Abstract
Postoperative non-small cell lung cancer (NSCLC) patients require adjuvant therapy to improve their prognosis. In this study, we investigated the efficacy of a sequential combination of autologous cellular immunotherapy (CIT) and chemotherapy for postoperative NSCLC. This retrospective study included 120 postoperative NSCLC patients: 60 cases received only chemotherapy; 33 cases received chemotherapy and sequential CIT with cytokine-induced killer (CIK) cells; and 27 cases received chemotherapy and sequential CIT with alternate CIK and natural killer (NK) cells. Survival analysis showed significantly higher overall survival rates in the CIT group compared with the control group. Overall survival was higher in patients who received CIT with alternate CIK and NK cells than those who received treatment with only CIK cells. Multivariate analysis showed that adjuvant CIT was an independent prognostic factor for overall survival of patients with NSCLC. In subgroup analyses, adjuvant CIT significantly improved the overall survival of patients with less than 60 y old and positive lymph node. In conclusions, these data indicate that adjuvant CIT, especially with alternate application of CIK and NK cells, is an effective therapeutic approach to prolong survival of patients with NSCLC, particularly for patients ≤60 y old with positive lymph nodes.
Abbreviations:
- CIK, cytokine-induced killer cells
- CIT, cellular immunotherapy
- CTLs, cytotoxic T lymphocytes
- GP, gemcitabine and cisplatin regimen
- MHC, major histocompatibility complex molecules
- NK, natural killer cells
- NLR, neutrophil-to-lymphocyte ratio
- NP, navelbine and cisplatin regimen
- NSCLC, non-small cell lung cancer
- PBMCs, peripheral blood mononuclear cells
- TP, paclitaxel and cisplatin regimen
Introduction
Lung cancer has been considered as the most commonly diagnosed cancer, as well as the leading cause of cancer death among men in both developed and developing countries.Citation1 In 2008, lung cancer accounted for 13% (1.6 million) of the total new cancer cases and 18% (1.8 million) of the cancer deaths worldwide.Citation1 Approximately 80% to 85% of lung malignancies are NSCLC, with a 5-y survival rate of only 15%.Citation2,3 Surgery is the most effective treatment for NSCLC. However, 60% to 70% of patients with NSCLC experience postoperative recurrence and metastasis, resulting in a poor prognosis.Citation4 Therefore, postoperative NSCLC patients require adjuvant therapy to improve their prognosis. Chemotherapy and radiotherapy have been adopted as basic postoperative treatment strategies in NSCLC; however, their effect is limited and seems to have reached an efficacy plateau in the past decade.Citation5-7 Therefore, the identification of more effective therapies for patients with NSCLC is still an important clinical challenge.
In recent years, multiple studies have shown that cancer formation and progression in patients with NSCLC are particularly influenced by tumor immune responses,Citation8-13 indicating that immune-based therapy could be an effective treatment option for patients with NSCLC. Current approaches to activate the immune system focus on vaccination, such as dendritic cell vaccines to increase the frequency of tumor-specific cytotoxic T lymphocytes (CTLs) and adoptive transfer of immune effector cells to promote tumor regression.Citation14,15 However, cancer cells often escape from immune attack through downregulation expression of major histocompatibility complex (MHC) molecules and costimulatory molecules.Citation16 This may be compensated by adoptive transfer of immune effector cells that mediate MHC-unrestricted cytolytic activity against tumor cells. A series of clinical studies have shown that adjuvant CIT, such as CIK cells and NK cells, can lead to promising antitumor effects on various cancers,Citation17-21 including lung cancer.Citation3,22-26 Several reviews and meta-analysis reports have also demonstrated the safety and effectiveness of adjuvant CIT in clinics.Citation27-30 However, the patterns of CIT require thorough investigation in order to achieve optimal outcome and cost-effectiveness.
As a part of the innate immune response, NK cells can recognize and lyse cells lacking MHC molecules through their activating receptors, such as NKG2D, NKp30, NKp40, and NKp46. Thus, tumor cells are more susceptible to NK cells due to their lack of MHC class I.Citation31 CIK cells are a group of heterogeneous immune-active host effector cells, including CD3+CD56+ NKT cells, CD3–CD56+ NK cells and CD3+CD56– typical T cells. The high cytotoxic activity of CIK cells is determined by the high proliferation of CD3+CD56+ cell population.Citation32 CIK cells can kill a broad spectrum of tumor cells through a MHC-unrestricted, NK-like mechanism.Citation33 This suggests that a combined application of NK and CIK cells may exhibit a synergistic antitumor immune response due to their selective cytotoxic activity against diverse tumor cells.Citation34,35 Thus, it is worthwhile to investigate the efficacy of the combined application of CIK and NK cells to treat NSCLC.
In this study, we retrospectively assessed the clinical efficacy of adjuvant CIT combined with chemotherapy in patients with resected NSCLC to provide more supportive information on whether CIT could improve the clinical outcomes in patients with NSCLC after tumor resection. More importantly, we evaluated the clinical efficacy of adjuvant CIT with alternate application of CIK and NK cells to find a potential therapeutic pattern for the future clinical application of CIT.
Results
Patient demographics and clinical characteristics
In total, 120 patients with NSCLC were retrospectively analyzed. 86 (71.1%) were men and 34 (28.3%) were women. The mean age ± SD was 57.94 ± 10.80 y (range, 34–89). The demographic data were well matched between the control and CIT groups (). No statistically significant differences were found between the 2 groups in terms of variables, such as age, sex, smoking index, pathological category, pathologic grade, tumor stage, and lymph node metastasis (; p > 0.05). The characteristics of the CIK and CIK + NK groups were evaluated as well, and no significant differences were observed between the two groups (; p > 0.05).
Table 1. Distribution of demographic and clinical characteristics of patients in the control and cellular immunotherapy (CIT) groups
Table 2. Distribution of demographic and clinical characteristics of patients in the CIK and CIK+NK groups
Quality of the cultured immune cells
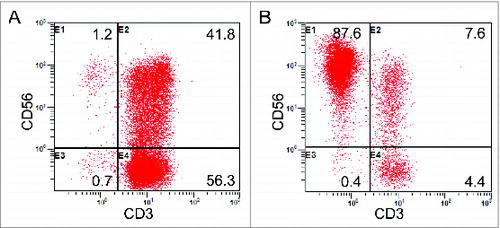
After culturing and expansion, the final number of cells was approximately 8.0 × 109 to 1.3 × 1010 for CIK cells, and 3.0 × 109 to 4.5 × 109 for NK cells. The viable immune cells were found to exceed 95% without any bacterial, fungal, and mycoplasma contamination. The result of the endotoxin test was less than 5 EU. The median percentage of CD3+CD56+ populations in the CIK cells was 30.63% (range, 24.1%–48.0%). The median percentage of CD3–CD56+ populations in the NK cells was 80.1% (range, 60.3%–90.6%). Representative results from one of the study patients are shown in . Following detection, all cultured immune cells were infused back into the patients.
Side effects of CIT infusion
Among the CIT patients, nine patients developed chills and a fever after immune cell infusion. Of them, the peak body temperature was 38°C and recovered naturally within 24 h without any medical treatment. There were no other toxic effects observed in the CIT group.
Survival analysis
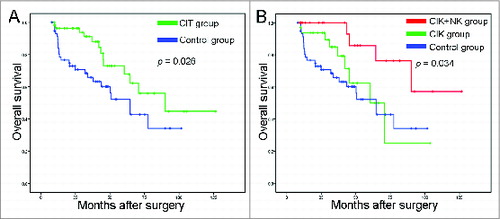
All 120 patients included in this study were assessed for overall survival. The median follow-up time of all patients was 33.0 months (range, 8–127 months). The 1-, 3-, and 5-y overall survival rates were 96.4%, 88.1%, and 67.8%, respectively, in the CIT group, and were 91.2%, 65.9%, and 52.2%, respectively, in the control group. The patients who received adjuvant CIT exhibited a better overall survival rate than the control group (; p = 0.026). Further analysis found that patients in the CIK + NK group showed significantly better prognosis than those in the CIK group (; p = 0.034).
Figure 2. Survival analysis in patients with NSCLC. (A) Overall survival curves for NSCLC patients (n = 120) who received adjuvant cellular immunotherapy (CIT) combined with chemotherapy (CIT group, n = 60) or chemotherapy alone (control group, n = 60). (B) Overall survival curves for NSCLC patients who received adjuvant CIK cell treatment (CIK group, n = 33), adjuvant CIK cell and NK cell treatment (CIK + NK group, n = 27), or chemotherapy alone (control group, n = 60).
Univariate and multivariate analysis
The effects of adjuvant CIT on the prognosis of patients with postoperative NSCLC were further assessed in univariate and multivariate Cox proportional hazards regression analysis. Early stage (p = 0.04), lymph node negative (p = 0.022), and adjuvant CIT (p = 0.03) showed a significant association with improved overall survival in univariate analysis (). Multivariate survival analysis indicated that lymph node negative (p = 0.023) and adjuvant CIT (p = 0.031) remained associated with improved overall survival (). To investigate the role of the percentage of NK cell treatment in the CIK + NK subgroup, univariate and multivariate cox regression analysis of overall survival for patients in the CIK and CIK + NK groups was performed as well (). From multivariate analysis, we found that NK cell treatment was an independent prognostic factor for overall survival of patients in the CIK and CIK + NK groups (p = 0.041; ), which suggested that patients might benefit more from receiving CIT with alternate application of CIK and NK cells than from receiving only CIK cell treatment.
Table 3. Univariate and multivariate analysis of overall survival in patients with NSCLC
Table 4. Univariate and multivariate analysis of overall survival for patients in the CIK and CIK+NK groups
Subgroup analysis
Since lymph node metastasis has been associated with the prognosis of patients with postoperative NSCLC, we subsequently assessed which group of patients with NSCLC would benefit the most from adjuvant CIT. In the lymph node negative group, adjuvant CIT did not significantly improve the overall survival of patients with NSCLC (; p = 0.413). However, in the lymph node positive group, adjuvant CIT significantly improved the overall survival of patients with NSCLC compared with the control group (; p = 0.029).
Figure 3. Subgroup analysis to estimate the benefits of additional cellular immunotherapy (CIT). (A) Overall survival curves for NSCLC patients with lymph node negative (n = 61) who received adjuvant CIT combined with chemotherapy (CIT group, n = 29) or chemotherapy alone (control group, n = 32). (B) Overall survival curves for NSCLC patients with lymph node positive (n = 59) who received adjuvant CIT combined with chemotherapy (CIT group, n = 31) or chemotherapy alone (control group, n = 28). (C) The Kaplan-Meier method was used to compare the overall survival rates between the ≤ 60-y-old group (n = 36) and > 60 age group (n = 24).
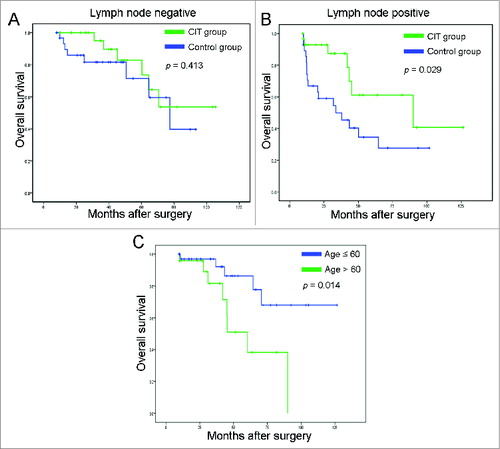
To investigate further the potential factors influencing immunotherapy outcome, we divided the patients with NSCLC in the CIT group into different groups according to age (≤60 and >60 y old), pathologic grade (grade 1, 2 and grade 3), and tumor stage (stage I and stages II–IV). Analysis of overall survival in the subgroups revealed that neither pathologic grade nor tumor stage had effect on the effectiveness of the adjuvant CIT. Overall survival in the ≤60-y-old group had a significantly better prognosis than that of the >60 y age group (; p = 0.014).
Discussion
Avoiding immune destruction has been considered an emerging hallmark of cancer, suggesting that the host immune system plays an important role as a barrier to tumor formation and progression.Citation13 Clinical epidemiology also provides evidence to support the existence of antitumoral immune responses in some forms of cancer, including lung cancer,Citation8-10 which provided the rationale of CIT for the treatment of NSCLC. Indeed, previous studies have increasingly confirmed the positive clinical efficacy of CIT in the treatment of NSCLC.Citation22–26 However, to date, the patterns of CIT have been rarely reported. Therefore, in the present study, through a retrospective analysis of 120 patients with NSCLC, we not only validated the efficacy of adjuvant CIT combined with chemotherapy, but also found that the efficacy of adjuvant CIT with alternate application of CIK and NK cells were superior to that of adjuvant CIT with CIK cells only.
Compared with the control group that received only postoperative chemotherapy, patients with NSCLC who received additional sequential CIT displayed an improved overall survival rate. This result is similar to the findings of Li et al., who also report that CIK cell treatment can improve the efficacy of chemotherapy in NSCLC patients.Citation24 More importantly, our results suggested that patients who received alternate CIK and NK cells treatment exhibited a better prognosis than those who received CIK cells only. These results indicate that adjuvant CIT, especially with the alternate application of CIK and NK cells, can improve the clinical outcome of NSCLC patients.
The possible mechanisms by which CIT enhances the therapeutic efficacy of chemotherapy may be as follows. First, CIT enhancing the efficacy of chemotherapy in patients with cancer is based on their synergistic effects. Many of the available anticancer drugs could enhance the immunogenic properties of malignant cells by subverting immunosuppressive circuitries, and increase the susceptibility of malignant cells to the cytotoxic activity of immune effector cells,Citation36 which would favor the antitumor functions of immune effector cells. Meanwhile, immune effector cells not only produce large amounts of inflammatory cytokines to alleviate immune damage caused by anticancer drugs and enhance the immunosurveillance capabilities of patients with cancer,Citation37 but additionally eliminate potential or residual tumor cells after chemotherapy, including even drug-resistant tumor cells and putative cancer stem cells.Citation38-40 Secondly, alternate application of CIK and NK cells exhibits a synergistic antitumor immunity via different mechanisms compared to the CIT with only CIK cells, which was also found by Maniar et al. and Cui et al.Citation34,35 On the other hand, it was reported that the circulating hematopoietic stem and progenitor cells from various patients with solid cancers exhibited a generalized myeloid bias with a skew toward granulocytic differentiation,Citation41 which increased the neutrophil-to-lymphocyte ratio (NLR), a poor prognostic indicator.Citation42 Adjuvant CIT could reverse the NLR, resulting in immune equilibrium to reduce tumor recurrence and metastasis.
To improve further the clinical application of CIT, the potential factors that could have had an impact on the outcome of the treatment were also evaluated in our study. In subgroup analyses, we found that adjuvant CIT did not significantly improve the prognosis of patients with NSCLC who were lymph node negative. However, adjuvant CIT was significantly associated with an improved overall survival rate in patients with NSCLC who were lymph node positive. The reason for such difference in survival benefit may be that patients with NSCLC who were lymph node negative already had better prognosis and might derive some benefit from adjuvant CIT, but this benefit would not be statistically significant. However, patients with lymph node positive exhibited worse overall survival, and adjuvant CIT could significantly improve the prognosis of this subset of patients. These data suggest that patients with lymph node metastasis positive are more recommended to receive additional CIT after their completion of postoperative chemotherapy. Besides, our results showed that the overall survival rate in the group aged ≤60 y was significantly better than that of the group aged >60 y. This improvement may be explained by the fact that the immune alteration was age dependent. Generally, the declining performance of immune cells that often occurs in elderly people may be correlated with the decreased antitumor immunity in these patients.Citation43 Aging may severely influence chemokine production and physical condition of immune cells.Citation44 For example, Albright, et al. found that NK/LAK cells obtained from young and old mouse spleen cells are different in chemokine production.Citation45 Schreiber, et al. also demonstrated that spleen cells from young but not old immunized mice could eradicate large established cancers.Citation46 Therefore, young patients with better immune function results show improved clinical outcome.
As this was a retrospective study, there were some limitations. First, the uniformity of patients between each group was not very good. Second, the frequency of follow-up in chemotherapy alone group was lower than adjuvant CIT group. Third, it was a retrospective study with all its inevitable defects. Despite these limitations, our study demonstrated that a combination of CIT and postoperative chemotherapy is a safe and potential treatment modality for patients with NSCLC.
In conclusion, in this single-center retrospective study, we have provided evidence that sequential CIT especially with alternate application of CIK and NK cells after surgery and chemotherapy show a better survival improvement for NSCLC patients. Furthermore, patients who are less than 60 y old with positive lymph nodes might benefit more from CIT. Prospective randomized studies are warranted to confirm the present findings and to further define optimal combinational treatment strategies for immunotherapy of NSCLC.
Patients and Methods
Study population
CIT is an observational clinical immunotherapy in the Sun Yat-sen University Cancer Center (SYSUCC, Guangzhou, PR China). It was approved by the institutional ethics committee of SYSUCC. Written informed consent was obtained from each patient before treatment. There were no special selection criteria regarding whether patients would receive adjuvant CIT and immune cell type. A multidisciplinary team of doctors from different departments, including surgeons, oncologists, physicians, and immunologists, made the treatment decisions.
From January 2004 to November 2013, the medical records of patients with NSCLC from a computerized database in the SYSUCC were reviewed after obtaining institutional review board approval. NSCLC was histologically proven according to the World Health Organization criteria. All study patients had an Eastern Cooperative Oncology Group performance status score of ≤2, adequate liver and renal functions, and were free of cardiac disease. Patients were excluded from the study based on the following criteria: with autoimmune disease or active infections at diagnosis, a history of other malignancy, previous cancer treatment, recruitment in other clinical trial, or postoperative dysfunction in any organ. After review, 60 NSCLC patients met these criteria received adjuvant CIT after surgery and chemotherapy (CIT group), whereas the other 60 patients diagnosed at the same or near day but without CIT were used as the control group for comparisons. Out of the 60 patients in the CIT group, 33 had received only CIK cell treatment (CIK group), and 27 had received alternate application of CIK and NK cell treatment (CIK + NK group). The characteristics of patients in each group are summarized in .
Treatment schedule
Following surgery, all patients in the control and CIT groups received four cycles of chemotherapy with TP regimen (paclitaxel and cisplatin), NP regimen (navelbine and cisplatin), or GP regimen (gemcitabine and cisplatin). One month after completion of chemotherapy, CIT group patients received immune cell infusions. The cell preparation and infusion processes are described below.
Generation of immune cells
In the CIT group, 2 weeks after the patients had completed chemotherapy treatment and when routine blood examination results had returned to normal, a 50–60 mL sample of heparinized peripheral blood was obtained from each patient. Mononuclear cells separated from peripheral blood mononuclear cells (PBMCs) by Ficoll-Hypaque density centrifugation were used to induce CIK and NK cells using different cytokines, respectively, in a good manufacturing practice-compliant facility.
To generate autologous CIK cells, PBMCs were cultured for the first 24 h in X-VIVO 15 serum-free medium (Lonza) supplemented with 1,000 U/mL recombinant human IFNγ (Shanghai Clone Company). Then, the following were added: 100 ng/mL mouse anti-human CD3 monoclonal antibody (R&D Systems), 100 U/mL IL-1α (Life Technologies), and 1,000 U/mL IL-2 (Beijing Sihuan). Fresh IL-2 and fresh medium were added periodically and the CIK cells were harvested at 14 d.
For expansion of autologous NK cells, PBMCs were cultured in an anti-HER2 monoclonal antibodyCitation47-49 (Roche Pharma) coated 75 cm2 flask with X-VIVO 15 serum-free medium supplemented with 1,000 U/mL IL-2 for 24 h. Next, the cells were centrifuged, and the supernatant was discarded. The cells were cultured again in X-VIVO 15 serum-free medium supplemented with 1,000 U/mL IL-2 for 2 weeks.
Immune cell infusion
The CIT protocol is shown in Fig. S1. After being cultured for 14 d, all numbers of autologous CIK or NK cells were harvested and washed three times with normal saline. Autologous immune cells were resuspended in 100 mL normal saline supplemented with 1% human serum albumin, respectively, and were administered to patients via intravenous infusion over 30 min. Before administration, a fraction of the immune cells were used to evaluate the number, viability (by dye exclusion test), and possible contamination by bacteria, fungi, or endotoxins. Patients received at least six cycles of immune cell infusions with a 2-week interval between each cycle. The next cycle of PBMC collection started 1 d before the last infusion of the previous cycle. If disease was stable and patients wanted, more cycles of CIT were administered using the above protocol. Otherwise, the CIT was discontinued when the disease was in progression or when patients refused further CIT. Subsequently, an alternative therapy was recommended by physicians.
Follow-up
After surgery, all the NSCLC patients were followed-up regularly at our outpatient department. Generally, patients were observed once every 2 months during the first year, every 3 months during the second year, and every 6 months thereafter. Additionally, telephone inquiries were carried out regularly for each patient at our follow-up center. At each follow-up visit in the outpatient department, physical examination, blood chemistry, and chest radiography were carried out. Chest computed tomography, bone scintigraphy, and positron emission tomography were performed when tumor recurrence or metastasis were suspected. Treatments for recurrent tumors were determined by our multidisciplinary team. Overall survival was defined as the interval between surgery and death or the last known follow-up. The correlating treatments and survival status of the patients were entered into the medical records after follow-up and updated accordingly in the database.
Statistical analysis
To evaluate the basic characteristics of the two groups, the Mann-Whitney U test was used to compare continuous variables; the Pearson χ2 test and Fisher's exact test were used to compare categorical variables. Overall survival was defined as the interval between surgery and death or the last known follow-up. Overall survival curves were constructed according to the Kaplan-Meier method and compared using the log-rank test. The multivariate Cox proportional hazard model was used to analyze factors found to be statistically significant by univariate analysis. Statistical analyses were performed using SPSS version 16.0 (SPSS, Inc., Chicago, IL, USA). All tests were two-sided with a statistical significance level set at p < 0.05.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Supplemental Material
Supplemental data for this article can be accessed on the publisher's website.
1038017_supplemental_data.zip
Download Zip (159.1 KB)Funding
This work was supported by grants from the Health Industry Scientific Research Project, China [grant number 200902002-2], the Guangdong Province Science and Technology Plan Project, China [grant number 2011A030400004], and Guangzhou City Plan Project, China [grant number 2013J4500005].
References
- Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin 2011; 61:69-90; PMID:21296855; http://dx.doi.org/10.3322/caac.20107
- Chen Z, Fillmore CM, Hammerman PS, Kim CF, Wong KK. Non-small-cell lung cancers: a heterogeneous set of diseases. Nat Rev Cancer 2014; 14:535-46; PMID:25056707; http://dx.doi.org/10.1038/nrc3775
- Yang L, Ren B, Li H, Yu J, Cao S, Hao X, Ren X. Enhanced antitumor effects of DC-activated CIKs to chemotherapy treatment in a single cohort of advanced non-small-cell lung cancer patients. Cancer Immunol Immunother 2013; 62:65-73; PMID:22744010; http://dx.doi.org/10.1007/s00262-012-1311-8
- Zhao M, Li H, Li L, Zhang Y. Effects of a gemcitabine plus platinum regimen combined with a dendritic cell-cytokine induced killer immunotherapy on recurrence and survival rate of non-small cell lung cancer patients. Exp Ther Med 2014; 7:1403-7; PMID:24940447
- Pisters KM. Adjuvant chemotherapy for non-small-cell lung cancer-the smoke clears. N Engl J Med 2005; 352:2640-2; PMID:15972872; http://dx.doi.org/10.1056/NEJMe058110
- Keller SM, Adak S, Wagner H, Herskovic A, Komaki R, Brooks BJ, Perry MC, Livingston RB, Johnson DH. A randomized trial of postoperative adjuvant therapy in patients with completely resected stage II or IIIA non-small-cell lung cancer. Eastern cooperative oncology group. N Engl J Med 2000; 343:1217-22; PMID:11071672; http://dx.doi.org/10.1056/NEJM200010263431703
- Felip E, Martinez-Marti A, Martinez P, Cedres S, Navarro A. Adjuvant treatment of resected nonsmall cell lung cancer: state of the art and new potential developments. Curr Opin Oncol 2013; 25:115-20; PMID:23262832; http://dx.doi.org/10.1097/CCO.0b013e32835ca1b0
- Aerts JG, Hegmans JP. Tumor-specific cytotoxic T cells are crucial for efficacy of immunomodulatory antibodies in patients with Lung Cancer. Cancer Res 2013; 73:2381-8; PMID:23580578; http://dx.doi.org/10.1158/0008-5472.CAN-12-3932
- Lv M, Xu Y, Tang R, Ren J, Shen S, Chen Y, Liu B, Hou Y, Wang T. miR-141-CXCL1-CXCR2 signaling-induced Treg cells recruitment regulates metastases and survival of non-small cell lung cancer. Mol Cancer Ther 2014; 13:3152-62; PMID:25349304; http://dx.doi.org/10.1158/1535-7163.MCT-14-0448
- Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, Powderly JD, Carvajal RD, Sosman JA, Atkins MB et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med 2012; 366:2443-54; PMID:22658127; http://dx.doi.org/10.1056/NEJMoa1200690
- Thomas A, Hassan R. Immunotherapies for non-small-cell lung cancer and mesothelioma. Lancet Oncol 2012; 13:e301-10; PMID:22748269; http://dx.doi.org/10.1016/S1470-2045(12)70126-2
- Hasegawa T, Suzuki H, Yamaura T, Muto S, Okabe N, Osugi J, Hoshino M, Higuchi M, Ise K, Gotoh M. Prognostic value of peripheral and local forkhead box P3 regulatory T cells in patients with non-small-cell lung cancer. Mol Clin Oncol 2014; 2:685-94; PMID:25054031; http://dx.doi.org/10.3892/mco.2014.299
- Hanahan D, Weinberg RA. Hallmarks of Cancer: The Next Generation. Cell 2011; 144:646-74; PMID:21376230; http://dx.doi.org/10.1016/j.cell.2011.02.013
- Dudley ME, Rosenberg SA. Adoptive-cell-transfer therapy for the treatment of patients with cancer. Nat Rev Cancer 2003; 3:666-75; PMID:12951585; http://dx.doi.org/10.1038/nrc1167
- Finn OJ. Cancer vaccines: between the idea and the reality. Nat Rev Immunol 2003; 3:630-41; PMID:12974478; http://dx.doi.org/10.1038/nri1150
- Drake CG, Jaffee E, Pardoll DM. Mechanisms of immune evasion by tumors. Adv Immunol 2006; 90:51-81; PMID:16730261; http://dx.doi.org/10.1016/S0065-2776(06)90002-9
- Wang D, Zhang B, Gao H, Ding G, Wu Q, Zhang J, Liao L, Chen H. Clinical research of genetically modified dendritic cells in combination with cytokine-induced killer cell treatment in advanced renal cancer. Bmc Cancer 2014; 14:251; PMID:24720900; http://dx.doi.org/10.1186/1471-2407-14-251
- Li JJ, Gu MF, Pan K, Liu LZ, Zhang H, Shen WX, Xia JC. Autologous cytokine-induced killer cell transfusion in combination with gemcitabine plus cisplatin regimen chemotherapy for metastatic nasopharyngeal carcinoma. J Immunother 2012; 35:189-95; PMID:22306907; http://dx.doi.org/10.1097/CJI.0b013e318241d9de
- Pan K, Guan XX, Li YQ, Zhao JJ, Li JJ, Qiu HJ, Weng DS, Wang QJ, Liu Q, Huang LX et al. Clinical Activity of Adjuvant Cytokine-Induced Killer Cell Immunotherapy in Patients with Post-Mastectomy Triple-Negative Breast Cancer. Clin Cancer Res 2014; 20:3003-11; PMID:24668644; http://dx.doi.org/10.1158/1078-0432.CCR-14-0082
- Pan K, Li Y, Wang W, Xu L, Zhang Y, Zheng H, Zhao J, Qiu H, Weng D, Li J et al. The Efficacy of Cytokine-Induced Killer Cell Infusion as an Adjuvant Therapy for Postoperative Hepatocellular Carcinoma Patients. Ann Surg Oncol 2013; 20:4305-11; PMID:23892527; http://dx.doi.org/10.1245/s10434-013-3144-x
- Ljunggren HG, Malmberg KJ. Prospects for the use of NK cells in immunotherapy of human cancer. Nat Rev Immunol 2007; 7:329-39; PMID:17438573; http://dx.doi.org/10.1038/nri2073
- Wu C, Jiang J, Shi L, Xu N. Prospective study of chemotherapy in combination with cytokine-induced killer cells in patients suffering from advanced non-small cell lung cancer. Anticancer Res 2008; 28:3997-4002; PMID:19192663
- Zhong R, Han B, Zhong H. A prospective study of the efficacy of a combination of autologous dendritic cells, cytokine-induced killer cells, and chemotherapy in advanced non-small cell lung cancer patients. Tumor Biol 2014; 35:987-94; PMID:24006222; http://dx.doi.org/10.1007/s13277-013-1132-1
- Li R, Wang C, Liu L, Du C, Cao S, Yu J, Wang SE, Hao X, Ren X, Li H. Autologous cytokine-induced killer cell immunotherapy in lung cancer: a phase II clinical study. Cancer Immunol Immunother 2012; 61:2125-33; PMID:22581306; http://dx.doi.org/10.1007/s00262-012-1260-2
- Zhong R, Teng J, Han B, Zhong H. Dendritic cells combining with cytokine-induced killer cells synergize chemotherapy in patients with late-stage non-small cell lung cancer. Cancer Immunol Immunother 2011; 60:1497-502; PMID:21681372; http://dx.doi.org/10.1007/s00262-011-1060-0
- Han RX, Liu X, Pan P, Jia YJ, Yu JC. Effectiveness and safety of chemotherapy combined with dendritic cells co-cultured with cytokine-induced killer cells in the treatment of advanced non-small-cell lung cancer: a systematic review and meta-analysis. PLoS One 2014; 9:e108958; PMID:25268709; http://dx.doi.org/10.1371/journal.pone.0108958
- Mesiano G, Todorovic M, Gammaitoni L, Leuci V, Giraudo DL, Carnevale-Schianca F, Fagioli F, Piacibello W, Aglietta M, Sangiolo D. Cytokine-induced killer (CIK) cells as feasible and effective adoptive immunotherapy for the treatment of solid tumors. Expert Opin Biol Ther 2012; 12:673-84; PMID:22500889; http://dx.doi.org/10.1517/14712598.2012.675323
- Thanendrarajan S, Kim Y, Schmidt-Wolf I. New adoptive immunotherapy strategies for solid tumours with CIK cells. Expert Opin Biol Ther 2012; 12:565-72; PMID:22444075; http://dx.doi.org/10.1517/14712598.2012.668879
- Hui KM. CIK cells-current status, clinical perspectives and future prospects-the good news. Expert Opin Biol Ther 2012; 12:659-61; PMID:22500927; http://dx.doi.org/10.1517/14712598.2012.676037
- Ma Y, Zhang Z, Tang L, Xu YC, Xie ZM, Gu XF, Wang HX. Cytokine-induced killer cells in the treatment of patients with solid carcinomas: a systematic review and pooled analysis. Cytotherapy 2012; 14:483-93; PMID:22277010; http://dx.doi.org/10.3109/14653249.2011.649185
- Ljunggren HG, Karre K. In search of the ‘missing self’: MHC molecules and NK cell recognition. Immunol Today 1990; 11:237-44; PMID:2201309; http://dx.doi.org/10.1016/0167-5699(90)90097-S
- Schmidt-Wolf IG, Lefterova P, Mehta BA, Fernandez LP, Huhn D, Blume KG, Weissman IL, Negrin RS. Phenotypic characterization and identification of effector cells involved in tumor cell recognition of cytokine-induced killer cells. Exp Hematol 1993; 21:1673-9; PMID:7694868
- Linn YC, Lau LC, Hui KM. Generation of cytokine-induced killer cells from leukaemic samples with in vitro cytotoxicity against autologous and allogeneic leukaemic blasts. Br J Haematol 2002; 116:78-86; PMID:11841399; http://dx.doi.org/10.1046/j.1365-2141.2002.03247.x
- Maniar A, Zhang X, Lin W, Gastman BR, Pauza CD, Strome SE, Chapoval AI. Human gammadelta T lymphocytes induce robust NK cell-mediated antitumor cytotoxicity through CD137 engagement. Blood 2010; 116:1726-33; PMID:20519625; http://dx.doi.org/10.1182/blood-2009-07-234211
- Cui J, Wang N, Zhao H, Jin H, Wang G, Niu C, Terunuma H, He H, Li W. Combination of radiofrequency ablation and sequential cellular immunotherapy improves progression-free survival for patients with hepatocellular carcinoma. Int J Cancer 2014; 134:342-51; PMID:23825037; http://dx.doi.org/10.1002/ijc.28372
- Zitvogel L, Galluzzi L, Smyth MJ, Kroemer G. Mechanism of action of conventional and targeted anticancer therapies: reinstating immunosurveillance. Immunity 2013; 39:74-88; PMID:23890065; http://dx.doi.org/10.1016/j.immuni.2013.06.014
- Hoyle C, Bangs CD, Chang P, Kamel O, Mehta B, Negrin RS. Expansion of Philadelphia chromosome-negative CD3(+)CD56(+) cytotoxic cells from chronic myeloid leukemia patients: in vitro and in vivo efficacy in severe combined immunodeficiency disease mice. Blood 1998; 92:3318-27; PMID:9787169
- Zhang YS, Yuan FJ, Jia GF, Zhang JF, Hu LY, Huang L, Wang J, Dai ZQ. CIK cells from patients with HCC possess strong cytotoxicity to multidrug-resistant cell line Bel-7402/R. World J Gastroenterol 2005; 11:3339-45; PMID:15948236; http://dx.doi.org/10.3748/wjg.v11.i22.3339
- Schmidt-Wolf IG, Lefterova P, Johnston V, Scheffold C, Csipai M, Mehta BA, Tsuruo T, Huhn D, Negrin RS. Sensitivity of multidrug-resistant tumor cell lines to immunologic effector cells. Cell Immunol 1996; 169:85-90; PMID:8612299; http://dx.doi.org/10.1006/cimm.1996.0094
- Gammaitoni L, Giraudo L, Leuci V, Todorovic M, Mesiano G, Picciotto F, Pisacane A, Zaccagna A, Volpe MG, Gallo S et al. Effective activity of cytokine-induced killer cells against autologous metastatic melanoma including cells with stemness features. Clin Cancer Res 2013; 19:4347-58; PMID:23794732; http://dx.doi.org/10.1158/1078-0432.CCR-13-0061
- Wu WC, Sun HW, Chen HT, Liang J, Yu XJ, Wu C, Wang Z, Zheng L. Circulating hematopoietic stem and progenitor cells are myeloid-biased in cancer patients. Proc Natl Acad Sci U S A 2014; 111:4221-6; PMID:24591638; http://dx.doi.org/10.1073/pnas.1320753111
- Templeton AJ, McNamara MG, Seruga B, Vera-Badillo FE, Aneja P, Ocana A, Leibowitz-Amit R, Sonpavde G, Knox JJ, Tran B et al. Prognostic role of neutrophil-to-lymphocyte ratio in solid tumors: a systematic review and meta-analysis. J Natl Cancer Inst 2014; 106:u124; PMID:24875653; http://dx.doi.org/10.1093/jnci/dju124
- Kozlowska E, Biernacka M, Ciechomska M, Drela N. Age-related changes in the occurrence and characteristics of thymic CD4(+) CD25(+) T cells in mice. Immunology 2007; 122:445-53; PMID:17627771; http://dx.doi.org/10.1111/j.1365-2567.2007.02667.x
- Muller L, Pawelec G. Aging and immunity - impact of behavioral intervention. Brain Behav Immun 2014; 39:8-22; PMID:24315935; http://dx.doi.org/10.1016/j.bbi.2013.11.015
- Albright JW, Bream JH, Bere EW, Young HA, Winkler-Pickett R, Ortaldo JR. Aging of innate immunity: functional comparisons of NK/LAK cells obtained from bulk cultures of young and aged mouse spleen cells in high concentrations of interleukin-2. Exp Gerontol 2004; 39:73-82; PMID:14724067; http://dx.doi.org/10.1016/j.exger.2003.09.017
- Schreiber K, Arina A, Engels B, Spiotto MT, Sidney J, Sette A, Karrison TG, Weichselbaum RR, Rowley DA, Schreiber H. Spleen cells from young but not old immunized mice eradicate large established cancers. Clin Cancer Res 2012; 18:2526-33; PMID:22415314; http://dx.doi.org/10.1158/1078-0432.CCR-12-0127
- Repka T, Chiorean EG, Gay J, Herwig KE, Kohl VK, Yee D, Miller JS. Trastuzumab and interleukin-2 in HER2-positive metastatic breast cancer: a pilot study. Clin Cancer Res 2003; 9:2440-6; PMID:12855616
- Carson WE, Parihar R, Lindemann MJ, Personeni N, Dierksheide J, Meropol NJ, Baselga J, Caligiuri MA. Interleukin-2 enhances the natural killer cell response to Herceptin-coated Her2/neu-positive breast cancer cells. Eur J Immunol 2001; 31:3016-25; PMID:11592078; http://dx.doi.org/10.1002/1521-4141(2001010)31:10%3c3016::AID-IMMU3016%3e3.0.CO;2-J
- Parihar R, Dierksheide J, Hu Y, Carson WE. IL-12 enhances the natural killer cell cytokine response to Ab-coated tumor cells. J Clin Invest 2002; 110:983-92; PMID:12370276; http://dx.doi.org/10.1172/JCI0215950