Abstract
Fusobacterium nucleatum is present in colon cancers where it was shown to generate a proinflammatory microenvironment that supports colorectal neoplasia progression. Remarkably, alongside with proinflammatory stimulation, fusobacteria also inhibit cytotoxicity of immune cells. Thus, it appears as if tumors exploit fusobacteria to generate a favorable proinflammatory and anti-cytotoxic microenvironment.
Viruses were the first infectious agents shown able to induce cancer. Over a century ago, in 1911, Rous demonstrated that sarcoma can be induced in chickens by a virus.Citation1 Fifty-five years later (1966) the association of viruses with human cancer was reported by Epstein, Achong and Barr who used electron microscopy to demonstrate the existence of Epstein-Barr Virus in Burkitt lymphoma cells.Citation2 Following studies linked hepatitis B and C viruses with liver cancer, papillomavirus with cervical cancer and herpesviruses with Kaposi sarcoma. Currently about 20% of cancer incidence are linked to infectious agents.Citation3
In contrast to the rising understanding of the oncogenic potential of viruses, no substantial evidence linking bacteria with cancer progression were available until the end of the twentieth century. In fact, bacteria were used as agents for anticancer treatment. In the early 1890s, a surgeon named William Coley pioneered the use of bacteria (mainly Streptococcus pyogenes) and their extracts to evoke antitumor immunity and successfully treat cancer patients. Later, radiotherapy, chemotherapy and immunotherapy became the main strategies for cancer treatment.
The realization that Helicobacter pylori is a causative agent of gastric cancers in the 1990s indicated, for the first time, that bacteria is involved in tumor promotion. Indeed, it was shown recently that mice that are genetically susceptible to colorectal cancer (CRC) develop significantly fewer tumors under germ-free conditions than when they have a conventional microbiota.Citation4 Among the potential bacterial cancer drivers are Escherichia coli strains that generate a mutagenic toxin called colibactin that can induce single-strand DNA breaks; and fragilysin expressing Bacteroides fragilis that is both genotoxic and capable of cleavage of the tumor suppressor protein E-cadherin.Citation4 A recent and surprising bacterial species added to cancer associated ones is F. nucleatum.
F. nucleatum is a common oral non-spore forming gram-negative anaerobe. It is also involved in extraoral infections including preterm births. Recent genomics and transcriptomics evidence demonstrated that F. nucleatum is also prevalent in colorectal carcinoma.Citation4
Tumors are monitored and controlled by the immune system. Natural Killer (NK) cells are large granular lymphocytes that comprise ∼10% of peripheral blood lymphocytes. They are best known for their ability to kill transformed and virally infected cells and for secreting cytokines, specifically TNF-α and IFNγ. NK cell activity is regulated through a balance of signals derived from inhibitory and activating receptors. Direct recognition of bacteria by NK cells was also demonstrated and interestingly bacterial pathogens have adapted and evolved mechanisms to escape or manipulate the NK cell recognition. For example, NK cells control urinary pathogenic E. coli (UPEC) in the urinary tract. However, UPEC was found to escape this control by type1 fimbriae mediated attachment to NK cells followed with a rapid killing of the NK cells via the hemolysinA toxin.Citation5 Whether adherent-invasive E. coli kills NK cells similar to uropathogenic E. coli in order to promote colitis-associated CRC is yet to be determined.
In a mouse model of periodontal disease NK cells were shown to specifically recognize an unknown proteinous ligand on F. nucleatum via their killer receptor NCR1 (NKp46 in humans). This recognition is followed by TNF-α secretion from the NK cells and results in the aggravation of periodontal disease in mice challenged with fusobacteria.Citation6 Since NK cells play a major role in tumor control and fusobacteria are abundant in colorectal adenocarcinoma, it was rational to test the effect of fusobacteria on the ability of NK cells to kill tumors.
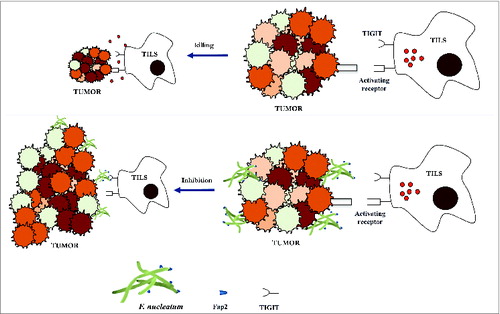
Surprisingly, killing of various tumors by NK cells was inhibited by the presence of various F. nucleatum strains. Inhibition of tumor-killing by fusobacteria was found to be mediated by human, but not by mouse TIGIT, an inhibitory receptor present on all human NK cells and on various T cells. Using a library of F. nucleatum transposon-inserted mutants, the Fap2 lectin of F. nucleatum previously associated with lymphocyte apoptosis by fusobacteria,Citation7 was found to directly interact with TIGIT. This TIGIT activation by fusobacteria leads to the inhibition of NK cell cytotoxicity. TIGIT inhibition was dependent on the hemagglutination ability of the bacterium; strains able to cause hemagglutination via Fap2 activate TIGIT, whereas strains unable to cause hemagglutination do not activate TIGIT. TIGIT is expressed on a variety of T cells including tumor-infiltrating lymphocytes (TILs). The TIGIT expressing T cells were also inhibited by F. nucleatum via Fap2 ().Citation8 Thus, a bacterium-dependent, tumor immune evasion mechanism in which tumors exploit the Fap2 protein of F. nucleatum to inhibit immune cell activity via TIGIT was revealed.
Figure 1. Fusobacterium nucleatum inhibits immune cell activity through the interaction with TIGIT. Tumor-infiltrating lymphocytes (TILs, including NK cells) are recruited to tumors, activated and commence in tumor regression. This immune response results in tumor regression (top). The Fap2 lectin expressed by fusobacteria that attach to tumors, activate TIGIT suppressive ability. TIGIT activation by fusobacteria leads to the inhibition of the TIL's cytotoxicity thus enabling tumor growth (bottom panel).
Prospective
Whether fusobacteria initiate CRC or colonize preexisting tumors remains to be determined. So far, evidence suggests that fusobacteria colonize adenomas or pre-existing tumors rather than driving tumor initiation.Citation8-10 It will be interesting to investigate whether other tumor promoting bacteria interact with TIGIT. It also remains to be determined whether blocking the ability of fusobacteria to activate TIGIT will improve tumor control by the host's immunity. Finally, since F. nucleatum efficiently penetrates colon cancer, perhaps it can be armed with an anticancer drug and used as a novel modality for anti-CRC treatment.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- Rous P. A sarcoma of the fowl transmissible by an agent separable from the tumor cells. J Exp Med 1911; 13:397-411; PMID:19867421; http://dx.doi.org/10.1084/jem.13.4.397
- Epstein MA, Achong BG, Barr YM. Virus particles in cultured lymphoblasts from Burkitt's lymphoma. Lancet 1964; 1:702-703; PMID:14107961; http://dx.doi.org/10.1016/S0140-6736(64)91524-7
- zur Hausen H. The search for infectious causes of human cancers: where and why (Nobel lecture). Angew Chem Int Ed Engl 2009; 48:5798-5808; PMID:19588476; http://dx.doi.org/10.1002/anie.200901917
- Tjalsma H, Boleij A, Marchesi JR, Dutilh BE. A bacterial driver-passenger model for colorectal cancer: beyond the usual suspects. Nat Rev Microbiol 2012; 10:575-582; PMID:22728587; http://dx.doi.org/10.1038/nrmicro2819
- Gur C, Coppenhagen-Glazer S, Rosenberg S, Yamin R, Enk J, Glasner A, Bar-On Y, Fleissig O, Naor R, Abed J et al. Natural killer cell-mediated host defense against uropathogenic E. coli is counteracted by bacterial hemolysinA-dependent killing of NK cells. Cell Host Microbe 2013; 14:664-674; PMID:24331464; http://dx.doi.org/10.1016/j.chom.2013.11.004
- Chaushu S, Wilensky A, Gur C, Shapira L, Elboim M, Halftek G, Polak D, Achdout H, Bachrach G, Mandelboim O. Direct recognition of Fusobacterium nucleatum by the NK cell natural cytotoxicity receptor NKp46 aggravates periodontal disease. PLoS Pathog 2012; 8:e1002601; PMID:22457623; http://dx.doi.org/10.1371/journal.ppat.1002601
- Kaplan CW, Lux R, Huynh T, Jewett A, Shi W, Haake S.K. Fusobacterium nucleatum apoptosis-inducing outer membrane protein. J Dent Res 2005; 84:700-704; PMID:16040725; http://dx.doi.org/10.1177/154405910508400803
- Gur C, Ibrahim Y, Isaacson B, Yamin R, Abed J, Gamliel M, Enk J, Bar-On Y, Stanietsky-Kaynan N, Coppenhagen-Glazer S et al. Binding of the Fap2 protein of Fusobacterium nucleatum to human inhibitory receptor TIGIT protects tumors from immune cell attack. Immunity 2015; 42:344-355; PMID:25680274; http://dx.doi.org/10.1016/j.immuni.2015.01.010
- Rubinstein MR, Wang X, Liu W, Hao Y, Cai G, Han YW. Fusobacterium nucleatum promotes colorectal carcinogenesis by modulating E-cadherin/β-catenin signaling via its FadA adhesin. Cell Host & Microbe 2013; 14:195-206; PMID:23954158; http://dx.doi.org/10.1016/j.chom.2013.07.012
- Kostic AD, Chun E, Robertson L, Glickman JN, Gallini CA, Michaud M, Clancy TE, Chung DC, Lochhead P, Hold GL et al. Fusobacterium nucleatum potentiates intestinal tumorigenesis and modulates the tumor-immune microenvironment. Cell Host & Microbe 2013; 14:207-215; PMID:23954159; http://dx.doi.org/10.1016/j.chom.2013.07.007
