Abstract
Success in eliciting tumor rejection by therapeutic blockade of T cell checkpoint inhibitors, such as PD-1/PD-L1 and CTLA-4, has spurred re-evaluation of additional receptors that regulate antitumor responses. Here we summarize our recent studies characterizing the role of co-inhibitory receptor TIGIT in antitumor and other chronic CD8+ T cell responses.
In 2006, Ahmed and colleagues elegantly demonstrated that the co-inhibitory receptor PD-1 functions as a central checkpoint inhibitor that limits the persistence and magnitude of CD8+ T cell responses.Citation1 Subsequent preclinical studies revealed that during chronic viral infection, cancer, or other cases of persistent immune stimulation, PD-1 and other T cell co-inhibitory receptors, including CTLA-4, LAG-3, and TIM-3, can progressively “exhaust” protective CD8+ T cell responses.Citation2 In the clinic antagonistic antibodies targeting PD-1, its ligand PD-L1, and CTLA-4 have all been shown to revitalize durable antitumor immune responses.Citation3 These checkpoint inhibitor immunotherapies promise to revolutionize the therapeutic strategies and outcomes for many cancers.
The number of co-inhibitory receptors with the potential to suppress T cell or other immune responses is large and growing. In support of efforts to determine which of these receptors are most relevant in tumor microenvironments, we probed gene expression data from lung squamous carcinoma and other cancers to identify those receptors that were best associated with T cell tumor infiltration.Citation2 Alongside PD-1 and CTLA-4, we found TIGIT (T cell Immunoglobulin and ITIM domain) to be highly correlated with T cell tumor infiltration; we confirmed by flow cytometry that NSCLC and CRC-infiltrating T cells – particularly CD8+ T cells – co-expressed TIGIT and PD-1. TIGIT, a member of the small family of the poliovirus receptor (PVR)-nectin family of immunoreceptors, was first identified and characterized by our group as a suppressor of CD4+ T cell priming and autoimmunity.Citation3,4 Subsequent studies corroborated the importance of TIGIT on CD4+ T cells and identified additional activity in limiting NK cell cytotoxicity and potentiating regulatory T cell suppressive activity.Citation5–7 Until now, TIGIT's expression and function on CD8+ T cells and in CD8+ T cell-dependent responses was unknown, prompting us to characterize TIGIT's role in pre-clinical models of cancer and viral infection.
To assess the role of TIGIT in antitumor immune responses, we treated wildtype mice bearing subcutaneous syngeneic CT26 or EMT6 tumors with blocking antibodies against TIGIT alone, PD-L1 alone, or a combination of TIGIT and PD-L1. Co-blockade of TIGIT and PD-L1 was necessary to elicit tumor rejection, and conferred lasting antigen-specific immunity against tumor re-challenge. We found that these responses were CD8+ T cell-dependent, and that TIGIT collaborated with PD-1/PD-L1 to selectively and synergistically suppress tumor-infiltrating CD8+ T cell effector function within the tumor microenvironment. TIGIT played a similar role in mice chronically infected with lymphocytic choriomeningitis virus (LCMV), supporting the notion that TIGIT functions as specialized inhibitor of CD8+ T cell effector function during chronic immune responses.
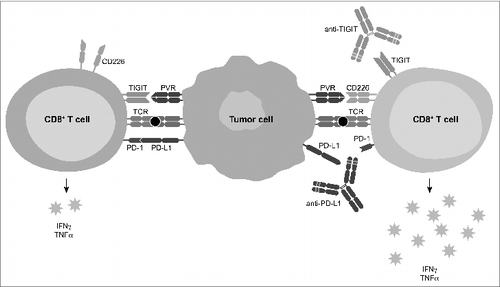
Co-inhibitory receptors can suppress T cells by direct signaling, by serving as ligands for other receptors, and by competing with complementary co-stimulatory receptors for ligand engagement. TIGIT functions as a high affinity ligand to PVR and related receptors,Citation3,5,6 competes with the co-stimulatory receptor CD226,Citation3,8 and in at least some settings signals directly through ITIM and ITT motifs.Citation9 Here, we found that the effects of anti-TIGIT on CD8+ T cells in vitro and CD8+ T cell-dependent responses in vivo were dependent on CD226. Strikingly, we found that TIGIT could directly interact with CD226 in cis on the cell surface, resulting in the disruption of CD226 homodimerization. These results indicated that TIGIT-mediated disruption of CD226 co-stimulatory activity is likely central to TIGIT's ability to suppress chronic T cell responses, and suggest that up-regulation expression of TIGIT on CD8+ T cells leads to a gradual abrogation of CD226 activity and, consequently, a loss of effector function (Fig. 1).
It is important to note, however, that TIGIT's role in limiting antitumor immune responses is almost certainly not limited to CD8+ T cells, or to interfering with CD226. Additional studies are needed to integrate these mechanisms of action and TIGIT's activity on CD4+ T cell subsets and NK cells into a comprehensive model of the influence TIGIT exerts on chronic immune responses.
Antagonistic antibodies against PD-1/PD-L1 and CTLA-4 have exhibited transformative potential as tumor immunotherapies, and the blockade of multiple checkpoint inhibitors holds even greater promise. Several other co-inhibitory receptors expressed by tumor-infiltrating T cells – most notably LAG-3 and TIM-3 – have been shown to act as PD-1 “collaborators” in that they function in concert with PD-1 to suppress chronic immune responses. However, it remains unclear which combinations of receptors should be targeted – and in which patients – to elicit optimal antitumor responses while incurring a minimum of immune pathology. One approach may be to differentiate between the members of this growing coterie on the basis of their functional effects on dysfunctional T cells. TIGIT appears to predominantly limit the cytokine competency and effector function of CD8+ T cells, while TIM-3 and LAG-3 may primarily regulate apoptosis and cell cycle progression, respectively.Citation10 In this simplified case, targeting PD-1/PD-L1 and TIGIT may be optimal in patients with extensive but highly dysfunctional antitumor T cell responses, whereas other therapeutic strategies may be preferable in patients with fewer tumor-infiltrating T cells. Assessing and optimizing such therapeutic combination rationales will be a key step in any future clinical development of antibodies targeting TIGIT and other PD-1 collaborators.
Figure 1. TIGIT as a co-inhibitory receptor that critically limits anti-tumor and other CD8+ T cell-dependent chronic immune responses. TIGIT competes with CD226 for binding the receptor PVR, and disrupts CD226 dimerization in cis at the cell surface. TIGIT collaborates with PD1 to limit chronic CD8+ T cell responses, and anti-antibody co-blockade of TIGIT and PD-L1 enhance CD8+ T cell effector function, resulting in enhanced tumor and chronic viral clearance.
Disclosure of Potential Conflicts of Interest
All authors are employed by Genentech, Inc., a for profit company.
References
- Barber DL, Wherry EJ, Masopust D, Zhu B, Allison JP, Sharpe AH, Freeman GJ, Ahmed R. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature 2006; 439:682-7; PMID:16382236; http://dx.doi.org/10.1038/nature04444
- Johnston RJ, Comps-Agrar L, Hackney J, Yu X, Huseni M, Yang Y, Park S, Javinal V, Chiu H, Irving B et al. The immunoreceptor TIGIT regulates antitumor and antiviral CD8(+) T cell effector function. Cancer Cell 2014; 26:923-37; PMID:25465800; http://dx.doi.org/10.1016/j.ccell.2014.10.018
- Yu X, Harden K, Gonzalez LC, Francesco M, Chiang E, Irving B, Tom I, Ivelja S, Refino CJ, Clark H et al. The surface protein TIGIT suppresses T cell activation by promoting the generation of mature immunoregulatory dendritic cells. Nat Immunol 2009; 10:48-57; PMID:19011627; http://dx.doi.org/10.1038/ni.1674
- Stengel KF, Harden-Bowles K, Yu X, Rouge L, Yin J, Comps-Agrar L, Wiesmann C, Bazan JF, Eaton DL, Grogan JL. Structure of TIGIT immunoreceptor bound to poliovirus receptor reveals a cell-cell adhesion and signaling mechanism that requires cis-trans receptor clustering. Proc Natl Acad Sci U S A 2012; 109:5399-404; PMID:22421438; http://dx.doi.org/10.1073/pnas.1120606109
- Stanietsky N, Simic H, Arapovic J, Toporik A, Levy O, Novik A, Levine Z, Beiman M, Dassa L, Achdout H et al. The interaction of TIGIT with PVR and PVRL2 inhibits human NK cell cytotoxicity. Proc Natl Acad Sci U S A 2009; 106:17858-63; PMID:19815499; http://dx.doi.org/10.1073/pnas.0903474106
- Levin SD, Taft DW, Brandt CS, Bucher C, Howard ED, Chadwick EM, Johnston J, Hammond A, Bontadelli K, Ardourel D et al. Vstm3 is a member of the CD28 family and an important modulator of T-cell function. Eur J Immunol 2011; 41:902-15; PMID:21416464; http://dx.doi.org/10.1002/eji.201041136
- Joller N, Lozano E, Burkett PR, Patel B, Xiao S, Zhu C, Xia J, Tan TG, Sefik E, Yajnik V et al. Treg cells expressing the coinhibitory molecule TIGIT selectively inhibit proinflammatory Th1 and Th17 cell responses. Immunity 2014; 40:569-81; PMID:24745333; http://dx.doi.org/10.1016/j.immuni.2014.02.012
- Lozano E, Dominguez-Villar M, Kuchroo V, Hafler DA. The TIGIT/CD226 axis regulates human T cell function. J Immunol 2012; 188:3869-75; PMID:22427644; http://dx.doi.org/10.4049/jimmunol.1103627
- Li M, Xia P, Du Y, Liu S, Huang G, Chen J, Zhang H, Hou N, Cheng X, Zhou L et al. T-cell immunoglobulin and ITIM domain (TIGIT) receptor/poliovirus receptor (PVR) ligand engagement suppresses interferon-gamma production of natural killer cells via beta-arrestin 2-mediated negative signaling. JBiol Chem 2014; 289:17647-57; PMID:24817116; http://dx.doi.org/10.1074/jbc.M114.572420
- Zhu C, Anderson AC, Schubart A, Xiong H, Imitola J, Khoury SJ, Zheng XX, Strom TB, Kuchroo VK. The Tim-3 ligand galectin-9 negatively regulates T helper type 1 immunity. Nat Immunol 2005; 6:1245-52; PMID:16286920; http://dx.doi.org/10.1038/ni1271
