity by nuclear staining with 7-AAD. Numbers in the dot plots correspond to the percentage of cells in each quadrant. Bar diagrams represent the mean ± SD of all experiments performed, at least three for each experimental condition, showing the specific percentages of annexin-V-FITC and/or 7-AAD positive cells.
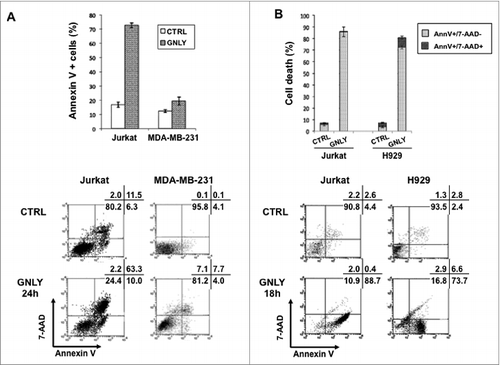
In contrast, NCI-H929 cells showed a high sensitivity to the cytotoxic effect of granulysin. After 18 h of treatment with 50 µM granulysin, cell death observed in H929 cells arrived approximately to 80%, similar to that observed in Jurkat cells ().
These data are in agreement with a previous study on the sensitivity of several human multiple myeloma cell lines to recombinant granulysin.Citation9
In vivo effect of recombinant granulysin on MDA-MB-231-induced tumors
Before performing the in vivo experiments, several human cell lines with different degree of sensitivity to granulysin were tested for their ability to induce tumors in athymic “nude” mice. 1 × 106 to 10 × 106 Jurkat cells, or multiple myeloma cell lines NCI-H929, MM1.S, and RPMI-8226, or MDA-MB-231 breast adenocarcinoma cells, were inoculated by subcutaneous (s.c.) injection, either resuspended in PBS or in Matrigel. Jurkat, MM1.S or RPMI-8226 cells did not induce detectable tumors at least after 6 mo of the injections. NCI-H929 cells induced detectable tumors after around 2 mo in 60% of the mice, but only when 10 × 106 cells were injected resuspended in Matrigel. Finally, 1 × 106 or 10 × 106 MDA-MB-231 cells resuspended in PBS induced detectable tumors in 100% of the mice after around 2 weeks of tumor injection, showing a high aggressiveness.
In spite of MDA-MB-231 cells were only partially sensitive to granuysin-induced cell death, they were used in the initial in vivo experiments due to their high efficiency of tumor induction.
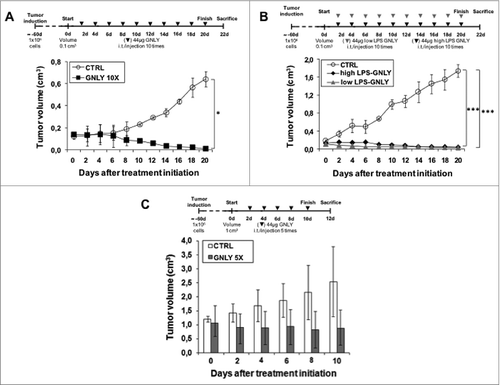
For tumor induction in the MDA-MB-231-xenograft model, 1 × 106 cells were injected s.c., and treatments were started when tumor volume arrived to 0.1 cm3, between 2 to 4 weeks after tumor injection. Mice were then randomly divided into two subgroups based on matched tumor volumes: the group treated with granulysin and the control group, treated with PBS. 44 μg of granulysin in 50 µL PBS was injected intratumorally every 2 d for five times and after that, mice were sacrificed and tumors were resected. As shown in , the mean tumor volume in the group treated with granulysin was around 50% of that observed in the group treated with PBS at the end of the treatment, with clear differences in tumor volume from the 3rd injection on. The mean ± SD of tumor volume after the 5th injection in the control group was of 0.60 ± 0.2 cm3, while it was of 0.34 ± 0.1 cm3 in the granulysin group, being the difference statistically significant. These significant differences were confirmed when measuring the weight and size of the resected tumors at the end of the experiment (data not shown).
Figure 2. Granulysin treatment of nude mice bearing MDA-MB-231 human mammary cancer xenografts. (A) 1 × 106 MDA-MB-231 mammary cancer cells were injected s.c. in 10 nude mice. After around 15 d, when tumor volume had arrived to 0.1 cm3, mice were divided in two groups, CTRL and GNLY-treated, n = 5 for each experimental group. Mice in the GNLY group received intra-tumoral injections of GNLY at 44 μg in 50 µL of PBS every 2 d for five times and then mice were sacrificed. Mice in the CTRL group received injections of 50 µL PBS with the same time schedule. (B) the same protocol as in A was followed, with the difference that granulysin-treated mice were divided in two groups, in one of them (GNLY 10X), mice were treated for 10 times with intra-tumoral granulysin, and in the other group (GNLY 5X), mice were treated for only five times, leaving them without any treatment until the end of the experiment. Data are the mean ± SD of the tumor volume in each group of the study. *, p < 0.05. (C) Left panels: Cells obtained from tumors resected in the experiment shown in A, were stained with a FITC-labeled anti-EpCAM mAb and with PE-labeled Annexin-V staining and analyzed by flow cytometry as shown in the dot plots. Numbers in the dot plots correspond to the percentage of cells in each quadrant. Right panel: Percentage of apoptotic tumoral cells in the resected tissue from each experimental group.
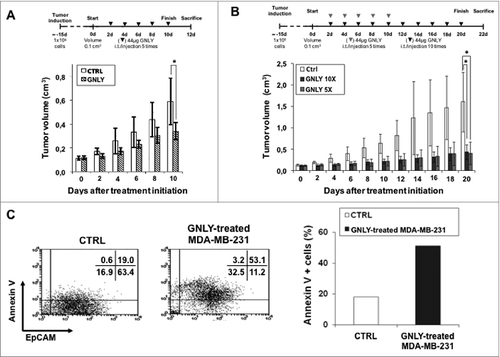
In a second set of experiments, we wanted to evaluate the effect of granulysin even if the treatment was stopped at a given moment of the experiment along the time. For this purpose, we followed the protocol described above, but mice treated with granulysin were divided into two groups: one group of mice was treated for 10 times while the other group was treated only for five times and left without any treatment until the end of the experiment. As shown in , granulysin was able to substantially inhibit tumor growth in both groups of mice and at a similar extent, around a 70%, being the differences statistically significant in both cases. These significant differences were confirmed when measuring the weight and size of the resected tumors at the end of the experiment (data not shown).
In order to analyze if the reduction of tumor growth observed in the MDA-MB-231 in vivo model was associated with apoptosis induction, and as a first approach, we took one representative tumor from the control and from the granulysin group of the experiment shown in , excised a small part, and minced it carefully into pieces, collecting cells by using a mechanical spill out method. The collected cells from each tumor tissue were stained with the apoptotic marker annexin-V-PE and also with a monoclonal antibody against the epithelial cell adhesion molecule (EpCAM), which is a membrane glycoprotein highly expressed on most carcinomas including breast adenocarcinoma and therefore allows us to identify tumor cells. As shown in , the percentage of Annexin V+ tumor cells (EpCAM+ cells) was substantially higher in the tumor treated with granulysin than in the control tumor, suggesting that granulysin treatment in vivo induce apoptosis efficiently on MDA-MB-231 adenocarcinoma cells.
Histological studies of resected MDA-MB-231-derived tumors
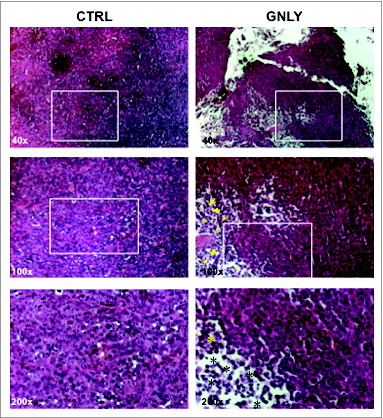
For histological studies, H&E staining was carried out using paraffin-embedded tumor samples from the experiments previously described. As shown in (left panels), untreated tumors from the control group presented a compact tissue structure, with high cellularity. On the contrary, granulysin-treated tumors showed a much less compact organization, with empty areas clearly observed inside the tumor mass (; right panels). Regarding possible infiltrations of the tumors with immune cells, this is only observed in the tumors treated with granulysin. In the higher magnification images it can be observed that in this case the infiltrate is mostly mononuclear (marked with asteriks), with small contribution of a polymorphonuclear infiltrate (marked with arrows). In these experiments, developed during at least two weeks, although the initial infiltration could correspond at least in part to an “acute” one, mostly polymorphonuclear, that observed at the end of the experiments should rather be of a “chronic” type, that is usually mediated by mononuclear cells, normally T cells, but, given that in this mice T cells are absent, it was explored the possibility of being mediated by NK cells (see below).
Figure 3. H&E staining in histological sections of MDA-MB-231-derived tumors. Representative images of resected MDA-MB-231-derived tumors from the control group (CTRL) or from the granulysin-treated group (GNLY) of the experiment in are shown. Paraffin tissue sections were stained with standard H&E coloration, as indicated in Material and Methods. Magnifications were of 40X in the upper panels, 100X in the middle panels and 200X in the lower panels. Squares correspond in each image to the zone that is enlarged below, arrows to the polymorphonuclear infiltrate, and asterisks to the mononuclear infiltrate.
In order to analyze apoptotic features in vivo, we first stained tumor tissue sections with Hoechst 33342 to evaluate nuclear morphology. As shown in , top panels, untreated tumors from the control group displayed flattened living cells with non-condensed chromatin, while granulysin-treated tumors showed less cellularity, with shrinked cells, most of them with condensed chromatin, and in some cases with fragmented nuclei characteristic of apoptosis induction
Figure 4. Nuclear staining and immunostaining for caspase-3 and NK1.1 in histological sections of MDA-MB-231-derived tumors. (A, B) Representative images of resected MDA-MB-231-derived tumors from the control group (CTRL) or from the granulysin-treated group (GNLY) of the experiment in are shown. Nuclei were stained using the Hoechst 33342 nuclear dye (upper panels in A) or immunostained with anti-caspase-3 antibody (lower panels in A) or with anti-NK1.1 antibody (B), as indicated, and photographed in a fluorescence microscope at 400X (A) or 200X (B) magnification. (C) Fluorescent intensity in the caspase-3 or NK1.1 immunostained sections was quantified using the freeware ImageJ v1.33 and results expressed as the mean±SD of the different sections analyzed (at least 10 in each experimental condition) as arbitraty units folllowing the recommendations of the software. **p < 0.02.
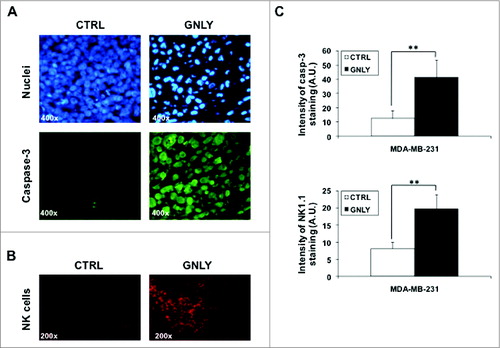
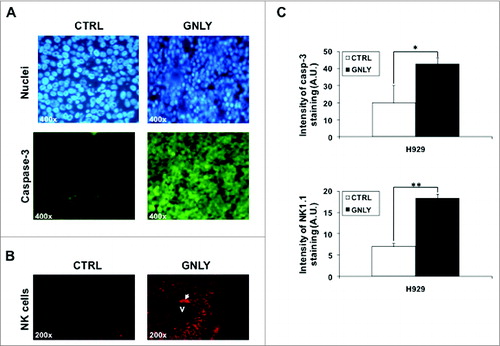
To further evaluate apoptosis markers, anti-caspase 3 immunostaining was also performed, using an antibody that detects both the pro-enzyme and the active form of cleaved caspase-3. As shown in , lower panels, tumors from the CRTL group showed a slight staining with the antibody, but the intensity of caspase-3 fluorescent immunostaining in tumors from the GLNY group was clearly higher. This higher intensity probably indicates that the antibody recognizes the cleaved active caspase-3 form with higher affinity, indicating that caspase-3 is being activated due to granulysin treatment.
It is remarkable the significant in vivo effect of granulysin on the development of MDA-MB-231-derived tumors (), since this tumor is not very sensitive to granulysin-induced cytotoxicity in vitro (). Hence, it is possible that granulysin would induce an immunogenic response against the tumor, resulting finally in apoptosis induction by recruited immune cells. However, athymic mice used in the study are mostly devoid of T cells, and the antibody response is also very limited due to the absence of T cell help. On the other hand, innate immune cells (neutrophils, macrophages, NK cells) are present in normal amounts in these mice. Among them, taking into account the histological stainings performed (see ), and since NK cells are important anti-tumoral cells,Citation24 we decided to study NK cell infiltration in granulysin-treated tumors by immunostaining with an anti-NK1.1 antibody. As shown in , in tumors from the control group, the presence of some NK cells was detected, although most of the tumor mass was free of NK cell infiltration. However, in tumors from the granulysin group, NK cell infiltration was substantially increased, with NK cell spots distributed throughout the whole tumor mass, demonstrating an increased NK cell infiltration into the tumor associated with granulysin treatment.
Fluorescence intensity of both caspase-3 staining and NK cell infiltration was analyzed using ImageJ software (). In both cases, the intensity of fluorescence was higher in tumors from the granulysin group when compared to tumors from the control group, being the difference statistically significant for both parameters. In both anti-caspase-3 and anti-NK1.1 immunostainings, negative controls were performed using an irrelevant antibody of the same isotype and the same secondary antibodies labeled with FITC or with PE, respectively, demonstrating the specificity of the labelings with the primary antibodies (data not shown).
In vivo effect of recombinant granulysin on NCI-H929-induced tumors
In this model, 10 × 106 NCI-H929 cells were resuspended in Matrigel and inoculated s.c. in nude mice. Treatments were initiated when NCI-H929-derived tumors arrived to 0.1 cm3 of volume. Mice in the control group were treated with 50 μL PBS and mice in the granulysin group were treated with recombinant granulysin (44 μg in 50 μL PBS) by intratumoral administration each two d for 10 times. Since we detected the presence of bacterial LPS in recombinant granulysin preparations (see ), we used in the control groups in these experiments PBS containing LPS, obtained in the final granulysin concentration step. As shown in , granulysin induced a detectable inhibition of tumor growth from the 4th injection on, being the differences between tumor volumes in the control and in the treated group statistically significant from the 5th injection on. In fact, NCI-H929-derived tumors were practically eradicated after treatment with the 10-granulysin injections. Tumor eradication was confirmed measuring the weight and size of the resected tumors at the end of the experiment (data not shown).
Figure 5. Granulysin treatment of nude mice bearing NCI-H929 human multiple myeloma xenografts. (A) 10 × 106 NCI-H929 multiple myeloma cells resuspended in Matrigel were injected s.c. in 10 nude mice. After around 2 mo, when tumor volume had arrived to 0.1 cm3, mice were divided in two groups, CTRL (n = 2) and GNLY-treated (n = 3). Mice in the GNLY group received intra-tumoral injections of GNLY at 44 μg in 50 µL of PBS every 2 d for 10 times and then mice were sacrificed. Mice in the CTRL group received injections of 50 µL PBS containing LPS with the same time schedule. (B) The same protocol as in A was followed, but using two different granulysin preparations with high or low LPS content, as indicated, and treating mice for only five times, leaving them without any treatment until the end of the experiment, n = 2 for each experimental group. (C) The same protocol as in A was followed, with the difference that tumors were left to arrive to a volume of 1 cm3 before beginning the treatments, that were administered five times and then mice were sacrificed. Data are the mean ± SD of the tumor volume in each group of the studies. *p < 0.05; ***P<0.01.
LPS is frequently present in recombinant protein preparations. We tested the presence of bacterial LPS using the Limulus amoebocyte lysate test, obtaining a concentration of 20 EU/mL of LPS in our stock recombinant granulysin preparations. LPS removal was performed using a high-efficiency resin based on the affinity matrix of modified polymyxin B (PMB). LPS concentration was reduced by the resin by around 50%, but the remaining granulysin stock had still an LPS concentration of 11 EU/mL. Hence, we used the two granulysin preparations, with high or low LPS content, in in vitro and in vivo experiments. First, we demonstrated that both granulysin preparations were equally cytotoxic against Jurkat cells in 4 h and 24 h assays (data not shown).
Next, we used a similar protocol as described in , but only five intratumoral injections were performed each two d, and mice left for 10 d more without treatment. In this experiment, both high and low LPS-containing preparations were tested. As shown in , tumor development in mice treated with either low- or high-LPS containing granulysin declined after 10 d of treatment and the tumors were almost eradicated at the end of the experiment. Tumor eradication independently of the LPS content in the granulysin preparation was confirmed measuring the weight and size of the resected tumors at the end of the experiment (data not shown).
Finally, we tested granulysin on H929-derived tumors, but leaving tumors to arrive to a 10-fold bigger size (1 cm3) before beginning the treatments. Treatments were performed every 2 d for five times and then, animals were sacrificed. The time of the experiment was not prolonged due to ethical reasons, given the exacerbated tumor growth in control mice. As shown in , the mean volume of the tumors was reduced around a 60% in the granulysin group compared to the control group. Differences did not arrive to be statistically significant, probably due to the small sample size. However, the experiment demonstrated that granulysin is able to stop the growth of H929-derived tumors even if the treatment was initiated when the tumor development was exacerbated.
Histological studies on NCI-H929-derived tumors
These histological studies could be done only on samples from the last experiment, shown in , since in the other experiments performed, H929-derived tumors were almost completely eradicated and did not allow to perform further histological studies. In order to analyze apoptotic features in vivo we first stained tumor tissue sections with Hoechst 33342 to evaluate nuclear morphology. As shown in , top panels, untreated control tumors displayed cells with non-condensed chromatin, while granulysin-treated tumors showed shrinked cells, most of them with condensed chromatin. To further evaluate apoptosis markers, we performed anti-caspase 3 immunostaining, using the same antibody as in . As shown in , lower panels, although control tumors stained with the antibody, the intensity of caspase-3 fluorescent staining in granulysin-treated tumors was much higher. Finally, as indicated above for MDA-MB-231-derived tumors, we also explored NK cell infiltration in the tumor mass of resected H929-derived tumors. As shown in , in tumors from the control group, most of the tumor mass was free of NK cell infiltration. However, in tumors from the granulysin group, NK cell infiltration was massive, with NK cell spots distributed throughout the whole tumor mass. A concentration of NK cell infiltration was sometimes observed in the borders of blood vessels irrigating the tumor tissue, as indicated in the right panel of with an arrow. These data demonstrate a substantial increase of NK cell infiltration in H929-derived tumors associated with granulysin treatment.
Figure 6. Nuclear staining and immunostaining for caspase-3 and NK1.1 in histological sections of NCI-H929-derived tumors. (A, B) Representative images of resected NCI-H929-derived tumors from the control group (CTRL) or from the granulysin-treated group (GNLY) of the experiment in are shown. Nuclei were stained using the Hoechst 33342 nuclear dye (upper panels in A) or immunostained with anti-caspase-3 antibody (lower panels in A) or with anti-NK1.1 antibody (B), as indicated, and photographed in a fluorescence microscope at 400X (A) or 200X (B) magnification. Arrow indicates an accumulation of NK cells in the border of a blood vessel (v). (C) Fluorescent intensity in the caspase-3 or NK1.1 immunostained sections was quantified using the freeware ImageJ v1.33 and results expressed as the mean ± SD of the different sections analyzed (at least 10 in each experimental condition) as arbitraty units following the recommendations of the software. *p < 0.05; **p < 0.02
As previously indicated for MDA-MB-231-xenografted tumors, fluorescence intensity of both caspase-3 staining and NK cell infiltration was also quantified in H929-xenografted tumors (). The intensity of fluorescence was higher in tumors from the granulysin group when compared to tumors from the control group, being the difference statistically significant both for caspase-3 staining and for NK cell infiltration.
Analysis of in vivo side effects of granulysin
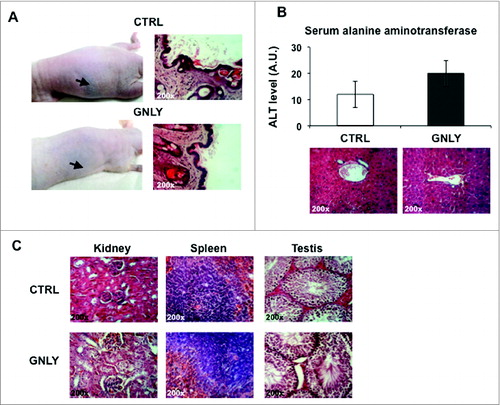
Finally, we analyzed in vivo the possible side effects as a result of granulysin administration. First of all, no significant changes in body weight or in mice behavior was observed during the experiments (data not shown). It has been reported that granulysin is implicated in the Stevens–Johnson syndrome, an exacerbated inflammatory reaction against some chemicals, which results in epidermis destruction due to massive keratinocyte death.Citation25 Hence, we first tested if s.c. granulysin injection following the protocols used in our experiments was deleterious against the skin of mice. We observed neither macroscopic nor histological changes after five injections () when compared with the control group (injected with PBS), and mice looked fine and healthy during the experiment. Next, we studied possible hepatotoxicity, the most common pharmacological side effect, by analyzing the levels of the hepatic enzyme alanine aminotransferase (ALT) in mice serum after intra-peritoneal injection of granulysin for five times. As shown in , top panel, a small increase in basal ALT serum levels was observed after the five intraperitoneal (systemic) granulysin injections. However, this increase was not statistically significant. The absence of liver damage was confirmed by H&E staining (, lower panel). Finally, other internal organs were also analyzed by H&E staining in the same experiments. As shown in , no morphological differences were found in kidney, spleen, or testis tissue in granulysin-treated mice as compared with control mice. These data indicate no overt toxicity of the treatment with granulysin, even if it is given systemically by intraperitoneal injection.
Figure 7. Study of the possible adverse effects of granulysin alone. (A, left panels) Representative images of nude mice that received five s.c. PBS injections (CTRL) or five s.c. granulysin injections (GNLY) in the absence of tumor development. Arrows indicate the place were injections were performed. Right panels, representative histological images of skin sections of both groups of mice stained with H&E. n = 5 in each experimental group. (B) Nude mice were injected intraperitoneally (i.p.) with PBS (CTRL) or with granulysin (GNLY) for five times with a 3-d interval. Upper panel, determination of hepatic serum alanine aminotransferase (ALT) in sera of mice from both groups. Data are the mean ± SD of ALT levels in both groups of mice, n = 5 in each experimental group. Lower panel, representative histological section of liver tissue sections from both groups of mice stained with H&E. (C) Representative photomicrographs of several tissues (kidney, spleen, and testis) in granulysin or PBS-treated mice from the same experiment shown in B after H&E staining, as indicated.
Discussion
The rationale of the present work was to initiate the in vivo experiments to test the use of granulysin as a novel anti-tumoral therapy. It has been clearly demonstrated that the physiological role of granulysin is the elimination of intracellular bacteria in concert with perforin, such as M. Tuberculosis and L. Monocytogenes.Citation3,8 However, the ability of recombinant granulysin to kill tumor cells directly, without needing participation of perforin, was already described in the first paper in which granulysin was characterized.Citation1 Afterwards, several indirect lines of evidence obtained in cancer patients pointed out that there is a good correlation between granulysin expression and control of tumor growth, as indicated in the Introduction.Citation17-20 Regardless of a possible physiological role of granulysin in anti-tumor immunity, 9 kDa recombinant granulysin could be used as an anti-tumoral agent. In a previous study, our group demonstrated the in vitro cytotoxicity of recombinant granulysin not only against the human acute lymphoblastic leukemia (ALL) Jurkat, used in previous mechanistic studies Citation10,11,13, but also against several human multiple myeloma cell lines.Citation9 In addition, it was showed that granulysin was also cytotoxic against primary tumor cells from B-CLL patients, while having no effect against normal peripheral blood lymphocytes from healthy donors.Citation9 This in vivo experimentation was initiated using the aggressive human breast adenocarcinoma MDA-MB-231 in immunodeficient athymic mice. This tumor was selected because, due to its high aggressiveness, induced detectable tumors in all athymic mice after just two weeks of injection of 106 cells, shortening the time of the in vivo experiments compared with other tumor cell lines. However, MDA-MB-231 cells were only partially sensitive to granulysin toxicity, at least in vitro. The results obtained show that the intratumoral injection of granulysin was able to arrest the growth of MDA-MB-231-xenografted tumors, not only when granulysin was injected 10 times, but also with five injections and leaving mice without any other treatment until the end of the experiment. Control of tumor growth by granulysin correlated with a less packed structure than in control tumors, with decreased cellularity and with empty zones in the inner tumoral core. This also correlated with some signs characteristic of apoptosis such as nuclear morphology changes and intense staining with an anti-caspase-3 mAb. Finally, control of tumor growth by granulysin also correlated with NK cell infiltration inside the tumor, a point that we will discuss below.
The results obtained with the breast adenocarcinoma xenograft model were the proof of concept about the possible use of recombinant granulysin for cancer treatment. The next step of our research was to perform similar in vivo experiments using in this case a multiple myeloma model. Multiple myeloma is one of the hematological malignancies with worst prognosis, and novel treatments in order to improve the disease outcome are needed.Citation26 As indicated above, our group tested the sensitivity of different multiple myeloma cell lines to granulysin, showing that H929 cells were the most sensitive.Citation9 In addition, H929 cells were able to generate tumors in athymic mice, although the tumor was less aggressive than MDA-MB-231 and s.c. injection of at least 10 × 106 cells was needed to induce detectable tumors after 2 mo of injection in around 60% of the mice. In addition, injections should be done in Matrigel, while MDA-MB-231 cells could be injected directly resuspended in PBS. Using this model, we demonstrated that intra-tumoral injection of granulysin was able to completely eradicate H929-xenografted tumors when treatment started with a tumor size around 0.1 cm3. This result was achieved with either 10 or with only 5 injections and leaving mice without any additional treatment until the end of the experiment. When the treatment was initiated with an exacerbated tumor growth (tumor size around 1 cm3) granulysin was able to control tumor growth. In this case, we could also demonstrate the presence of an apoptotic phenotype in the treated tumors, and also the presence of a substantial NK cell infiltration.
We have shown that recombinant granulysin preparations have bacterial LPS, a common feature of recombinant proteins. LPS can act as an immune adjuvant through their binding to TLR4 on the surface of antigen-presenting cells, activating macrophage and dendritic cell migration, cytokine production and antigen presentation.Citation27,28 In our in vivo model, antigen-presenting cell activation is not quite relevant, since no T cells are present, but inflammatory cytokine production could be relevant and could be implicated in promoting NK cell migration to the site of the tumor. We used a commercial kit to eliminate LPS from granulysin preparations, but this elimination was not complete. First, we corroborated that the granulysin preparations with high or low LPS content killed Jurkat cells in vitro at the same level. And second, we demonstrated that both preparations showed a similar efficiency in in vivo eradication of H929-derived tumors. Moreover, we also assessed that PBS obtained from the granulysin production process, that contained similar amounts of LPS, was not toxic against Jurkat or H929 cells in vitro. In addition, in in vivo H929 experiments, we always used this filtered PBS containing LPS for injections in the control group, and no effect on tumor growth was observed. Hence, LPS by itself does not seem cytotoxic in our experimental models, and in vivo results can be attributed solely to granulysin. In addition, in vivo results were more evident in the H929 model than in the MDA-MB-231 model, correlating with the relative sensitivity of both cell lines to granulysin toxicity in vitro.
However, in vivo results were better than expected from in vitro results, especially in the case of MDA-MB-231-derived tumors, indicating that some additional immunogenic effect could be acting. Recruitment of NK cells to the tumor tissue after treatment with granulysin was observed, a fact that probably contribute to the anti-tumoral effects observed, supporting this hypothesis. Immunogenic tumor cell death mediated by some cytotoxic drugs normally used in chemotherapy, such as anthracyclines, is a concept recently developed in cancer treatment.Citation29,30 Although, we did not analyze the established markers of immunogenic cell death in granulysin-induced apoptosis, it is very possible that granulysin was able to induce immunogenic tumor cell death in vivo, resulting in our mouse model in a NK cell recruitment and activation. This would explain the more potent effect of granulysin in vivo than in vitro, especially in the case of MDA-MB-231-derived tumors. In this regard, it was also plausible that LPS was acting as an adjuvant, promoting a stronger anti-tumoral immune response, limited in this case to NK cells. However, granulysin eradication of H929-derived tumors was not dependent on the LPS concentration present in the recombinant granulysin preparations and LPS alone was without effect. In any case, further studies are necessary to demonstrate the ability of granulysin preparations devoid of LPS to eradicate H929-derived tumors or to stop tumoral growth in the MDA-MB-231 xenograft model.
Finally, it is noteworthy that s.c. or systemic injection of granulysin did not have overt adverse effects. Although, it has been shown that granulysin is implicated in the deleterious effects of uncontrolled inflammation in the Stevens–Johnson syndrome, resulting in epidermis destruction,Citation25 we demonstrated that several s.c. injections of granulysin on healthy mice did not result in any side effect on skin. This seems to indicate that Stevens–Johnson syndrome is more dependent on the uncontrolled polymorphonuclear and mononuclear inflammatory infiltration, than on granulysin itself. On the other hand, it has been also described that the main mediator of that syndrome is 15 kDa granulysin,Citation25 that behaves as an immune alarmin,Citation2 augmenting inflammatory recruitment and infiltration, rather than the cytotoxic 9 kDa granulysin used in this work. In addition, we have also demonstrated that five systemic administration of granulysin was not hepatotoxic without affecting the normal tissue structure of liver and other organs such as kidney, spleen, and testis.
As a main conclusion, the results presented in this work open the door to translational research on the possible use of granulysin as a novel anti-tumoral treatment in the clinic, with special emphasis in the case of multiple myeloma.
Material and Methods
Cell culture
Jurkat and NCI-H929 cell lines, both obtained from the ATCC, were cultured in RPMI 1640 medium supplemented with 5% FCS and penicillin/streptomycin (all of them from Pan Biotech, Aidenbach, Germany) and GlutaMAX (Invitrogen, Barcelona), at 37°C and 5% CO2 using standard procedures. Dr. Joan Massagué, Memorial Sloan Kettering Cancer Center, New York, USA kindly provided the MDA-MB 231-Luciferase cell line, and it was cultured in DMEM medium (PAN Biotech GmbH) supplemented with 15% fetal bovine serum (FBS, Sigma). All cell lines were routinely tested for mycoplasma contamination by PCR.
Expression and purification of recombinant functional granulysin
Recombinant granulysin was produced and purified as described in Ref. 9. Briefly, competent Escherichia coli BL21 (DE3) were transfected with the pET28a vector containing the polyhistidine-tagged recombinant 9-kDa granulysin, kindly provided by Dr. Alan Krensky, NIH, Bethesda.Citation1 The recombinant protein was over-expressed by incubating E. coli with IPTG for 3 h. Granulysin was purified under denaturing conditions from a guanidinium hydrochloride lysate, using a Ni-NTA resin (Qiagen, Valencia, California, USA). On-column renaturation and purification were performed following the protocol described by Oganesyan et al.Citation31 and adapted to granulysin in.Citation9 The refolded protein was then eluted with Buffer A containing 450 mM imidazole and protein concentration was measured using a spectrophotometer (NanoVue, GE Healthcare, Barcelona). Fractions containing the highest levels of protein were passed through a desalting column (PD-10 Desalting Columns, GE Healthcare, Barcelona) following manufacturer's instructions and protein was recovered in PBS. Protein was then concentrated by centrifugation using 3,000 MWCO Centrifugal Filter Units (Amicon, Millipore, Madrid) until a final concentration of about 1 mM (measured with NanoVue).
Protein purity was always checked by SDS-PAGE in 12% polyacrylamide gels and Coomassie blue staining and by immunoblot with specific anti-granulysin antibodies, a kind gift of Dr. Alan Krensky, NIH, Bethesda.
Quantification and removal of LPS in recombinant granulysin
LPS content in recombinant granulysin preparations was tested using the Limulus amoebocyte lysate (LAL) test (ToxinSensor Chromogenic LAL Endotoxin Assay Kit; GenScript, USA). The concentration of LPS present in the samples was determined by diluting ten- or two-fold recombinant granulysin preparations, and performing calibration curves using E. Coli standards provided with the kit. LPS removal was performed using a high-efficiency resin (ToxinEraser Endotoxin Removal Kit; GenSript, USA), following manufacturer's instructions. The resin is based on the affinity matrix of modified polymyxin B, and has a binding capacity up to 2 × 106 EU/mL.
In vitro cytotoxicity assays
100 µL aliquots of 1 × 106/mL cell suspensions in complete medium were seeded per well in 96 well plates and granulysin was added at the indicated concentrations. In control wells, instead of granulysin, the same volume of PBS or of the protein-free concentration fraction was added. Cells were then incubated for the indicated times at 37°C and then cell death was analyzed. PS exposure and loss of membrane integrity was analyzed simultaneously by flow cytometry using respectively Annexin-V-FITC (BD Biosciences, Madrid) and 7AAD (Inmunostep, Salamanca, Spain). Cells were washed with PBS and incubated with Annexin-V and 7AAD in annexin-binding buffer (140 mMNaCl, 2.5 mMCaCl2,10 mM Hepes/NaOH, pH 7.4) for 15 min. After that, they were analyzed using a FACSCalibur (BD, Madrid).
In vivo experiments
Immune-deficient athymic mice, Swiss nu/nu strain, six-week-old males, were used in the present study. Those mice were purchased from Charles River. Mice experiments were performed according to the European recommendations on animal ethics and the University of Zaragoza Animal Experimentation Ethical Commission previously approved the housing and experimental protocols. Mice were kept under specific standard pathogen-free conditions (average ambient temperature 24°C, 12/12-h light/dark cycle) with water and food provided ad libitum throughout the study.
MDA-MB-231 mammary adenocarcinoma xenograft model
1 × 106 MDA-MB-231 tumor cells were injected subcutaneously in nude mice. When the tumors arrived to a volume of 0.1 cm3 (around 2 or 3 weeks), the treatments began on the experimental groups, normally of five mice each. 44 μg of granulysin suspended in 50 μL of PBS were injected intra-tumorally every 2 d in the granulysin-treated group for five times, while mice in the control group were injected in the same way with 50 μL of PBS. In other experiments, the granulysin-treated mice group was subdivided into two subgroups (each one with five mice), one subgroup received 44 μg granulysin in 50 μL of PBS for 10 times with a two-d interval and the other subgroup, in which mice were injected intra-tumorally every 2 d with the same volume and concentration of granulysin for just five times and then, the tumors were left for 12 d without treatment until the end of the experiment. Injected mice were examined every 2 d for data collection. After the treatments, all mice were euthanized and the tumors were surgically excised 2 d after the last injection, stored in a proper preservation solution (10% buffered formalin) until examination in histological studies.
NCI-H929 multiple myeloma xenograft model
10 × 106 NCI-H929 viable tumor cells were harvested and resuspended in 100 μL of Matrigel (Sigma) and subcutaneously injected in nude mice. These cells induce detectable tumors in around a 60% of nude mice after at least two months of tumor injection. When tumors reached a volume of 0.1 cm3, a similar protocol as described above in the MDA-MB-231 xenograft model was followed. In some experiments, treatments were initiated when tumor growth was exacerbated and tumor volume was around 10-fold higher (1 cm3), performing only five injections, because of ethical reasons regarding tumor development in the control group.
In both models, tumor growth was analyzed by measuring the tumor daily with a caliper of precision. To calculate the tumor volume, the width (A) and length (L) of the tumor were measured, and the following formula was applied:
Histological studies
Hematoxylin–eosin staining
Tissue for light microscopy was fixed in 4% formaldehyde and embedded in paraffin using routine procedure. Sections 5-μm thick were cut from the tissue blocks and deparaffinized by immersion in two changes of xylene for 5 min each. After that, tissue sections were rehydrated by immersion in decreasing concentrations of ethanol (immersion in separated baths of 100%, 95% and 70% of ethanol for 2 min/each bath). The nuclei of the cells in tissue sections were stained by immersing in GILL II Hematoxylin for 3 min. Sections were rinsed in HCl 0.1% for 5 s and then, in tap water for 3–5 min. Cell cytoplasm was stained by immersing in 0.5% of eosin, which contains 0.2% of glacial acetic acid, for 3 min and dipped once quickly in water for 10 seconds. After eosin staining, sections were dehydrated by immersion in ascending alcohol solutions (70%, 95% and absolute alcohol 100%) for 5 s/each. Finally, all sections were cleaned by xylene for 5 s, dried and mounted with DPX using glass coverslips.
Immunofluorescence study of apoptotic nuclei, caspase-3 and NK1.1 staining
Tissue for fluorescence microscopy was fixed in 4% formaldehyde and embedded in paraffin using routine procedure. Sections (5 μm thick) of tumor samples were deparaffinized in xylene, hydrated through a graded series of ethanol, and then immersed in 3% hydrogen peroxide in 100% methanol for 30 min to inhibit endogenous peroxidase activity. For antigen retrieval, the sections were boiled in 10 mM citrate buffer, pH 6.0 for 30 min.
The expression of caspase-3 was investigated with immunofluorescence staining using affinity purified rabbit polyclonal antibodies against caspase-3 (Cell Signaling Technology). This antibody recognizes in principle both the pro-enzyme and the active, cleaved caspase-3. After being rinsed in PBS-Tween 20 for 2 times (2 min each), the sections were incubated with 200 μL blocking solution (5% rabbit or horse serum diluted in PBS) for 1 h at room temperature, and then incubated overnight at 4°C in humid chambers with the primary antibody to caspase-3 at 1/200 dilution. After three washes with PBS-Tween 20, the sections were then incubated with a goat anti-mouse IgG secondary conjugated with FITC (dilution 1:200; Santa Cruz Biotech, CA, USA) in 1% BSA/PBS for 1 h.
For study the NK cells infiltration to the site of tumor tissue, sections were stained with monoclonal antibody mouse anti-NK1.1 (BD Bioscience) and revealed in the same way as indicated for caspase-3 detection, but using secondary antibody conjugated with PE. After washing again with PBS Tween-20 for 3 times (2 mins each), the slides were dried, and mounted over 1 mL of Fluoromount-G (Southern Biotech) containing 10 mg/mL Hoechst 33342, for nuclear staining. Isotype control staining plus the secondary FITC- or PE-labeled secondary antibodies were also performed, giving no substantial fluorescence by themselves in the experimental conditions described.
Finally, the preparations were visualized in a fluorescence microscope (E600/E400, Nikon) equipped with a digital photograph (DXM1200F, Nikon).
For the quantification of caspase-3 staining and NK cell infiltration, the freeware ImageJ v1.33 downloaded from the NIH website (http://rsb.info.nih.gov/ij) were used (Anatomical Record 2013, Jensen EC, 2013). A minimum of 10 slides for each experimental condition was analyzed in each case.
Study of the status of tumoral cells obtained from mice
In some studies, MDA-MB-231-derived tumors obtained from mice were minced into small pieces with sterile scissors and scalpels, releasing the tumor cell aggregates. After repeated resuspension with a wide-bore pipette and further mincing, tumor cells were released into medium and collected after filtration through Nylon mesh. Cells were then analyzed by flow cytometry. Mammary tumoral cells were identified by analysis of surface EpCAM expression using a FITC-labeled mAb (Abcam, Madrid) and apoptosis was analyzed by Annexin-V-PE staining.
Statistical analysis
Computer-based statistical analysis was performed using GraphPad Prism 4.0 program (GrandPath Software Inc.). Results are shown as mean ± SD. Statistical significance was evaluated by using Student t test for non-paired variants. A p < 0.05 value was considered to be significant.
Disclosure of Potential Conflicts of Interest
The use of granulysin as an anti-tumoral treatment is protected by the patent application PCT/ES2014/070086 presented to the Spanish Bureau of Patents and Brands (OEPM) on 2/6/2013 and extended to international application on 2/6/2014, with the code PCT/ES2014/070086.
Acknowledgments
We gratefully acknowledge Dr. Joan Massagué, Memorial Sloan Kettering Cancer Center, New York, USA, for MDA-MB 231-Luciferase cells and Dr. Javier Azúa for processing of mice tumor tissues.
Funding
This work was supported by grants SAF2010-15341 and SAF2013-48626-C2-1-R from Ministerio de Economía y Competitividad (Spain) and by Gobierno de Aragón/Fondo Social Europeo. SAW was supported by a pre-doctoral fellowship from the Government of Yemen.
References
- Peña SV, Hanson DA, Carr BA, Goralski TJ, Krensky AM. Processing, subcellular localization, and function of 519 (granulysin), a human late T cell activation molecule with homology to small, lytic, granule proteins. J Immunol 1997; 158:2680-8; PMID:9058801
- Clayberger C, Finn MW, Wang T, Saini R, Wilson C, Barr VA, Sabatino M, Castiello L, Stroncek D, Krensky AM. 15 kDa Granulysin Causes Differentiation of Monocytes to Dendritic Cells but Lacks Cytotoxic Activity. J Immunol 2012; 188:6119-26; PMID:22586033; http://dx.doi.org/10.4049/jimmunol.1200570
- Stenger S, Hanson DA, Teitelbaum R, Dewan P, Niazi KR, Froelich CJ, Ganz T, Thoma-Uszynski S, Melián A, Bogdan C et al. An antimicrobial activity of cytolytic T cells mediated by granulysin. Science 1998; 282:121-5; PMID:9756476; http://dx.doi.org/10.1126/science.282.5386.121
- Ernst WA, Thoma-Uszynski S, Teitelbaum R, Ko C, Hanson DA, Clayberger C, Krensky AM, Leippe M, Bloom BR, Ganz T et al. Granulysin, a T cell product, kills bacteria by altering membrane permeability. J Immunol 2000; 165:7102-8; PMID:11120840; http://dx.doi.org/10.4049/jimmunol.165.12.7102
- Ma LL, Spurrell JCL, Wang JF, Neely GG, Epelman S, Krensky AM, Mody CH. CD8 T cell-mediated killing of Cryptcoccus neoformans requires granulysin and is dependent on CD4 T cells and IL-15. J Immunol 2002; 169:5787-95; PMID:12421959; http://dx.doi.org/10.4049/jimmunol.169.10.5787
- Hata A, Zerboni L, Sommer M, Kaspar A, Clayberger C, Krensky A, Arvin AM. Granulysin blocks replication of varicella-zoster virus and triggers apoptosis of infected cells. Viral Immunol 2001; 14:125-33; PMID:11398808; http://dx.doi.org/10.1089/088282401750234501
- Ochoa MT, Stenger S, Sieling PA, Thoma-Uszynski S, Sabet S, Cho S, Krensky AM, Rollinghoff M, Nunes Sarno E, Burdick AE et al. T-cell relese of granulysin contributes to host defense in leprosy. Nature Med 2001; 7:174-9; PMID:11175847; http://dx.doi.org/10.1038/84620
- Walch M, Dotiwala F, Mulik S, Thiery J, Kirchhausen T, Clayberger C, Krensky AM, Martinvalet D, Lieberman J. Cytotoxic Cells Kill Intracellular Bacteria through Granulysin-Mediated Delivery of Granzymes. Cell 2014; 157:1309-23; PMID:24906149; http://dx.doi.org/10.1016/j.cell.2014.03.062
- Aporta A, Catalán E, Galán-Malo P, Ramírez-Labrada A, Pérez M, Azaceta G, Palomera L, Naval J, Marzo I, Pardo J et al. Granulysin induces apoptotic cell death and cleavage of the autophagy regulator Atg5 in human hematological tumors. Biochem Pharmacol 2014; 87:410-23; PMID:24269628; http://dx.doi.org/10.1016/j.bcp.2013.11.004
- Gamen S, Hanson DA, Kaspar A, Naval J, Krensky AM, Anel A. Granulysin-induced apoptosis. I. Involvement of at least two distinct pathways. J Immunol 1998; 161:1758-64; PMID:9712041
- Pardo J, Pérez-Galán P, Gamen S, Marzo I, Monleón I, Kaspar AA, Susín SA, Kroemer G, Krensky AM, Naval J et al. A role of the mitochondrial apoptosis-inducing factor (AIF) in granulysin-induced apoptosis. J Immunol 2001; 167:1222-9; PMID:11466337; http://dx.doi.org/10.4049/jimmunol.167.3.1222
- Tewary P, Yang D, de la Rosa G, Li Y, Krensky AM, Clayberger C, Oppenheim JJ. Granulysin activates antigen-presenting cells through TLR4 and acts as an immune alarmin. Blood 2010; 116:3465-74; PMID:20660289; http://dx.doi.org/10.1182/blood-2010-03-273953
- Kaspar AA, Okada S, Kumar J, Poulain FR, Drouvalakis KA, Kelekar A, Hanson DA, Kluck RM, Hitoshi Y, Johnson DE et al. A distinct pathway of cell-mediated apoptosis initiated by granulysin. J Immunol 2001; 167:350-6; PMID:11418670; http://dx.doi.org/10.4049/jimmunol.167.1.350
- Okada S, Li Q, Whitin JC, Clayberger C, Krensky AM. Intracellular mediators of granulysin-induced cell death. J Immunol 2003; 171:2556-62; PMID:12928406; http://dx.doi.org/10.4049/jimmunol.171.5.2556
- Saini RV, Wilson C, Finn MW, Wang T, Krensky AM, Clayberger C. Granulysin delivered by cytotoxic cells damages endoplasmic reticulum and activates caspase-7 in target cells. J Immunol 2011; 186:4497-3504; PMID:21296981; http://dx.doi.org/10.4049/jimmunol.1003409
- Dunn GP, Bruce AT, Ikeda H, Old LJ, Schreiber RD. Cancer immunoediting : from immunosurveillance to tumor escape. Nature Immunol 2002; 3:991-8; PMID:12407406; http://dx.doi.org/10.1038/ni1102-991
- Kishi A, Takamori Y, Ogawa K, Takano S, Tomita S, Tanigawa M, Niman M, Kishida T, Fujita S. Differential expression of granulysin and perforin by NK cells in cancer patients and correlation of impaired granulysin expression with progression of cancer. Cancer Immunol Immunother 2002; 50:604-14; PMID:11807624; http://dx.doi.org/10.1007/s002620100228
- Pagès F, Berger A, Camus M, Sanchez-Cabo F, Costes A, Molidor R, Mlecnik B, Kirilovsky A, Nilsson M, Damotte D et al. Effector memory T cells, early metastasis, and survival in colorectal Cancer. New Eng J Med 2005; 353:2654-66; PMID:16371631; http://dx.doi.org/10.1056/NEJMoa051424
- Saigusa S, Ichikura T, Tsujimoto H, Sugasawa H, Majima T, Kawabayashi N, Chochi K, Ono S, Kinoshita M, Seki S et al. Serum granulysin level as a novel prognostic marker in patients with gastric carcinoma. J Gastroenterol Hepatol 2007; 22:1322-7; PMID:17688669; http://dx.doi.org/10.1111/j.1440-1746.2006.04796.x
- Nagasawa M, Kawamoto H, Tsuji Y, Mizutani S. Transient increase of serum granulysin in a stage IVs neuroblastoma patient during spontaneous regression: case report. Int J Hematol 2005; 82:456-7; PMID:16533752; http://dx.doi.org/10.1532/IJH97.05091
- Huang LP, Lyu SC, Clayberger C, Krensky AM. Granulysin-mediated tumor rejection in transgenic mice. J Immunol 2007; 178:3688-94; PMID:17182542
- Ellerby HM, Lee S, Ellerby LM, Chen S, Kiyota T, del Rio G, Sugihara G, Sun Y, Bredesen DE, Arap W et al. An artificially designed pore-forming protein with anti-tumor effects. J Biol Chem 2003; 278:35311-6; PMID:12750379; http://dx.doi.org/10.1074/jbc.M300474200
- Li Q, Dong C, Deng A, Katsumata M, Nakadai A, Kawada T, Okada S, Clayberger C, Krensky AM. Hemolysis of erythrocytes by granulysin-derived peptides but not by granulysin. Antimicrob Agents Chemother 2005; 49:388-97; PMID:15616319; http://dx.doi.org/10.1128/AAC.49.1.388-397.2005
- Anel A, Aguiló JI, Catalán E, Garaude J, Rathore MG, Pardo J, Villalba M. Protein kinase C-q (PKC-q) in natural killer cell function and anti-tumor immunity. Front Immunol 2012; 3:187; PMID:22783260; http://dx.doi.org/10.3389/fimmu.2012.00187
- Chung WH, Hung SI, Yang JY, Su SC, Huang SP, Wei CY, Chin SW, Chiou CC, Chu SC, Ho HC et al. Granulysin is a key mediator for disseminated keratinocyte death in Stevens-Johnson syndrome and toxic epidermal necrolysis. Nature Med 2008; 14:1343-50; PMID:19029983; http://dx.doi.org/10.1038/nm.1884
- Palumbo A, Anderson K. Multiple myeloma. New Eng J Med 2011; 364:1046-60; PMID:21410373; http://dx.doi.org/10.1056/NEJMra1011442
- Poltorak A, He X, Smirnova I, Liu M, Van Huffel C, Du X, Birdwell D, Alejos E, Silva M, Galanos C et al. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science 1998; 282:2085-8; PMID:9851930; http://dx.doi.org/10.1126/science.282.5396.2085
- Takeuchi O, Hoshino K, Kawai T, Sanjo H, Takada H, Ogawa T, Takeda K, Akira S. Differential roles of TLR2 and TLR4 in recognition of gramnegative and gram-positive bacterial cell wall components. Immunity 1999; 11:443-51; PMID:10549626; http://dx.doi.org/10.1016/S1074-7613(00)80119-3
- Casares N, Pequignot MO, Tesniere A, Ghiringhelli F, Roux S, Chaput N, Schmitt E, Hamai A, Hervas-Stubbs S, Obeid M et al. Caspase-dependent immunogenicity of doxorubicin-induced tumor cell death. J Exp Med 2005; 202:1691-701; PMID:16365148; http://dx.doi.org/10.1084/jem.20050915
- Vacchelli E, Aranda F, Eggermont A, Galon J, Sautès-Fridman C, Cremer I, Zitvogel L, Kroemer G, Galluzzi L. Trial watch: chemotherapy with immunogenic cell death inducers. OncoImmunol 2014; 3:e27878; PMID:24800173; http://dx.doi.org/10.4161/onci.27878
- Oganesyan N, Kim SH, Kim R. On-column protein refolding for crystallization. J Struct Func Genomics 2005; 6:177-82; PMID:16211516; http://dx.doi.org/10.1007/s10969-005-2827-3
