Abstract
Tumor-infiltrating T and B lymphocytes could have the potential to affect cancer prognosis. The objective of this study was to investigate the prognostic significance of tumor infiltration by CD8 and CD4 T cells, and B lymphocytes in patients with localized gastric cancer. In a retrospective cohort of 82 patients with localized gastric cancer and treated by surgery we quantitatively assessed by immunohistochemistry on surgical specimen, immune infiltrates of IL-17+, CD8+, Foxp3+, Tbet+ T cells and CD20+ B cells both in the tumor core and at the invasive margin via immunohistochemical analyses of surgical specimens. We observed that CD8+ and IL17+ T-cell densities were not significantly associated with gastric cancer prognosis. In contrast, high infiltration of Tbet+ T cells, high numbers of CD20+ B-cell follicles, and low infiltration of Foxp3+ T cells, were associated with better relapse-free survival. Interestingly, treatment with neoadjuvant chemotherapy or histological tumor type (diffuse versus intestinal) did not influence type and density of immune infiltrates or their prognostic value. Immunohistochemical analysis of the gastric cancer stromal microenvironment revealed organized T and B cell aggregates, with strong structural analogies to normal secondary lymphoid organs and which could be considered as tertiary lymphoid structures. Using transcriptomic data from an independent cohort of 365 localized gastric cancer, we confirmed that a coordinated Th1, and B cell stromal gene signature is associated with better outcome. Altogether, these data suggest that tumor infiltration by B and Th1 T cells could affect gastric cancer prognosis and may be used to better define the outcome of patients with localized gastric cancer.
Abbreviations
| APC | = | antigen presenting cell |
| DC | = | dendritic cell |
| NK | = | natural killer |
| OS | = | overall survival |
| RFS | = | relapse-free survival |
| Th1 | = | T helper type 1 |
| TLS | = | tertiary lymphoid structures |
| Treg | = | regulatory T cell. |
Introduction
Despite the steep reduction in gastric cancer incidence over the past 50 years, it remains the world's third leading cause of cancer mortality reported in 2012 to be only preceded by lung and liver cancers and estimated to be responsible for 723,000 deaths worldwide annually.Citation1 More than 70% of gastric cancers occur in developing countries,Citation2 and the vast majority of them are adenocarcinomas, which can be further subdivided into intestinal and diffuse types (this latter being associated with poorer prognosis).Citation3 Even diagnosed at a localized stage, the prognosis of gastric cancer remains poor,Citation4 such that the identification of new clinical, biological and molecular features affecting outcome is an important consideration in clinical trial design and evaluation of new treatments for gastric cancer.
It is now well recognized in most cancer types that immunosurveillance can control tumor progression.Citation5 This has been illustrated by the observations that immune infiltrates enriched in T helper type 1 (Th1) and CD8 effector T cells are associated with better clinical outcomes in different cancer types. A particularity of gastric cancer is the Helicobacter pylori-induced chronic gastritis, which constitutes a major risk factor of gastric cancer.Citation6 Due to the related persistent inflammatory response and expression of tumor-associated antigens, as well as antigens produced by the H. pylori infection itself, gastric cancer can induce adaptive immune responses conducive to either restraining or enhancing tumor growth.
In the present work, we analyzed the prognostic effect of T and B lymphocyte tumor infiltration in patients with localized gastric cancer. Interestingly, this study reveals that coordinated Th1 and B cell tumor infiltration in gastric cancer is associated with clinical outcome, and thus could be used to better define the prognosis of gastric cancer patients.
Results
Patient characteristics
Among the 82 patients analyzed in this study, 42 patients received neoadjuvant chemotherapy, whereas 40 patients did not. The two groups of patients did not significantly differ in terms of sex ratio, age, tumor size, local lymph node invasion, or histological type of tumor (Table S1). Furthermore, no significant difference in relapse-free survival (RFS) or overall survival (OS) was observed between these 2 groups of patients (not shown), despite a statistical trend in favor of neoadjuvant chemotherapy. We surmise that this observation could be accounted for by the lack of power in our retrospective study and/or insufficient follow-up.
Relationship between immune infiltrates and histological tumor type or neoadjuvant chemotherapy
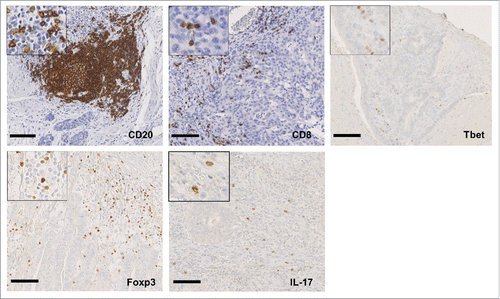
Gastric tumor infiltration by CD8+, Foxp3+, IL-17+ or T-bet+ immune cells were assessed using immunohistochemistry to analyze patient samples derived from the tumor core and from the tumor invasive margin. In addition, the presence of CD20+ lymphoid aggregates surrounding the tumor was also determined (representative stainings are depicted in ). The histological type (intestinal vs diffuse) is the most important prognostic factor for localized gastric cancer. We thus decided to investigate if immune infiltrates were comparable in these different tumor types. Surprisingly, we did not detect any difference between diffuse and intestinal tumors in the number of immune cells that infiltrate these distinct gastric neoplasms, suggesting comparable immune responses in both histological subtypes (Table S2).
Figure 1. Immune infiltrates in human gastric cancer. Representative immunohistochemical stainings of CD20+, CD8+, Tbet+, Foxp3+ and IL-17+ lymphocytes infiltrating gastric cancer. Scale bar indicates 100 µm.
In our cohort, half of the patients received neoadjuvant chemotherapy. Thus, we comparatively analyzed the treated vs untreated patients in order to determine if such preoperative treatment quantitatively or qualitatively affects aspects of gastric cancer immune infiltrates on surgical specimens. Here again, there was no significant difference in tumor immune cell infiltration regardless of the type of treatment (i.e., surgery alone or preceded by chemotherapy; Table S3).
Association between immune infiltrates and patients survival
Univariate Cox analysis indicated that neither IL-17+, nor CD8+ cell infiltrates in tumor were associated with an increased risk of relapse (i.e., RFS). By contrast, both a high number of CD20+ cell aggregates and a high degree of Tbet+ cell infiltration into the tumor stroma were associated with improved RFS. (P = 0.03; HR: 0.48 [95%CI: 0.24–0.9]; and P = 0.03; HR: 0.48 [95%CI: 0.2–0.98], respectively) ( and ). Considering that the presence of tumor-associated CD20+ cells aggregates and Tbet+ cells positively influenced the outcome of gastric cancer patients, we further stratified the patients into groups according to the high or low density of each marker (CD20Hi/TbetHi vs CD20Hi/TbetLo or CD20Lo/TbetHi vs CD20Lo/TbetLo). We observed that the patients with CD20Hi and TbetHi tumor infiltration had the lowest risk of relapse, whereas patients with low density of both CD20+ B cell aggregates, and Tbet expressing tumor-infiltrating cells were at the highest risk of relapse (). Altogether, these results suggest the prognostic importance of a coordinated Th1 and B cell response in gastric cancer. By contrast, high infiltration of Foxp3+ cells into the tumor stroma was associated with reduced RFS (P = 0.04; HR: 2 [95%CI: 1–4]) ( and ). Multivariate Cox regression modeling showed that only the presence of CD20+ cell peritumoral aggregates was independently associated with improved RFS (P = 0.04; HR: 0.44; 95% CI: 0.2–1) ().
Figure 2. Correlation between immune infiltrates and gastric cancer patient survival. Kaplan–Meier curves for recurrence-free survival (RFS) stratified according to (A) the number of CD20+ B cell aggregates (< or > to the median value), (B) the number of intratumoral Tbet+ cells, (C) the number of intratumoral Foxp3+ cells, and (D) according to both CD20+ B cell aggregates and T-bet+ cells. P-values were calculated using the log-rank test.
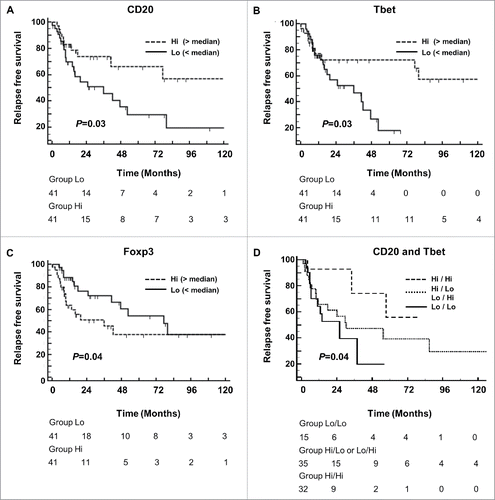
Table 1. Univariate and multivariate analyses of relapse-free survival according to clinical prognostic factors and immune infiltrates
Interestingly, in our population of localized gastric cancer, infiltrations of CD8+, Tbet+, Foxp3+ or IL-17+ immune cells at the invasive margin were not associated with changes in RFS. Moreover, our study found no correlations between the densities of these immune populations and the presence or absence of Helicobacter Pylori infection (not shown).
Presence of tertiary lymphoid structures in localized gastric cancer
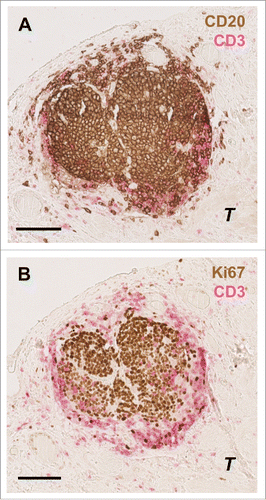
In order to better characterize these B cell peritumoral aggregates and their interactions with other immune cells, particularly T cells, we performed additional double stainings on the same gastric cancer samples. In so doing, we found that similar to normal lymph nodes, peritumoral B cell structures were always surrounded by CD3+ T cells (). Moreover, a significant correlation between the density of tertiary lymphoid structure (TLS) B cell aggregates and Tbet+ cells was found (R = 0.35, P = 0.0015). Akin to such structures in normal lymph nodes, these CD3+ T cells areas were selectively infiltrated by mature Lamp+ dendritic cells (DCs) (). Of note, in our series of gastric cancer patients, the density of Lamp+ DCs was not associated with improved survival (logrank test P = 0.12, data not shown). In gastric cancer tumor samples, DC-Lamp+ dendritic cells clusters were found exclusively in areas enriched in CD3+ T cells surrounding B cell follicles. B cell follicles were characterized by the presence of centrally located Ki67+ proliferating B cells (), such as occurs in normal germinal centers. Moreover, these T/B peritumoral lymphoid structures were surrounded by peripheral node addressin (PNAd+) high endothelial venules (HEVs) () the expression of which allows the extravasation of lymphocytes from the blood to the tissue such as in normal lymph nodes.
Figure 3. Characterization of T and B cells interaction in the gastric cancer microenvironment. Double immunohistochemical staining of gastric cancer patient tumor samples. Similar to normal lymph node (A) B cells aggregates (CD20: brown) are surrounded by T cells (CD3: pink) also in gastric cancer (B). DC-Lamp+ mature dendritic cells (DC-Lamp: red) were exclusively found in T cell zones (CD3: blue) both in normal lymph nodes (C) and in gastric cancer (D). In gastric cancer, these so-called tertiary lymphoid structures (TLS) are surrounded by PNAd+ high endothelial venules (HEVs: red arrows) (PNAd: brown; CD20: blue) (F), comparable to normal secondary lymphoid organs (E). Scale bar indicates 100 µm. T indicates tumor cells.
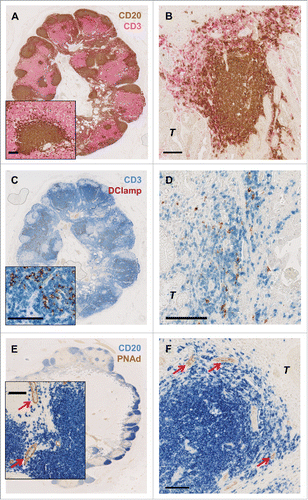
Figure 4. Characterization of T and B cells interaction in germinal centers in the gastric cancer microenvironment. Consecutive slides showing the same B cell zone (A) surrounded by T cells (CD20: brown; CD3: pink). (B) Some of these tumor-associated CD20+ B-cell follicles contained Ki67+ (Ki67: brown) proliferating germinal center B cells. Scale bar indicates 100 µm. T indicates tumor cells.
Collectively, these findings indicate that gastric cancer could be sustained by a complex network of tumor-infiltrating immune cells organized in TLS, exhibiting a structural organization similar to secondary lymphoid organs, allowing B/T cells coordination.
External validation using transcriptomic data
To confirm our observation of the prognostic impact of B- and T-cell responses in gastric cancer, we used an independent public large scale transcriptomic data set available on through GEO (GSE26253). As our initial population only included localized gastric cancer patients, we analyzed Stage I-III gastric cancers, corresponding to 365 patients (whose clinicopathologic characteristics are given in Table S4). Among the 24526 genes available on the chip, we selected 63 genes in accordance with their expression in immune cells of interest, including CD8 T lymphocytes, B cells, regulatory T cells (Tregs) and various T helper cells, specifically Th1, Th2, Th17 and follicular helper T cells, (Table S5). Based on the expression of these genes, a correlation matrix was constructed (, presented in higher magnification in Fig. S1). Using ClueGO software to analyze the non-redundant biological terms for large clusters of genes in a functionally grouped network, we identified that a highly correlated gene cluster of 19 genes (cluster 1: Table S6) enriched in Th1 and B cell related genes (). We thus named this cluster of correlated genes the Th1/B metagene signature. We dichotomized the 365 patients into 2 groups according to low or high expression of this metagene, and analyzed the RFS in these 2 groups. Patients harboring a high expression of Th1/B cell correlated genes had a better RFS than patients with a low expression (P = 0.0415; HR: 0.6999 [95%CI: 0.4902–0.9857] (). In contrast, isolated high expression of Th1-related genes, or B cell-related genes alone did not correlate with survival (), thus suggesting that only a coordinated B and Th1 immune response is associated with better survival.
Figure 5. Gastric cancer patient transcriptomic data analysis. Independent large-scale transcriptomic data set available on through GEO (GSE26253), analyzing Stage I-III gastric cancer patients. (A) A correlation matrix of gene expression in gastric cancer was constructed and submitted to unsupervised hierarchical clustering using complete linkage. Each dot represents a correlation coefficient represented by the color of the dot (green for negative correlation, red for positive correlation). Correlation matrix identified a cluster of 19 highly correlated genes enriched in T helper type 1 (Th1-) and B cell-specific genes (red rectangle). (B) The ClueGO analysis confirmed that this highly correlated cluster is enriched in pathways regulating T and B cells. (C) Kaplan–Meier curves for recurrence-free survival (RFS) stratified according to the present of a high or low expression of the Th1/B cell enriched cluster. (D) Kaplan–Meier curves for RFS stratified according to the present of a high or low expression of the Th1-related gene or B cell-related gene signatures. (E) P-values were calculated by using the log-rank test.
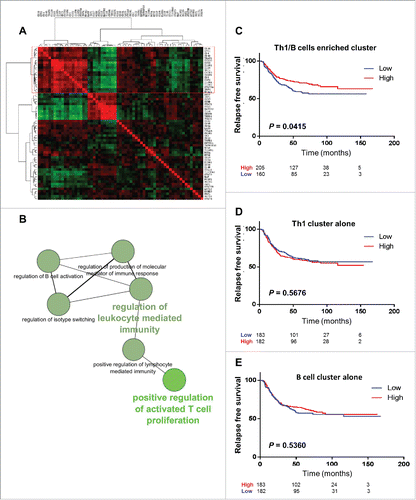
Of note, in the second cluster of genes (), 11 genes were grouped (cluster 2: Table S6). Four genes (CCL5, CEBPB, TMED1, STAT6) appeared to be specific for Th2 lymphocytes, 3 genes (IRF1, HAVCR2, IL18) were found to specify Th1 lymphocytes and 1 (STAT1) was shared by follicular T helper (Tfh) and Th1 cells. The last 3 genes indicated Th17 (BATF), Treg (CCR7) and CD8 (GZMB) cells. This metagene signature was not associated with survival in our validation cohort of gastric cancer patients.
Discussion
This study highlights the importance of the host immune response in the prognosis of localized gastric cancer, as well as the potential interactions between constituent immune cell populations. In our patient cohort, we found that high levels of tumor infiltration with Tbet+ lymphocytes, the presence of peritumoral CD20+ aggregates, and a low degree of Foxp3+ cell tumor stroma infiltration were associated with better RFS. These observations suggest that tumor infiltration by B and Th1 cells may contribute to the control of gastric cancer progression. These findings are corroborated by our transcriptomic analysis, which supports the fact that high expression of a Th1 and B cell stromal metagene signature is associated with better outcome, despite a modest statistical significance.
There is increasing evidence that the development of Th1 adaptive immunity is associated with improved outcome in patients afflicted with a variety of cancer types.Citation7-11 T-bet (TBX21) is a Tbox transcription factor known to be crucial for the development of effector Th1 cells,Citation12 and the most specific marker for this T-cell subset. In patients with colorectal carcinoma, the presence of mRNA encoding molecules expressed by Th1 cells (such as T-bet) has been shown to correlate with reduced metastatic invasion and increased survival.Citation7 In gastric cancer, a recent work conduced on 152 surgical specimens of gastrectomy found a more favorable outcome for patients with gastric tumors highly infiltrated with Tbet+ cells.Citation13 Tbet expression is not restricted to Th1 CD4+ T cells, but is also an essential transcription factor required for the differentiation and functions of CD8+ T cells, γδ T cells and natural killer (NK) cells.Citation14 Interestingly, flow cytometry analyses on cells derived from fresh gastric cancer patient tumor tissue demonstrated that CD4+ (T helper), as well as CD8+ (cytotoxic T) and CD56+ (NK) lymphocytes express Tbet in the gastric cancer microenvironment,Citation13 suggesting that the prognostic value of Tbet+ cells in gastric cancer relies on the contribution of several types of effector lymphoid cells.
In our study, we observed that CD20+ peritumoral lymphoid structures were also associated with better RFS upon univariate and multivariate analysis. We demonstrated here that these structures could be considered as TLS located at the tumor vicinity due to their structural organization, reminiscent of secondary lymphoid organs. Tertiary lymphoid structures were previously described in human lung cancer patients and associated with lung cancer prognosis.Citation15-17 As in our study, these structures present features of an ongoing immune reaction site and were characterized by: (i) ectopic and organized lymphoid aggregates containing anatomically distinct yet adjacent T and B cell enriched areas; (ii) the presence of high endothelial venules occurring within or surrounding the lymphoid aggregate that allow the extravasation of CD62L+ immune cells along with proliferating B cells; (iii) and finally by the presence of mature DCs within the T-cell zones. High density TLS-associated mature DCs has been previously described to be associated with improved outcome in cancer patients.Citation16 However, in our study, no survival correlation was found despite a statistical trend in favor of better outcome (P = 0.12). This question has been previously explored by Ishigami et al.Citation18 in a larger series of localized gastric cancer (n = 128), also with insignificant results.
CD20+ B cells contribute both to humoral immunity as antibody-producing cells, but also to cellular immunity by serving as antigen-presenting cells (APCs) and/or by providing costimulatory signals to T cells.Citation19,20 A favorable effect of tumor-infiltrating B cells on patient prognosis was previously reported in cutaneous melanoma,Citation21 breast cancerCitation22 and ovarian cancer,Citation23 but the mechanism linking the accumulation of these cells in the tumor microenvironment and better prognosis remains poorly understood. Nielsen et al. found that tumor-infiltrating B cells expressed markers of antigen presentation and could have a function of professional APCs to support the T cell-mediated immune anticancer response.Citation23 Another report underlines the capacity of B cells found in TLS to produce antibodies that specifically recognize tumor antigens.Citation24
In our series, we hypothesize that the anticancer effect of T-bet+ T cells and CD20+ B cells could be balanced by the immunosuppressive action of Foxp3+ Tregs since a high intratumoral density of these particular immune cells are significantly associated with reduced RFS. In most varieties of human cancer, a high density of tumor-infiltrating Tregs is associated with poorer prognosis, such as in ovarianCitation25,26 and breast cancer.Citation27 However, a paradoxical association of improved clinical prognosis and a high density of FoxP3+ tumor-infiltrating Tregs is usually observed in colorectal cancer patients. A possible explanation for this observation lies in the dense microbiological flora present in the large intestine with a tendency to translocate through the tumor. This microbiological hazard requires a T-cell mediated inflammatory anti-microbial response that involves inflammatory cells and inflammatory-driven molecular pathways in cancer cells, thereby promoting cancer growth.Citation28 Even if gastric cancer is frequently associated with Helicobacter pylori-induced gastritis, a high density of Foxp3+ cells is usually associated with a poor prognosis, as found in our present study and predominating the literature.Citation29-32 Only Haas et al. found a relationship between high levels of Foxp3+ cell tumor infiltration and improved prognosis in 52 patients bearing gastric adenocarcinomas of the cardia.Citation33
In the present work, we did not find a significant correlation between gastric cancer patient survival and the density of CD8+ or IL-17+ tumor-infiltrating cells. This seems at first in contradiction with data obtained using a larger series of 220 gastric cancer patients in which a high density of tumor-infiltrating lymphocytes (especially CD8+ cells) was found to be independently predictive both of regional lymph node metastasis and patient survival.Citation34 However, this study was conducted using tumor microarrays, and whether this approach gives a valid picture of both the tumor and tumor stroma landscape remains under discussion. This is one of the reasons we chose to perform our analyses on an entire slide of tumor sample for each patient. Of note, if tumor-infiltrating CD8+ T cell density is usually described as a favorable prognostic marker in the vast majority of cancers, some reports also indicate a reciprocal (unfavorable) prognostic value for CD8+ infiltration.Citation35 In any case, our results in gastric cancer concerning the absence of a favorable effect should be confirmed with a larger patient series.
Th17 cells, CD4+ T cells producing IL-17, have been poorly studied in gastric cancer. While Th17 cells have been reported to be heavily represented among the CD4+ T cell population in the gastric cancer stroma,Citation36 their exact function in tumor immunity remains controversial,Citation37 and, their effect on cancer patient survival has not been clearly demonstrated. Of note, a subpopulation of CD8+ T cells producing IL-17 has been recently isolated from gastric cancer patient's samples, and the level of these Tc17 (IL-17 expressing cytotoxic T) cells increased with tumor progression and was associated with reduced overall survival.Citation38 This later point clearly illustrates the complex phenotype of CD8+ and CD4+ T cells in cancer, especially in gastric cancer.
In this particular malignant landscape, our study suggests that gastric cancer, regardless of its histological type, can elicit spontaneous antitumor adaptive immune responses, especially the generation of peritumoral B lymphoid structures and Th1 T-cell infiltration, which can play an important role in patient prognosis.
Materials and Methods
Patients
We retrospectively studied formalin-fixed and paraffin-embedded (FFPE) cancer surgical specimens from 82 consecutive gastric cancer patients, who underwent gastric surgical resection between January 1993 and December 2013 in our institution (Center Gorges François Leclerc, Dijon, France). Before surgery, 42 of these patients received neoadjuvant 5-fluorouracil and cisplatin-based chemotherapy (physician's choice and/or evolution of clinical practice over the time). For each patient, the tumor stage was assigned according to the International Union against Cancer staging system (AJCC/UICC). Patients were excluded from this study if distant metastatic lesions (excluding local lymph node invasion) were discovered during surgical procedure.
Patients' clinical and tumor pathological characteristics are shown in Table S1. There was no significant difference between patients that did or did not receive neoadjuvant chemotherapy. The median follow-up at the time of analysis was 27 months. The study was approved by the local ethics committee and all patients gave written informed consent at the time of the diagnosis for the use of tumor samples for research purposes.
Immunohistochemistry
Antibodies used are listed in Table S7. Paraffin-embedded gastric tumors, and normal lymph nodes (used as control), were sectioned for immunohistochemistry: Briefly, serial 4-μm tissue sections were deparaffinized, rehydrated, and pretreated in 10 mmol/L of citrate buffer pH6 for antigen retrieval. Sections were incubated with 5% human serum before adding primary antibodies followed by secondary antibodies. Alkaline phosphatase and peroxidase activities were revealed using their respective substrates (Vector Laboratories Inc., Burlingame, CA), whereas the binding of biotinylated antibodies was revealed by streptavidin-peroxidase, or streptavidin-alkaline phosphatase. Tissue slides were counterstained (in case of single staining) with hematoxylin, or not (in case of double staining), and then mounted in Aquamount (Dako). Negative controls were established by adding nonspecific isotype controls as primary antibodies.
The presence of Helicobacter Pylori was assessed in each case by May-Grundwald Giemsa staining.
Quantification of lymphocyte infiltrates
The presence or absence of lymphocyte infiltration and their quantification in different areas on each tumor sample were evaluated by 2 independent physicians (FG and AH) over the entire tissue section. All samples were previously anonymized and blinded for clinicopathologic data. For CD8, T-bet, Foxp3 and IL-17 labeling, the number of positively stained cells was counted in 3 consecutive high power fields (40×) both in the tumor core and at the invasive margin. The mean count of 3 fields was used for statistical analysis. The results of the analyses conducted by each independent physician were subsequently compared. Major discrepancies between the 2 observers were reviewed jointly to reach a consensus when the mean count differed by more than 10%. For CD20+ stained cells, we counted the number of CD20+ lymphoid aggregates in the whole tumor area.
Statistical analyses
Qualitative variables were described as frequencies and percentages. The association of variables was evaluated with the χ2 test. The Wilcoxon-Mann-Whitney test was used to compare non-continuous variables in paired samples as appropriate. Relapse-free survival (RFS) was calculated from the date of diagnosis until the date of metastatic relapse (local or metastatic) or death, whichever came first, or the last follow-up. Alive or dead patients without relapse were censored at the last follow-up. Follow-up was calculated using the reverse Kaplan–Meier method. RFS probabilities were estimated using Kaplan–Meier method and were compared by the log-rank test. The hazards ratios (HRs) with 95% confidence interval (CI) were calculated using univariate Cox proportional hazards regression modeling. All variables with a univariate Cox P-value 0.20 were eligible for multivariate analyses. Correlations between co-variables were first tested for eligible variables. Finally, multivariate Cox proportional hazards regression modeling was applied to assess independent prognosis effect for RFS. All reported P-values are 2 sided. The statistical significance level was set at P < 0.05. Analyses were performed using Medcalc Software.
Data sets
We used the GSE26253 datasetCitation39 downloaded from GEO website. This data set contained stage, recurrence events and RFS information from 432 gastric cancer patients. For this work to be in accordance with the immunohistologically studied population, we only studied patients with localized gastric cancer (stages 1 -3 tumors), corresponding to 365 patients similarly to the previous cohort.
Correlation matrix construction
We isolated from the transcriptomic dataset, a combination of genes known to be highly expressed in Th1, Th2, T follicular helpers, Th17, Treg, CD8+ T, and B cells (genes are listed in Table S5) Then, a correlation matrix was constructed by calculation of the correlation coefficient between each gene, followed by unsupervised hierarchical clustering based on Euclidean distance calculation using Gene Cluster 3.0 software. The correlation matrix was visualized with Treeview software.
Metagene immune signature and prognosis
The expression of genes constituting the most highly correlated cluster of the correlation matrix was used for the classification of tumors. This cluster of 19 highly correlated genes (listed in Table S6) was enriched in Th1- and B cell-related genes, and was called the Th1/B metagene signature. Based on these 19 genes, an unsupervised hierarchical clustering of the 365 localized gastric cancer patients was performed. A value corresponding to the mean of the 19 gene coefficients obtained after clustering was calculated for each patient. This value was used for the dichotomization of tumors as low or high Th1/B metagene signature. Then, relapse-free survival (RFS) was defined as the time between the date of diagnosis and the date of relapse or death, whichever came first, or the last follow-up. Survival curves were generated using the Kaplan-Meier method, and the significance of differences between patient groups was obtained by the Mantel-Cox log-rank test. Only tests with P<0.05 were considered significant.
ClueGO analysis
To illustrate the immune pathways enriched in the highly correlated cluster, we performed functional enrichment analysis using ClueGO.Citation40 ClueGO facilitates the visualization of functionally related genes displayed as a clustered network and chart. The statistical test used for the enrichment was based on right-sided hypergeometric option with a Benjamini-Hochberg correction and kappa score of 0.3. Only significant pathways were conserved.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
1054598_supplemental_files.zip
Download Zip (133.2 KB)Supplemental Material
Supplemental data for this article can be accessed on the publisher's website.
References
- Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 2015; 136(5):E359-86; PMID:25220842
- Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin 2011; 61:69-90; PMID:21296855
- Lauren P. The two histological main types of gastric carcinoma: diffuse and so-called intestinal-type carcinoma. An attempt at a histo-clinical classification. Acta Pathol Microbiol Scand 1965; 64:31-49; PMID:14320675
- Bouvier AM, Sant M, Verdecchia A, Forman D, Damhuis R, Willem Coebergh J, Crocetti E, Crosignani P, Gafa L, Launoy G, et al. What reasons lie behind long-term survival differences for gastric cancer within Europe? Eur J Cancer 2010; 46:1086-92; PMID:20163952; http://dx.doi.org/10.1016/j.ejca.2010.01.019
- Vesely MD, Kershaw MH, Schreiber RD, Smyth MJ. Natural innate and adaptive immunity to cancer. Annu Rev Immunol 2011; 29:235-71; PMID:21219185; http://dx.doi.org/10.1146/annurev-immunol-031210-101324
- Polk DB, Peek RM, Jr. Helicobacter pylori: gastric cancer and beyond. Nat Rev Cancer 2010; 10:403-14; PMID:20495574; http://dx.doi.org/10.1038/nrc2857
- Galon J, Costes A, Sanchez-Cabo F, Kirilovsky A, Mlecnik B, Lagorce-Pages C, Tosolini M, Camus M, Berger A, Wind P, et al. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science 2006; 313:1960-4; PMID:17008531; http://dx.doi.org/10.1126/science.1129139
- Zhang L, Conejo-Garcia JR, Katsaros D, Gimotty PA, Massobrio M, Regnani G, Makrigiannakis A, Gray H, Schlienger K, Liebman MN, et al. Intratumoral T cells, recurrence, and survival in epithelial ovarian cancer. N Engl J Med 2003; 348:203-13; PMID:12529460; http://dx.doi.org/10.1056/NEJMoa020177
- Roepman P, Jassem J, Smit EF, Muley T, Niklinski J, van de Velde T, Witteveen AT, Rzyman W, Floore A, Burgers S, et al. An immune response enriched 72-gene prognostic profile for early-stage non-small-cell lung cancer. Clin Cancer Res 2009; 15:284-90; PMID:19118056; http://dx.doi.org/10.1158/1078-0432.CCR-08-1258
- Tartour E, Gey A, Sastre-Garau X, Lombard Surin I, Mosseri V, Fridman WH. Prognostic value of intratumoral interferon gamma messenger RNA expression in invasive cervical carcinomas. J Natl Cancer Inst 1998; 90:287-94; PMID:9486814; http://dx.doi.org/10.1093/jnci/90.4.287
- Marth C, Fiegl H, Zeimet AG, Muller-Holzner E, Deibl M, Doppler W, Daxenbichler G. Interferon-gamma expression is an independent prognostic factor in ovarian cancer. Am J Obstet Gynecol 2004; 191:1598-605; PMID:15547530; http://dx.doi.org/10.1016/j.ajog.2004.05.007
- Mullen AC, High FA, Hutchins AS, Lee HW, Villarino AV, Livingston DM, Kung AL, Cereb N, Yao TP, Yang SY, Reiner SL. Role of T-bet in commitment of TH1 cells before IL-12-dependent selection. Science 2001; 292:1907-10; PMID:11397944; http://dx.doi.org/10.1126/science.1059835
- Chen LJ, Zheng X, Shen YP, Zhu YB, Li Q, Chen J, Xia R, Zhou SM, Wu CP, Zhang XG, Lu BF, Jiang JT. Higher numbers of T-bet(+) intratumoral lymphoid cells correlate with better survival in gastric cancer. Cancer Immunol Immunother 2013; 62:553-61; PMID:23090288; http://dx.doi.org/10.1007/s00262-012-1358-6
- Glimcher LH. Trawling for treasure: tales of T-bet. Nat Immunol 2007; 8:448-50; PMID:17440449; http://dx.doi.org/10.1038/ni0507-448
- Dieu-Nosjean MC, Antoine M, Danel C, Heudes D, Wislez M, Poulot V, Rabbe N, Laurans L, Tartour E, de Chaisemartin L, et al. Long-term survival for patients with non-small-cell lung cancer with intratumoral lymphoid structures. J Clin Oncol 2008; 26:4410-7; PMID:18802153; http://dx.doi.org/10.1200/JCO.2007.15.0284
- Goc J, Germain C, Vo-Bourgais TK, Lupo A, Klein C, Knockaert S, de Chaisemartin L, Ouakrim H, Becht E, Alifano M, et al. Dendritic cells in tumor-associated tertiary lymphoid structures signal a Th1 cytotoxic immune contexture and license the positive prognostic value of infiltrating CD8+ T cells. Cancer Res 2014; 74:705-15; PMID:24366885; http://dx.doi.org/10.1158/0008-5472.CAN-13-1342
- Goc J, Fridman WH, Sautes-Fridman C, Dieu-Nosjean MC. Characteristics of tertiary lymphoid structures in primary cancers. Oncoimmunology 2013; 2:e26836; PMID:24498556; http://dx.doi.org/10.4161/onci.26836
- Ishigami S, Ueno S, Matsumoto M, Okumura H, Arigami T, Uchikado Y, Setoyama T, Arima H, Sasaki K, Kitazono M, et al. Prognostic value of CD208-positive cell infiltration in gastric cancer. Cancer Immunol Immunother 2010; 59:389-95; PMID:19760221; http://dx.doi.org/10.1007/s00262-009-0758-8
- Bouaziz JD, Yanaba K, Venturi GM, Wang Y, Tisch RM, Poe JC, Tedder TF. Therapeutic B cell depletion impairs adaptive and autoreactive CD4+ T cell activation in mice. Proc Natl Acad Sci U S A 2007; 104:20878-83; PMID:18093919; http://dx.doi.org/10.1073/pnas.0709205105
- Crawford A, Macleod M, Schumacher T, Corlett L, Gray D. Primary T cell expansion and differentiation in vivo requires antigen presentation by B cells. J Immunol 2006; 176:3498-506; PMID:16517718; http://dx.doi.org/10.4049/jimmunol.176.6.3498
- Ladanyi A, Kiss J, Mohos A, Somlai B, Liszkay G, Gilde K, Fejos Z, Gaudi I, Dobos J, Timar J. Prognostic impact of B-cell density in cutaneous melanoma. Cancer Immunol Immunother 2011; 60:1729-38; PMID:21779876; http://dx.doi.org/10.1007/s00262-011-1071-x
- Mahmoud SM, Lee AH, Paish EC, Macmillan RD, Ellis IO, Green AR. The prognostic significance of B lymphocytes in invasive carcinoma of the breast. Breast Cancer Res Treat 2012; 132:545-53; PMID:21671016; http://dx.doi.org/10.1007/s10549-011-1620-1
- Nielsen JS, Sahota RA, Milne K, Kost SE, Nesslinger NJ, Watson PH, Nelson BH. CD20+ tumor-infiltrating lymphocytes have an atypical CD27- memory phenotype and together with CD8+ T cells promote favorable prognosis in ovarian cancer. Clin Cancer Res 2012; 18:3281-92; PMID:22553348; http://dx.doi.org/10.1158/1078-0432.CCR-12-0234
- Germain C, Gnjatic S, Tamzalit F, Knockaert S, Remark R, Goc J, Lepelley A, Becht E, Katsahian S, Bizouard G, et al. Presence of B cells in tertiary lymphoid structures is associated with a protective immunity in patients with lung cancer. Am J Respir Crit Care Med 2014; 189:832-44; PMID:24484236; http://dx.doi.org/10.1164/rccm.201309-1611OC
- Curiel TJ, Coukos G, Zou L, Alvarez X, Cheng P, Mottram P, Evdemon-Hogan M, Conejo-Garcia JR, Zhang L, Burow M, et al. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med 2004; 10:942-9; PMID:15322536; http://dx.doi.org/10.1038/nm1093
- Wolf D, Wolf AM, Rumpold H, Fiegl H, Zeimet AG, Muller-Holzner E, Deibl M, Gastl G, Gunsilius E, Marth C. The expression of the regulatory T cell-specific forkhead box transcription factor FoxP3 is associated with poor prognosis in ovarian cancer. Clin Cancer Res 2005; 11:8326-31; PMID:16322292; http://dx.doi.org/10.1158/1078-0432.CCR-05-1244
- Xu L, Xu W, Qiu S, Xiong S. Enrichment of CCR6+Foxp3+ regulatory T cells in the tumor mass correlates with impaired CD8+ T cell function and poor prognosis of breast cancer. Clin Immunol 2010; 135:466-75; PMID:20181533; http://dx.doi.org/10.1016/j.clim.2010.01.014
- Ladoire S, Martin F, Ghiringhelli F. Prognostic role of FOXP3+ regulatory T cells infiltrating human carcinomas: the paradox of colorectal cancer. Cancer Immunol Immunother 2011; 60:909-18; PMID:21644034; http://dx.doi.org/10.1007/s00262-011-1046-y
- Shen Z, Zhou S, Wang Y, Li RL, Zhong C, Liang C, Sun Y. Higher intratumoral infiltrated Foxp3+ Treg numbers and Foxp3+/CD8+ ratio are associated with adverse prognosis in resectable gastric cancer. J Cancer Res Clin Oncol 2010; 136:1585-95; PMID:20221835; http://dx.doi.org/10.1007/s00432-010-0816-9
- Lu X, Liu J, Li H, Li W, Wang X, Ma J, Tong Q, Wu K, Wang G. Conversion of intratumoral regulatory T cells by human gastric cancer cells is dependent on transforming growth factor-beta1. J Surg Oncol 2011; 104:571-7; PMID:21695703; http://dx.doi.org/10.1002/jso.22005
- Ishigami S, Arigami T, Uenosono Y, Matsumoto M, Okumura H, Uchikado Y, Kita Y, Nishizono Y, Maemura K, Kijima Y, et al. Cancerous HLA class I expression and regulatory T cell infiltration in gastric cancer. Cancer Immunol Immunothera 2012; 61:1663-9; PMID:22374482; http://dx.doi.org/10.1007/s00262-012-1225-5
- Li Q, Li Q, Chen J, Liu Y, Zhao X, Tan B, Ai J, Zhang Z, Song J, Shan B. Prevalence of Th17 and Treg cells in gastric cancer patients and its correlation with clinical parameters. Oncol Rep 2013; 30:1215-22; PMID:23807713
- Haas M, Dimmler A, Hohenberger W, Grabenbauer GG, Niedobitek G, Distel LV. Stromal regulatory T-cells are associated with a favourable prognosis in gastric cancer of the cardia. BMC Gastroenterol 2009; 9:65; PMID:19732435; http://dx.doi.org/10.1186/1471-230X-9-65
- Lee HE, Chae SW, Lee YJ, Kim MA, Lee HS, Lee BL, Kim WH. Prognostic implications of type and density of tumour-infiltrating lymphocytes in gastric cancer. Br J Cancer 2008; 99:1704-11; PMID:18941457; http://dx.doi.org/10.1038/sj.bjc.6604738
- Giraldo NA, Becht E, Remark R, Damotte D, Sautes-Fridman C, Fridman WH. The immune contexture of primary and metastatic human tumours. Curr Opin Immunol 2014; 27:8-15; PMID:24487185; http://dx.doi.org/10.1016/j.coi.2014.01.001
- Yamada Y, Saito H, Ikeguchi M. Prevalence and clinical relevance of Th17 cells in patients with gastric cancer. J Surg Res 2012; 178:685-91; PMID:22940035; http://dx.doi.org/10.1016/j.jss.2012.07.055
- Martin F, Apetoh L, Ghiringhelli F. Controversies on the role of Th17 in cancer: a TGF-beta-dependent immunosuppressive activity? Trends Mol Med 2012; 18:742-9; PMID:23083809; http://dx.doi.org/10.1016/j.molmed.2012.09.007
- Zhuang Y, Peng LS, Zhao YL, Shi Y, Mao XH, Chen W, Pang KC, Liu XF, Liu T, Zhang JY, et al. CD8(+) T cells that produce interleukin-17 regulate myeloid-derived suppressor cells and are associated with survival time of patients with gastric cancer. Gastroenterology 2012; 143:951-62.e8; PMID:22710190; http://dx.doi.org/10.1053/j.gastro.2012.06.010
- Lee J, Sohn I, Do IG, Kim KM, Park SH, Park JO, Park YS, Lim HY, Sohn TS, Bae JM, et al. Nanostring-based multigene assay to predict recurrence for gastric cancer patients after surgery. PLoS One 2014; 9:e90133; PMID:24598828; http://dx.doi.org/10.1371/journal.pone.0090133
- Bindea G, Mlecnik B, Hackl H, Charoentong P, Tosolini M, Kirilovsky A, Fridman WH, Pages F, Trajanoski Z, Galon J. ClueGO: a Cytoscape plug-in to decipher functionally grouped gene ontology and pathway annotation networks. Bioinformatics 2009; 25:1091-3; PMID:19237447; http://dx.doi.org/10.1093/bioinformatics/btp101
