Abstract
Myeloid-derived suppressor cells (MDSCs) are a heterogeneous group of cells which comprise two subsets: granulocytic MDSCs (G-MDSCs) and monocytic MDSCs (M-MDSCs). MDSCs involve in tumor-associated immune suppression by remarkably blocking effector T-cell activation and inducing expansion of regulatory T cells in the tumor microenvironment. The treatment that alters the suppression of MDSCs can effectively facilitate the antitumor immune responses. Recently, we showed that the whole β-glucan particles (WGPs) are capable of altering the suppression of MDSCs. However, the regulatory mechanism of MDSCs by WGP remains unknown. In this study, we found that the expression of nuclear factor I-A (NFIA), an integral transcriptional component of myeloid differentiation and lineage commitment, was inhibited by WGP in G-MDSCs. The effect of WGP on expression of NFIA was the c-jun molecule dependent via Dectin-1 pathway in vitro. Moreover, NFIA knockdown could alter the suppressive function of G-MDSCs, promote the antitumor immune responses and delay the tumor progression in tumor-bearing mice. Taken together, our results demonstrate a critical role of NFIA during WGP regulating the immunosuppression of G-MDSCs, with potential implications as an antitumor immune therapeutic approach.
Introduction
Immune suppression is a major cause of tumor-associated immune evasion and accumulating evidences have demonstrated that MDSCs are closely associated with tumor-induced immunosuppression.Citation1,2 In mice, MDSCs are a heterogeneous group of cells characterized as CD11b+Gr1+ cells.Citation3 Under physiological conditions, these cells produced in bone marrow can quickly differentiate into granulocytes, macrophages or dendritic cells (DCs). However, in pathological conditions, MDSCs expand dramatically, especially in tumor-bearing hosts.Citation4 MDSCs in mice have recently been divided into two subsets for their different morphology and phenotype: G-MDSCs whose phenotype is CD11b+LY6G+LY6Clow, whereas M-MDSCs are CD11b+LY6G−LY6Chi.Citation5 G-MDSCs which are the majority population of MDSCs in tumor-bearing mice represent nearly 70%–80% of all MDSCs and they suppress the T-cell-mediated antitumor immune response.Citation6 During the close cell-cell contact, Arg1 and ROS released by G-MDSCs can result in the alterations of cell-surface molecules on T cells.Citation4 Additionally, G-MDSCs have also been defined in cancer patients recently.Citation7,8
β-glucans are glucose polymers with a backbone of linear β-1,3-linked D-glucose molecules (β-1,3-D-glucan) and β-1,6-linked side chains of varying sizes with distribution frequency.Citation9 They exist most commonly as cellulose in plants, the brain of cereal grains, the cell wall of yeast, certain fungi, mushrooms and bacteria.Citation10,11 β-1,3-D-glucans with β-1,6 side chains are characterized by the features of high molecular weight, low water solubility and high viscosity. In a recent study, it shows that there exists a unique cyclic structure in Candida albicans hyphal β-glucans while not found in yeast β-glucans.Citation12,13
The biological activity of β-glucans are often determined by the molecular weight, the degree of branching and the secondary structure of these compounds.Citation14 Plenty of research about immunomodulating, antitumor and antiinflammatory effects have conducted essentially for β-glucans. β-glucans belong to pathogen-associated molecular patterns (PAMPs), which are able to induce and enhance innate immune responses efficiently mainly via Dectin-1/Syk pathway.Citation9,15 Moreover, β-glucans have recently been reported to enhance Ag-specific T cell and B cell responses.Citation16–18 In our previous studies, it has been confirmed that WGPs accelerate the differentiation of MDSCs as well as inhibiting the suppressive function of MDSCs via Dectin-1/Syk pathway.Citation19 However, the molecular mechanism of regulation on MDSCs by WGP is still unknown.
NFIA is a member of the nuclear factor I (NFI) family as well as NFI-B, NFI-C, NFI-X. Typical NFI protein contains an N-terminal DNA-binding/dimerization domain and C-terminal transcriptional activation and/or repression domain.Citation20 Recent studies suggest that NFI-A is a relevant target of the myeloid regulator miR-223 in promyelocytic leukemia cells as over-expression of NFIA suppresses monocytic and granulocytic differentiation of HPCs but accelerates erythropoiesis.Citation21,22 All these data suggest that NFIA is a crucial transcription factor involved in the differentiation of myeloid cells, especially granulocyte.
Inhibition of MDSCs function is a feasible approach to enhance antitumor immune responses.Citation4,23,24 In this study, we found that NFIA played a critical role in regulating the immunosuppressive capacity of G-MDSCs. WGP downregulated NFIA expression via c-jun-dependent pathway. NFIA knockdown declined the suppression of G-MDSCs and promoted antitumor T-cell responses. These findings indicate NFIA may have potential therapeutic implications as a modulator of MDSCs suppressive function.
Results
WGP downregulates expression of NFIA in G-MDSCs via Dectin-1 pathway in vitro
Our previous studies have demonstrated that WGP is able to reduce the immunosuppression of MDSCs via Dectin-1 pathway.Citation19 To investigate the regulation of WGP on the expression of NFIA in G-MDSCs, we firstly confirmed whether there existed NFIA expression in G-MDSCs. G-MDSCs were isolated from splenocytes of tumor-bearing or wild-type C57BL/6 mice. qRT-PCR analysis reflected that NFIA expression increased in G-MDSCs isolated from tumor-bearing mice compared to cells from wild-type mice (). We also found that the expression of NFIA in G-MDSCs sorted from tumor tissue showed no significant difference compared to NFIA level in G-MDSCs derived from splenocytes in tumor-bearing mice (Fig. S1A). Then, WGP was used to stimulate G-MDSCs isolated from spleen and tumor tissue of tumor-bearing C57BL/6 mice in vitro. NFIA mRNA and protein levels were detected with qRT-PCR and protein gel blot respectively. As shown in , in G-MDSCs isolated from splenocytes of tumor-bearing mice, NFIA mRNA expression was significantly reduced after stimulation with WGP. Analogously, NFIA protein level in G-MDSCs was declined after WGP treatment (). The analogous results were also detected in G-MDSCs isolated from tumor tissue of tumor-bearing mice (Fig. S1B and C).
Figure 1. WGP downregulates expression of NFIA in G-MDSCs via Dectin-1 pathway in vitro. A total of 3 × 106 Lewis lung carcinoma cells (LLCs) were injected s.c. into C57BL/6 mice. After 4 weeks, splenocytes were collected and G-MDSCs were sorted. (A) Total RNA isolated from G-MDSCs was subjected to qRT-PCR to measure NFIA expression. (B, C) Sorted G-MDSCs were cultured in the presence or absence of WGP (100 μg/mL) for 24 h/48 h. (B) NFIA mRNA level in G-MDSCs was measured by qRT-PCR. (C) Protein gel blot analysis was developed with anti-NFIA antibody. Histone 3 served as a loading control. The amount of NFIA protein was calculated by gray scanning. (D, E) The purified G-MDSCs were pretreated with anti-Dectin-1 mAb or isotype rat IgG (5 μg/mL) for 1h at 37°C and then treated with 100 μg/mL WGP. After 24 h/48 h stimulation, cells were collected. (D) RNA isolated from collected G-MDSCs was subjected to qRT-PCR to measure NFIA mRNA expression. (E) NFIA protein in G-MDSCs was detected by protein gel blot. The amount of NFIA protein was calculated by gray scanning. ***p < 0.001, *p < 0.05, ns: no significance.
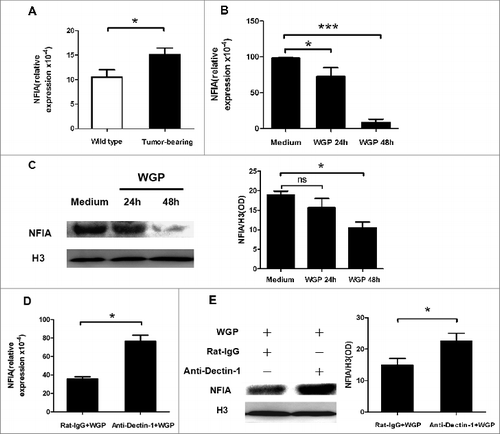
β-glucans recognized as PAMPs can mediate the innate immune responses by recognizing Dectin-1, which is a non-Toll-like pattern recognition receptor for β-glucans, and activate Syk or Raf-1 kinase signaling pathway.Citation9 In order to confirm whether NFIA expression in G-MDSCs was impaired by WGP through Dectin-1 pathway, anti-Dectin-1 antibody was used to block Dectin-1 and it was found that NFIA downregulation induced by WGP was subverted. As shown in , both NFIA mRNA and protein levels in G-MDSCs restored to high levels after Dectin-1 was blocked even treated with WGP. Collectively, these data demonstrate that WGP is able to decrease NFIA expression in G-MDSCs via Dectin-1 pathway.
The attenuated expression of NFIA in G-MDSCs induced by WGP is c-jun dependent
So far, we had already proved that WGP regulated NFIA expression in G-MDSCs via Dectin-1 pathway, and recently, we have found that WGP affects the suppressive function and differentiation of MDSCs mainly through Dectin-1/Syk pathway. To determine the potential molecular mechanism of WGP inhibiting NFIA expression in G-MDSCs, we detected phosphorylation of c-jun in G-MDSCs after treatment with WGP. The results indicated that WGP was capable of promoting the phosphorylation of c-jun molecular obviously in G-MDSCs ().
Figure 2. The attenuated expression of NFIA in G-MDSCs induced by WGP is c-jun molecule dependent. (A) G-MDSCs sorted from splenocytes of tumor-bearing mice were treated with or without WGP (100 μg/mL) for indicated times. Protein gel blot analysis developed with anti-phospho-c-jun antibody and anti-c-jun antibody, histone 3 served as a loading control. The amount of phospho-c-jun protein was calculated by gray scanning. (B, C) G-MDSCs sorted from splenocytes of tumor-bearing mice were pretreated with SP600125 (20 μmol/mL) which is the inhibitor of the phosphorylation of c-jun or DMSO at 37°Cfor 1 h and then stimulated with 100 μg/mL WGP. After 24 h/48 h stimulation, cells were collected. (B) The NFIA mRNA expression in collected G-MDSCs was detected by qRT-PCR. (C) Protein gel blot detection of NFIA protein in G-MDSCs. The amount of NFIA protein was calculated by gray scanning. **p < 0.01, *p < 0.05.
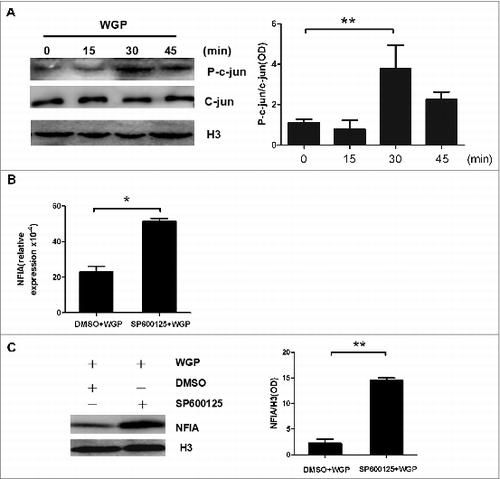
Since the phosphorylation of c-jun molecule which is the downstream of Dectin-1 in G-MDSCs was strengthened by WGP, we supposed that c-jun molecule might be involved in the pathway that WGP regulated NFIA in G-MDSCs. We firstly used SP600125, which is the inhibitor of the phosphorylation of c-jun, to suppress the phosphorylation of c-jun molecule in G-MDSCs and then stimulated G-MDSCs with WGP. Interestingly, NFIA mRNA and protein levels were increased manifestly after the phosphorylation of c-jun molecule was inhibited in G-MDSCs (). The data indicate that c-jun molecule is indeed involved in the Dectin-1 pathway that WGP mediates NFIA expression in G-MDSCs.
NFIA knockdown alters the suppressive capacity of G-MDSCs in vitro
As described previously, WGP can regulate the suppressive function of MDSCs via Dectin-1 pathway.Citation19 Meanwhile, NFIA acting as a crucial nuclear transcription factor has the ability to mediate lineage commitment and biology of myeloid cells.Citation21,22 Since NFIA expression in G-MDSCs was declined after WGP treatment, we hypothesized that NFIA may be related to the suppressive function of G-MDSCs. To determine a link between NFIA expression and the immunosuppression of G-MDSCs, G-MDSCs isolated from spleen and tumor tissue were transfected with NFIA siRNA respectively to inhibit NFIA expression. It was observed that compared with the control, NFIA protein expression in G-MDSCs transfected with siNFIA was inhibited apparently (; Fig. S2A). In addition, we found G-MDSC-mediated T-cell suppression was altered after NFIA knockdown (; Fig. S2B).
Figure 3. NFIA knockdown alters the suppressive capacity of G-MDSCs in vitro. G-MDSCs sorted from splenocytes of tumor-bearing mice were transfected with NFIA siRNA (100 nM). (A) Protein gel blot analysis confirmed NFIA knockdown in G-MDSCs transfected with siNFIA. (B) G-MDSCs transfected with NFIA siRNA, then cells were harvested and cocultured with CD4+ T cells at different ratios in the presence of anti-CD3 mAb and anti-CD28 mAb for 72 h. 3H-thymidine incorporation was used to detect T cells proliferation. (C) NADPH oxidase complex which consists of phox p47 and gp91 appears to be a major source of ROS. Here, p47 and gp91 were chosen to represent the ROS production. Arg1 and gp91/p47 mRNA levels in G-MDSCs transfected with siNFIA were detected by qRT-PCR. (D) Arg1 activity of G-MDSCs transfected with siNFIA was measured. Production of ROS in G-MDSCs was analyzed by flow cytometry. ***p < 0.001, **p < 0.01, *p < 0.05, ns: no significance, Geo MFI: geometric mean fluorescent intensity.
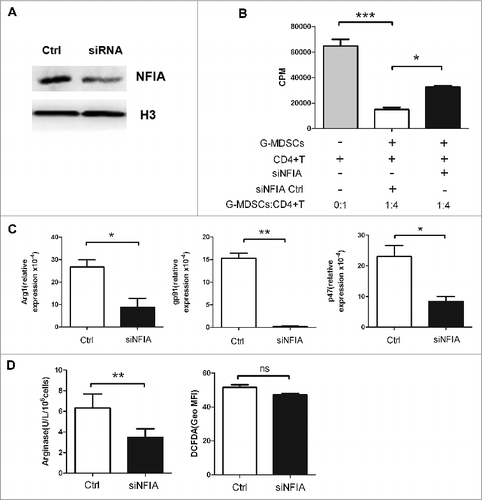
It has been reported that MDSCs suppress T-cell-induced antitumor immune responses through a variety of mechanisms, especially Arg1 activity, production of NO and ROS. NO and Arg1 are the main effector molecules of M-MDSCs while G-MDSC-induced suppression relies on ROS and Arg1.Citation4 NADPH oxidase complex which consists of phox p47 and gp91 appears to be a major source of ROS. Here, p47 and gp91 mRNA levels were chosen to represent the ROS. As shown in ; Fig. S2C, after transfected with siNFIA, Arg1 and ROS (p47 and gp91) mRNA levels were reduced in G-MDSCs sorted from spleen and tumor tissue. Meanwhile, in G-MDSCs derived from spleen of tumor-bearing mice, Arg1 activity was declined but ROS produced by G-MDSCs showed no significant difference (). However, Arg1 activity and ROS production were both decreased in G-MDSCs isolated from tumor tissue after NFIA knockdown (Fig. S2D). Together, these data indicate that NFIA expression is closely associated with the suppressive capacity of G-MDSCs in vitro.
NFIA knockdown declines the capability of G-MDSCs to accelerate the tumor progression and inhibit the antitumor immune responses
G-MDSCs isolated from spleen of tumor-bearing C57BL/6 mice were transfected with NFIA siRNA in vitro. Then, 3 × 106 G-MDSCs transfected with siNFIA were mixed with 0.9 × 106 Lewis lung carcinoma (LLC) cells, and these mixed cells were implanted s.c into C57BL/6 mice. As shown in , the tumor growth rate and tumor weight were significantly reduced in mice implanted with G-MDSCs transfected with siNFIA compared with mice implanted with G-MDSCs transfected with RNA control. Analogously, mice in group that implanted with G-MDSCs transfected with siNFIA survived longer compared with the group implanted with G-MDSCs transfected with RNA control ().
Figure 4. NFIA knockdown declines the capability of G-MDSCs to accelerate the tumor progression and inhibit the antitumor immune responses. Groups of mice were injected s.c. with the cells mix of G-MDSCs transfected with siNFIA and LLCs. (A) Tumor volume and weight were measured at indicated time. (B) Survival of mice in different groups. (C) The proportions of CD4+IFNγ+ Th1 and CD8+IFNγ+ CTL cells from spleens, draining lymph nodes and tumor tissue were analyzed by flow cytometry. ***p < 0.001, **p < 0.01, *p < 0.05.
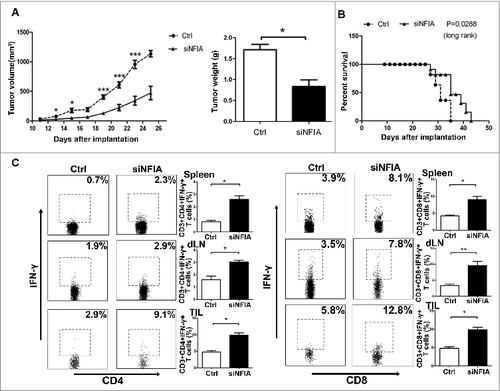
Figure 5. Administration of siNFIA into tumor tissue delays tumor progression and enhances antitumor immune responses. Groups of mice bearing established LLC were intratumorally injected with siNFIA every 3 d for 2 weeks. (A) The proportion of CD11b+Ly6G+ G-MDSCs in tumor tissue was analyzed by flow cytometry. (B) G-MDSCs from tumor tissue were isolated, NFIA protein level was detected by Protein gel blot. Arg1 activity and ROS production in G-MDSCs sorted from tumor tissues were detected. (C) Tumor volume and weight were measured at indicated time. (D) Survival of mice in different groups. (E) Ki67 expression in tumor tissue was detected by qRT-PCR. (F) The proportions of CD4+IFNγ+ Th1 and CD8+IFNγ+ CTL cells from spleens, draining lymph nodes and tumor tissue were analyzed by flow cytometry. **p < 0.01, *p < 0.05, ns: no significance, Geo MFI: geometric mean fluorescent intensity.
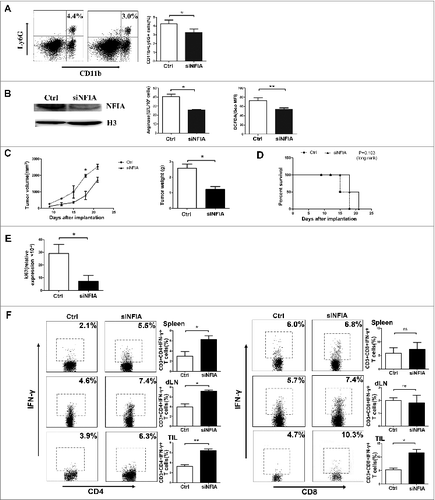
As T cells are the main effector cells suppressed by MDSCs in tumor microenvironment, we further investigated whether the alteration of NFIA expression in G-MDSCs could regulate the Th1 and CTL priming in vivo. Thus, the proportions of CD4+IFNγ+ Th1 and CD8+IFNγ+ CTL cells in spleens, draining lymph nodes and tumor tissue were detected by flow cytometry, we found that mice in group that implanted with G-MDSCs transfected with siNFIA had augmented Th1 and CTL cells responses compared with the group implanted with G-MDSCs transfected with RNA control ().
Administration of siNFIA into tumor tissue delays tumor progression and promotes antitumor T-cell responses
In order to investigate whether NFIA could impair the antitumor immune responses directly, we firstly prepared C57BL/6 mice implanted with 0.9 × 106 LLC cells. After palpable tumors were formed, tumor-bearing mice were treated by intratumoral injection of NFIA siRNA every 3 d for 2 weeks. At the third week, G-MDSCs in tumor tissue were isolated and NFIA expression along with Arg1 activity and ROS level in G-MDSCs was measured. As shown in , along with the proportion of CD11b+Ly6G+ cells declined, NFIA expression was dropped. At the same time, Arg1 activity and ROS production were also reduced in G-MDSCs sorted from tumor tissue after NFIA knockdown.
Besides that, tumor volume and weight as well as survival rate were detected. As shown in , NFIA knockdown could delay tumor progression and enhance the survival. To confirm the malignant degree of tumor, Ki67 antigen which is associated with carcinoma cells proliferation was detected. We found Ki67 expression was declined in tumor tissue after NFIA knockdown (). We also found NFIA knockdown was capable of reinforcing the CD4+IFNγ+ Th1 priming in spleens, draining lymph nodes and tumor tissue. However, NFIA knockdown enhanced CD8+IFNγ+ CTL priming only in tumor tissue (). The results suggest that NFIA knockdown enhances the antitumor T-cell responses.
High level of NFIA in human lung cancer tissue
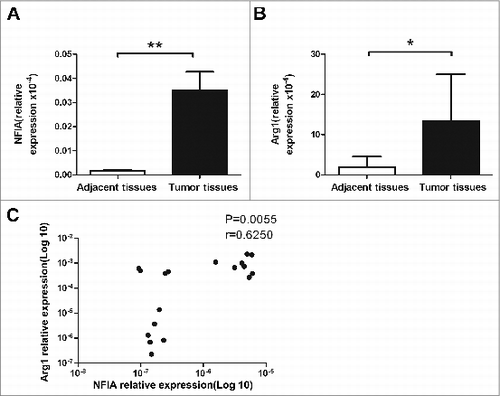
Finally, to define whether NFIA expression has clinical outcome in human tumor biology, we detected the NFIA expression in tumor and adjacent tissues from lung cancer patients. We found that NFIA and Arg1 expression significantly increased in tumor tissue compared with adjacent tissue derived from lung cancer patients (). Moreover, NFIA expression was positively correlated with the expression of Arg1 in tumor tissue of lung cancer patients (). The data suggest that NFIA expression is associated with MDSC-derived factor in human lung cancer.
Figure 6. High level of NFIA in human lung cancer tissue. NFIA expression (A) and Arg1 expression (B) of tumor and adjacent tissues from lung cancer patients were detected by qRT-PCR. (C) Correlation of the expression of NFIA and Arg1 in tumor tissue from lung cancer patients. **p < 0.01, *p < 0.05.
Discussion
β-glucans have been defined as the immunomodulator for a long time.Citation25 β-glucans belong to PAMPs which can bind to their receptors such as Dectin-1 and CR3 and effectively induce immune responses.Citation26,27 β-glucans involved in antitumor immune responses mainly depend on their capacity of regulating the release of cytokines, nitric oxide and arachidonic acid.Citation28 It has been demonstrated that β-glucans, acting as immnunomodulators, can promote antitumor effect and inhibit metastasis.Citation29 During antitumor immune responses, β-glucans can activate macrophages and neutrophil complement receptor 3 (CR3) to kill iC3b-opsonized tumor cells.Citation30 Furthermore, it has been recently reported β-glucans enhance Ag-specific T cells and B cells responses by promoting the maturation and antigen presentation of DCs.Citation12,16-18,31 Thus, β-glucans play a key role in the innate and adaptive immune responses. We have indicated that WGP can regulate the differentiation and immunosuppression of MDSCs as this NF-κB dependent regulation is through Dectin-1/Syk pathway.Citation19 However, there are still many problems unsolved about the mechanism that WGP mediates MDSCs. Here, we firstly demonstrate that NFIA plays a critical role during WGP mediating the suppressive function of G-MDSCs and involves in Dectin-1 pathway. In this study, we observed that either NFIA mRNA or protein expression in G-MDSCs was significantly inhibited by WGP through Dectin-1 pathway.
As another important receptor that β-glucans can recognize and bind to, CR3 composed with CD11b/CD18 is an integrin dimer which is widely expressed by monocytes, DCs, macrophages, natural killer (NK) cells and neutrophils. CR3 acts as the main receptor which is responsible for phagocytosis of complement-opsonized particles through binding complement component iC3b. β-glucans recognize the lectin domain on the αM chain of CR3 and enhance the innate immune reponses. However, the consociation of CR3 and Dectin-1 is indispensable for neutrophil responses to β-glucans when macrophage recognition by β-glucans only relys on the Dectin-1. During this procedure, β-glucans recognize Dectin-1 and facilitate Vav1,3 activation which results in enabling CR3 binding and internalization of β-glucans by neutrophils. Moreover, soluble β-glucans binding to human neutrophils depend on iC3b-mediated β-glucans opsonization which can accelerate CR3 binding. All these imply the importance of CR3 involved in the β-glucans recognition.Citation12,14 Whether its cooperation with Dectin-1 may participate in the process WGP regulates the immune suppressive function of G-MDSCs will be defined in our following work.
After β-glucans recognize and bind to Dectin-1 on the surface of myeloid cells such as DCs, monocytes/macrophages and neutrophils, Syk and Raf-1 kinase signaling pathways are activated resulting in the phosphorylation of MAP kinase, AP-1 and NF-κB which finally cause a variety of cellular responses.Citation19,32 When trying to determine the specific mechanism that WGP-mediated NFIA expression in G-MDSCs, we found c-jun molecule which is a component of AP-1 could cooperate with NFIA. Besides that, NFIA is also identified as a molecular target of mutually antagonistic TGF-β and TNF-α regulation while TGF-β and TNF-α mediate NFIA expression in NIH3T3 cells is probably via c-jun molecule.Citation33,34 Interestingly, NFIA mRNA and protein levels in G-MDSCs were increased after the inhibition of the phosphorylation of c-jun even treated with WGP. These results indicate the attenuated expression of NFIA in G-MDSCs induced by WGP is c-jun molecule dependent, and NFIA may be associated with the procedure that WGP mediates the function of G-MDSCs. Additionally, NFIA siRNA was used to downregulate the NFIA expression in G-MDSCs, and the immunosuppression of G-MDSCs was significantly declined after NFIA knockdown. Thus, NFIA knockdown can inhibit the suppressive function of G-MDSCs in vitro.
Next, we investigated the relationship between NFIA in G-MDSCs and the antitumor immune responses in vivo. The cells mixed with G-MDSCs transfected with NFIA siRNA and LLCs were implanted to C57BL/6 mice, the tumor progression and both CD4+IFNγ+ and CD8+IFNγ+ T cells proportions in spleens, draining lymph nodes and tumor tissues were detected. NFIA knockdown declines the capability of G-MDSCs to accelerate the tumor progression and inhibit the antitumor immune responses. Then, to confirm whether NFIA could directly influence the antitumor immune responses, NFIA siRNA was intratumorally injected into tumor tissue and we found NFIA knockdown could delay the progression of tumor and enhance the priming of CD4+IFNγ+ T cells in spleens, draining lymph nodes and tumor tissue. However, the percentage of CD8+IFNγ+ T cells only increased in tumor tissue. Thus, NFIA knockdown in G-MDSCs can delay the tumor progression and enhance the antitumor T cell responses. Furthermore, NFIA expression significantly increased and was associated with the level of Arg1 which is the main effector molecular of G-MDSCs in tumor tissue derived from lung cancer patients. In summary, it appears that NFIA knockdown in G-MDSCs can enhance the antitumor immune responses, which may be a modulator of immune suppression with potential therapeutic target for G-MDSCs in lung cancer.
Materials and Methods
Cell line, mice and tumor models
The LLC cells were obtained according to American Type Culture Collection. Specific pathogen-free male C57BL/6 mice which were 6–8 weeks old were purchased from Yangzhou University. Mice were implanted s.c. with LLC (0.9–3 × 106/mouse) to construct the tumor models. All experiments were approved by the Institutional Committee on the Use of Animals for Research and Teaching.
Whole β-glucan particles (WGPs)
WGP (Biothera, Eagan, M N) purified from the cell wall of S. cerevisiae via a strand of alkaline and acid extractions to generate hollow yeast cell wall ghosts is constituted mainly by β −1,3/1,6-glucan. The constituents of WGP contain β-glucan (>85%), protein (<3.5%), mannan (< 0.01%), and moisture (<8%) as described previously.Citation19 WGP was kindly provided by Dr Jun Yan from the University of Louisville School of Medicine.
Isolation of G-MDSCs
Murine G-MDSCs from the spleen and tumor tissue of tumor-bearing mice were isolated using a mouse MDSC isolation kit (Miltenyi Biotec, Auburn, CA) following the manufacturer's protocol.
Reverse transcript PCR and quantitative real-time PCR
The RNA isolation, reverse transcript PCR and quantitative real-time PCR (qRT-PCR) were performed as described previously.Citation19 The sequences for the primers used are: NFIA, 5′-TGGCCAAGTTACGGAAAGAT-3′ (forward), 5′-GCGCTCGCCATCAGT
ACT-3′ (reverse); Arg1, 5′-ATGGAAGAGACCTTCAGCTAC-3′ (forward), 5′-GCTG
TCTTCCCAAGAGTTGGG-3′ (reverse); p47, 5′-GCCCAAAGATGGCAAGAATAA
CG-3′ (forward), 5′-CTCGTCGGGACTGTCAAGGG-3′(reverse); gp91, 5′-GAACG
CTACAGAAGAAGCCAACA-3′ (forward),5′-TGGTCATCCCACTCGTGAAAAG
-3′ (reverse); Ki67, 5′-CAAGGAAGTGTTGGTGGACA-3′ (forward), 5′-GCAAAGC
CCTGGTTCTCAC-3′ (reverse); β-actin, 5′-TGGAATCCTGTGGCATCCATGAAAC
-3′ (forward), 5′-TAAAACGCAGCTCAGTAACAGTCCG-3′ (reverse); 18s, 5′-TCC
GGAGAGGGAGCCTGAGA-3′ (forward), 5′-GCACCAGACTTGCCCTCCAA-3′ (reverse). Relative quantification of mRNA expression was calculated by the comparative threshold cycle (Ct) method.
Protein gel blot analysis
Proteins extracted from cells were prepared as described previously.Citation19 Protein was resolved by 12% SDS-PAGE and transferred onto immobilon polyvinylidene fluoride (PVDF) membranes (BioRad, Hercules, CA). Membranes were blocked with 5% skimmed milk before probed overnight at 4°C with specific rabbit anti-NFIA antibody (Abcam, Cambridge, UK), rabbit anti-phospho-c-jun antibody (CST, Danvers, MA), rabbit anti c-jun antibody (CST), rabbit anti-histone 3 antibody (CST) and with secondary HRP-conjugated goat anti-rabbit antibody (CST) followed by chemiluminescent detection (Champion Chemical, Whittier, CA).
Transfection
G-MDSCs were planted into 24-well plates and then transfected with 100 nM NFIA siRNA or its negative control (Ribobio Co., Guangzhou, China) following the manufacturer's protocol.
Detection of ROS levels and arginase activity
ROS produced by G-MDSCs was measured using the oxidation-sensitive dye 2, 7-dichlorofluorescin diacetate (Invitrogen, Carlsbad, CA). G-MDSCs were harvested and cultured with PMA (30 ng/mL) and oxidation-sensitive dye 2,7-dichlorofluorescin diacetate (2.5 nM) for 0.5 h. After that, cells were detected by flow cytometry.
Arginase activity was detected with the QuantiChrom Argianse Assay kit (BioAssay systems, Hayward, CA). The arginase activity was computed following the manufacturer's instructions.
Flow cytometry
Cells were stimulated with PMA (Sigma-Aldrich, St. Louis, MO, 50 ng/mL), ionomycin (eBioscience, San Diego, CA, 1 μg/mL), monensin (eBioscience, 2 μg/mL) for 5 h. After that, cells were stained with anti-CD3 and anti-CD8 mAbs (eBioscience), fixed, permeabilized and stained with anti-IFNγ mAb (eBioscience) according to the Intracellular Staining Kit (Invitrogen, Carlsbad, CA) instructions. Cells obtained from tumor tissues were stained with anti-CD11b and anti-Ly6G mAbs (BioLegend).
G-MDSCs suppression assay in vitro
G-MDSCs isolated were transfected with NFIA siRNA or negative control. After that, different ratios of G-MDSCs were cocultured with CD4+ T cells sorted from wild-type C57BL/6 mice spleens using CD4 microbeads (MiltenyiBiotec, Bergisch Gladbach, Germany) in U-bottomed 96-well plates (Costar, Corning, NY) in the presence of 10 μg/mL anti-CD3 mAb and 5 μg/mL anti-CD28 mAb (Biolegend, SanDiego, CA) for 72 h and pulsed with 3H-thymidine (Pharmacia, Stockholm, Sweden, 1 μCi/well) for the last 16 h of culture.
In vivo experiments
G-MDSCs isolated from spleens of tumor-bearing C57BL/6 mice were transfected with NFIA siRNA. Then, 3 × 106 G-MDSCs transfected with siNFIA mixed with 0.9 × 106 LLC cells, and these mixed cells were implanted into C57BL/6 mice. Tumor volume was calculated using the formula V=1/2a2b with “ b” as the larger diameter and “a” as the smaller diameter in succession. The weight of tumor was measured when the mice were sacrificed. Tumor tissues obtained were cut into small pieces (1–2 mm3) and digested with collagenase at 37°C for 2 h on a rotating platform to get the single-cell suspension.
To investigate whether NFIA could directly impair antitumor immune responses, C57BL/6 mice were implanted with 0.9 × 106 LLC cells. After palpable tumors were formed, tumor-bearing mice were treated by intratumoral injection of 15 nmol NFIA siRNA every 3 d for 2 weeks as described previously.Citation35 Tumor volume and weight were measured, and the single-cell suspension was collected for analysis.
Patients and tissue samples
Lung cancer tissue samples and adjacent tissue samples were obtained from 18 lung cancer patients in Affiliated People's Hospital of Jiangsu University. All samples were collected with the informed consent obtained from each patient and approved by the institutional review board of the hospital.
Graphing and statistical analysis of data
Data are presented as Mean ± SD. Data from all experiments were entered into GraphPad Prism 5.0 (GraphPad, San Diego, CA) to generate bar graphs or graphs of tumor regression. The statistical significance of differences between groups was determined by the Student's t-test. For analyzing survival of mice in different groups, long-rank test was used with the p values indicated. Correlations between variables were determined by Spearman's correlation coefficient. Differences were considered significant at a p level less than 0.05.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Supplemental Material
Supplemental data for this article can be accessed on the publisher's website.
1038687_Supplemental_data.pdf
Download PDF (236.9 KB)Funding
This work was supported by the National Natural Science Foundation of China (Grant No. 31170849, 31470881), Specialized Project for Clinical Medicine of Jiangsu Province (Grant No. BL2014065), Specialized Research Fund for the Doctoral Program of Higher School (Grant No. 20133227110008), Health Department Foundation of Jiangsu Province (Grant No.Z201312), Science and Technology Support Program (Social Development) of Zhenjiang (Grant No. SH2014039), Medical Science and Technology Foundation of Jiangsu University (Grant No. JLY20140004), Jiangsu Province “333” Project, Priority Academic Program Development of Jiangsu Higher Education Institutions.
References
- Watanabe S, Deguchi K, Zheng R, Tamai H, Wang LX, Cohen PA, Shu S. Tumor-induced CD11b+Gr-1+ myeloid cells suppress T cell sensitization in tumor-draining lymph nodes. J Immunol, 2008; 181(5): 3291-3300; PMID:18714001; http://dx.doi.org/10.4049/jimmunol.181.5.3291
- Lee JM, Kim EK, Seo H, Jeon I, Chae MJ, Park YJ, Song B, Kim YS, Kim YJ, Ko HJ et al. Serum amyloid A3 exacerbates cancer by enhancing the suppressive capacity of myeloid-derived suppressor cells via TLR2-dependent STAT3 activation. Eur J Immunol, 2014; 44(6):1672-1684; PMID:24659444; http://dx.doi.org/10.1002/eji.201343867
- Ostrand-Rosenberg S, Sinha P. Myeloid-derived suppressor cells: linking inflammation and cancer. J Immunol, 2009; 182(8):449-4506; PMID:19342621; http://dx.doi.org/10.4049/jimmunol.0802740
- Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol, 2009; 9(3):162-174; PMID:19197294; http://dx.doi.org/10.1038/nri2506
- Li L, Zhang J, Diao W, Wang D, Wei Y, Zhang CY, Zen K. MicroRNA-155 and MicroRNA-21 promote the expansion of functional myeloid-derived suppressor cells. J Immunol, 2014; 192(3):1034-1043; PMID:24391219; http://dx.doi.org/10.4049/jimmunol.1301309
- Movahedi K, Guilliams M, Van den Bossche J, Van den Bergh R, Gysemans C, Beschin A, De Baetselier P, Van Ginderachter JA. Identification of discrete tumor-induced myeloid-derived suppressor cell subpopulations with distinct T cell-suppressive activity. Blood, 2008; 111(8):4233-4244; PMID:18272812; http://dx.doi.org/10.1182/blood-2007-07-099226
- Youn JI, Collazo M, Shalova IN, Biswas SK, Gabrilovich DI. Characterization of the nature of granulocytic myeloid-derived suppressor cells in tumor-bearing mice. J Leukoc Biol, 2012; 91(1):167-181; PMID:21954284; http://dx.doi.org/10.1189/jlb.0311177
- Ko JS, Zea AH, Rini BI, Ireland JL, Elson P, Cohen P, Golshayan A, Rayman PA, Wood L, Garcia J et al. Sunitinib mediates reversal of myeloid-derived suppressor cell accumulation in renal cell carcinoma patients. Clin Cancer Res, 2009; 15(6):2148-2157; PMID:19276286; http://dx.doi.org/10.1158/1078-0432.CCR-08-1332
- Qi C, Cai Y, Gunn L, Ding C, Li B, Kloecker G, Qian K, Vasilakos J, Saijo S, Iwakura Y et al. Differential pathways regulating innate and adaptive antitumor immune responses by particulate and soluble yeast-derived β-glucans. Blood, 2011; 117(25):6825-6836; PMID:21531981; http://dx.doi.org/10.1182/blood-2011-02-339812
- Chihara G, Hamuro J, Maeda Y, Arai Y, Fukuoka F. Fractionation and purification of the polysaccharides with marked antitumor activity, especially lentinan, from Lentinus edodes (Berk.) Sing. (an edible mushroom). Cancer Res, 1970; 30(11):2776-2781; PMID:5530561.
- McIntosh M, Stone BA, Stanisich VA. Curdlan and other bacterial (1->3)-β-D-glucans. Appl Microbiol Biotechnol 2005; 68(2):163-173; PMID:15818477; http://dx.doi.org/10.1007/s00253-005-1959-5
- Barreto-Bergter E, Figueiredo RT. Fungal glycans and the innate immune recognition. Front Cell Infect Microbiol 2014; 4: 145; PMID:25353009; http://dx.doi.org/10.3389/fcimb.2014.00145
- Lowman DW, Greene RR, Bearden DW, Kruppa MD, Pottier M, Monteiro MA, Soldatov DV, Ensley HE, Cheng SC, Netea MG et al. Novel structural features in Candida albicans hyphal glucan provide a basis for differential innate immune recognition of hyphae versus yeast. J Biol Chem, 2014; 289(6):3432-3443; PMID:24344127; http://dx.doi.org/10.1074/jbc.M113.529131
- Schwartz B, Hadar Y. Possible mechanisms of action of mushroom-derived glucans on inflammatory bowel disease and associated cancer. Ann Transl Med, 2014; 2(2):19; PMID:25332995; http://dx.doi.org/10.3978/j.issn.2305-5839.2014.01.03
- Brown GD, Gordon S. Immune recognition. A new receptor for β-glucans. Nature, 2001; 413(6851):36-37; PMID:11544516; http://dx.doi.org/10.1038/35092620
- Harnack U, Eckert K, Fichtner I, Pecher G. Oral administration of a soluble 1-3, 1-6 β-glucan during prophylactic survivin peptide vaccination diminishes growth of a B cell lymphoma in mice. Int Immunopharmacol, 2009; 9(11):1298-1303; PMID:19664725; http://dx.doi.org/10.1016/j.intimp.2009.07.013
- Chen Y, Dong L, Weng D, Liu F, Song L, Li C, Tang W, Chen J. 1,3-β-glucan affects the balance of Th1/Th2 cytokines by promoting secretion of anti-inflammatory cytokines in vitro. Mol Med Rep, 2013; 8(2):708-712; PMID:23799616; http://dx.doi.org/10.3892/mmr.2013.1553
- Li B, Cai Y, Qi C, Hansen R, Ding C, Mitchell TC, Yan J. Orally administered particulate β-glucan modulates tumor-capturing dendritic cells and improves antitumor T-cell responses in cancer. Clin Cancer Res, 2010; 16(21):5153-5164; PMID:20855461; http://dx.doi.org/10.1158/1078-0432.CCR-10-0820
- Tian J, Ma J, Ma K, Guo H, Baidoo SE, Zhang Y, Yan J, Lu L, Xu H, Wang S. β-Glucan enhances antitumor immune responses by regulating differentiation and function of monocytic myeloid-derived suppressor cells. Eur J Immunol, 2013; 43(5):1220-1230; PMID:23424024; http://dx.doi.org/10.1002/eji.201242841
- Gronostajski RM. Roles of the NFI/CTF gene family in transcription and development. Gene, 2000; 249(1-2):31-45; PMID:10831836; http://dx.doi.org/10.1016/S0378-1119(00)00140-2
- Starnes LM, Sorrentino A, Pelosi E, Ballarino M, Morsilli O, Biffoni M, Santoro S, Felli N, Castelli G, De Marchis ML. NFI-A directs the fate of hematopoietic progenitors to the erythroid or granulocytic lineage and controls β-globin and G-CSF receptor expression. Blood, 2009; 114(9):1753-1763; PMID:19542302; http://dx.doi.org/10.1182/blood-2008-12-196196
- Glasgow SM, Laug D, Brawley VS, Zhang Z, Corder A, Yin Z, Wong ST, Li XN, Foster AE, Ahmed N. The miR-223/nuclear factor I-A axis regulates glial precursor proliferation and tumorigenesis in the CNS. J Neurosci, 2013; 33(33):13560-13568; PMID:23946414; http://dx.doi.org/10.1523/JNEUROSCI.0321-13.2013
- Talmadge JE, Hood KC, Zobel LC, Shafer LR, Coles M, Toth B. Chemoprevention by cyclooxygenase-2 inhibition reduces immature myeloid suppressor cell expansion. Int Immunopharmacol, 2007; 7(2):140-151; PMID:17178380; http://dx.doi.org/10.1016/j.intimp.2006.09.021
- Serafini P, Meckel K, Kelso M, Noonan K, Califano J, Koch W, Dolcetti L, Bronte V, Borrello I. Phosphodiesterase-5 inhibition augments endogenous antitumor immunity by reducing myeloid-derived suppressor cell function. J Exp Med, 2006; 203(12):2691-2702; PMID:17101732; http://dx.doi.org/10.1084/jem.20061104
- Goodridge HS, Wolf AJ, Underhill DM. Beta-glucan recognition by the innate immune system. Immunol Rev, 2009; 230(1):38-50; PMID:19594628; http://dx.doi.org/10.1111/j.1600-065X.2009.00793.x
- Leentjens J, Quintin J, Gerretsen J, Kox M, Pickkers P, Netea MG. The effects of orally administered Beta-glucan on innate immune responses in humans, a randomized open-label intervention pilot-study. PLoS One, 2014; 9(9):e108794; PMID:25268806; http://dx.doi.org/10.1371/journal.pone.0108794
- Karumuthil-Melethil S, Gudi R, Johnson BM, Perez N, Vasu C. Fungal β-glucan, a Dectin-1 ligand, promotes protection from type 1 diabetes by inducing regulatory innate immune response. J Immunol, 2014; 193(7):3308-3321; PMID:25143443; http://dx.doi.org/10.4049/jimmunol.1400186
- Lavi I, Nimri L, Levinson D, Peri I, Hadar Y, Schwartz B. Glucans from the edible mushroom Pleurotus pulmonarius inhibit colitis-associated colon carcinogenesis in mice. J Gastroenterol, 2012; 47(5):504-518; PMID:22187166; http://dx.doi.org/10.1007/s00535-011-0514-7
- Nosal'ova V, Bobek P, Cerna S, Galbavý S, Stvrtina S. Effects of pleuran (β-glucan isolated from Pleurotus ostreatus) on experimental colitis in rats. Physiol Res, 2001; 50(6):575-581; PMID:11829318.
- Liu J, Gunn L, Hansen R, Yan J. Combined yeast-derived β-glucan with anti-tumor monoclonal antibody for cancer immunotherapy. Exp Mol Pathol, 2009; 86(3):208-214; PMID:19454271; http://dx.doi.org/10.1016/j.yexmp.2009.01.006
- Leibundgut-Landmann S, Osorio F, Brown GD, Reis e Sousa C. Stimulation of dendritic cells via the dectin-1/Syk pathway allows priming of cytotoxic T-cell responses. Blood, 2008; 112(13):4971-4980; PMID:18818389; http://dx.doi.org/10.1182/blood-2008-05-158469
- Galan-Diez M, Arana DM, Serrano-Gomez D, Kremer L, Casasnovas JM, Ortega M, Cuesta-Domínguez A, Corbí AL, Pla J, Fernández-Ruiz E. Candida albicans β-glucan exposure is controlled by the fungal CEK1-mediated mitogen-activated protein kinase pathway that modulates immune responses triggered through dectin-1. Infect Immun, 2010; 78(4):1426-1436; PMID:20100861; http://dx.doi.org/10.1128/IAI.00989-09
- Waki H, Nakamura M, Yamauchi T, Wakabayashi K, Yu J, Hirose-Yotsuya L, Take K, Sun W, Iwabu M, Okada-Iwabu M. Global mapping of cell type-specific open chromatin by FAIRE-seq reveals the regulatory role of the NFI family in adipocyte differentiation. PLoS Genet, 2011; 7(10):e1002311; PMID:22028663; http://dx.doi.org/10.1371/journal.pgen.1002311
- Alevizopoulos A, Mermod N. Antagonistic regulation of a proline-rich transcription factor by transforming growth factor β and tumor necrosis factor alpha. J Biol Chem, 1996; 271(47):29672-29681; PMID:8939900; http://dx.doi.org/10.1074/jbc.271.47.29672
- Hou J, Zhou Y, Zheng Y, Fan J, Zhou W, Ng IO, Sun H, Qin L, Qiu S, Lee JM et al. Hepatic RIG-I predicts survival and interferon- α therapeutic response in hepatocellular carcinoma. Cancer Cell, 2014; 25(1):49-63; PMID:24360797; http://dx.doi.org/10.1016/j.ccr.2013.11.011
