ABSTRACT
Infiltrating myeloid derived suppressor cells and tumor-associated macrophages (TAMs) are important components of the immunosuppressive tumor microenvironment. We recently reported that tasquinimod, which binds to S100A9, impairs both infiltration and function of these cells. Here we discuss the underlying mechanisms responsible for targeting multiple suppressive populations and the modulation of the tumor microenvironment.
Suppressive myeloid cells (SMCs), including myeloid-derived suppressor cells (MDSCs) and TAMs, are associated with tumor progression and metastasis.Citation1 MDSCs expand and accumulate in many cancers,Citation1 whereas TAMs are differentiated from immature monocytes or MDSCs when these precursors infiltrate and adapt to the tumor environment.Citation2 Both MDSCs and TAMs are critical components of the immunosuppressive tumor microenvironment that can directly inhibit activation and function of T and NK cells.Citation3 Therefore, modulating and/or depleting these cells in the tumor environment is expected to help counteracting SMC-associated immunosuppression, tumor progression, and metastasis.
Previous reports have shown different approaches to deplete MDSCs or to affect their expansion.Citation4 While reduction of peripheral MDSCs has been observed, few of these efforts have been able to reduce infiltration of MDSCs in the tumor sites or impair their suppressive function.Citation4 Tasquinimod is an orally active synthetic quinoline-3-carboxamide derivate that has shown effect in a phase II clinical trial in patients with castration resistance prostate cancer (CRPC).Citation5 We have recently reported that tasquinimod reduced tumor-infiltrating MDSCs and M2 polarized macrophages and impaired the suppressive function of these infiltrating myeloid cells in two different experimental cancer models, namely the CR Myc-CaP prostate cancer model and the B16-5T4 melanoma model.Citation6 Combining tasquinimod with two different immunotherapy strategies in these models clearly showed an enhanced effect on tumor growth in both models. Interestingly, tasquinimod did not affect accumulation of MDSCs in the peripheral sites in CR Myc-CaP model, but slightly reduced MDSCs in spleen in the B16-5T4 model, suggesting that modulation of SMCs in tumors is sufficient to facilitate immunotherapies.
The composition and suppressive function of tumor-infiltrating myeloid cells are usually different from those of peripheral SMCs. In the CR Myc-CaP prostate cancer model, Gr1+CD11b+ MDSCs accumulate in peripheral blood with tumor growth. Gr1lowCD11b+ and Gr1− CD11b+ immature monocytes are also present in peripheral blood.Citation6 In CR Myc-CaP tumors, Gr1+CD11b+ MDSCs are one of the major populations, and Ly6G+Ly6C− granulocytic MDSCs are the major subpopulation. However, the majority of tumor-infiltrating Gr1− CD11b+ cells are F4/80+ macrophages (up to 80% of CD11b+ cells), and most of these are M2 polarized TAMs.Citation6 We have compared the suppressive function of peripheral and tumor-infiltrating myeloid cells. The CD11b+ cells isolated from Myc-CaP tumors efficiently inhibited T cell proliferation upon CD3 and CD28 stimulation,Citation6 whereas the CD11b+ cells from spleen did not have inhibitory effect on T cell proliferation upon the same stimulation (data not shown). These observations are consistent with another report showing that tumor and peripheral site MDSCs have different functions.Citation7 Importantly, we observed that tasquinimod treatment not only reduced CD206+Arginase-1high M2 macrophages, but also induced iNOShigh macrophages with M1 polarized phenotype. This indicates that tasquinimod may switch the differentiation of tumor-infiltrating macrophages from M2 polarized immunosuppressive phenotype to M1 phenotype. As compared to infiltrating MDSCs, M2 polarized TAM may represent an even more important component of tumor-promoting, immunosuppressive environment. To confirm our hypothesis, we isolated infiltrating myeloid cells from vehicle or tasquinimod-treated tumors from donor mice, and mixed them with fresh tumor cells to make inoculates in recipient mice. We observed that transferred SMCs from tasquinimod-treated tumor were less supportive of tumor growth.Citation6 The analysis of the composition of tumor immune microenvironment specific to different models or diseases of interest may be critical to develop effective strategies to modulate the environment and facilitate the development of antitumor therapies.
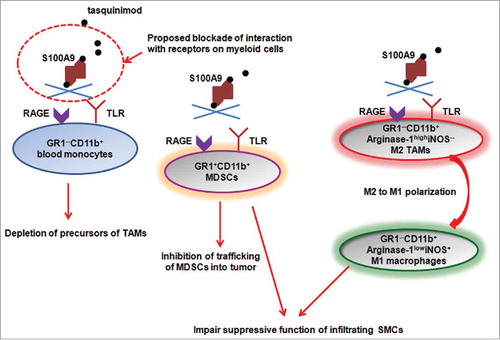
Our study provides clues to the underlying mechanism for myeloid cell modulation by tasquinimod (). S100A9 is a potential target of tasquinimod that may be involved in immunomodulatory activity,Citation8 and S100A9 has been shown to regulate accumulation of MDSCs.Citation4,9 Tasquinimod binds to S100A9,Citation10 and blocks the interaction of this ligand with its receptors, including receptor for advanced glycation end product (RAGE) and Toll-like receptor (TLR)-4 and EMMPRIN. In an additional metastatic model, we observed that tasquinimod was able to dramatically reduce M2 macrophages in renal cell tumors and at the metastatic site (lung; unpublished data). This observation suggests that tasquinimod may act through inhibiting the trafficking of MDSCs, via inhibition of S100A9, into the tumor in our tested models. However, a previous report showed that antibody against RAGE, mAbGB3.1, inhibited the accumulation of MDSCs in peripheral sites, but not in the metastatic tumor site (the primary tumors were resected).Citation4 The discrepancy between these results may originate from the fact that tasquinimod binds and inhibits the ligand, S100A9, whereas mAbGB3.1 blocks one of receptors for the ligand, RAGE. Therefore, the other receptor for S100A9, TLR4 and/or EMMPRIN, may mediate the SMC-targeting activity of tasquinimod. At the same time, other tumor or stroma-secreted inflammatory factors may still induce peripheral MDSC expansion when S100A9 protein ligand is blocked. We have also demonstrated that expression of suppressive function-related genes, Arginase-1 (reduction) and iNOS (induction) was regulated by tasquinimod treatment. It has been reported that TLR-4 pathway is an important regulator of Arginase-1 expression. This finding would suggest that tasquinimod may affect function and polarization of SMCs by inhibiting S100A9–TLR4–Arginase axis. Though tasquinimod did not change accumulation of Gr1+CD11b+ MDSCs in blood, it depleted Gr1−CD11b+ monocytes from peripheral blood. Therefore, tasquinimod may also reduce the CD206+ M2 TAMs through depletion of these Gr1−CD11b+ precursor cells from blood.
Figure 1. Proposed potential mechanisms underlying modulation of tumor microenvironment by tasquinimod. Tasquinimod binds to S100A9 homodimers in a 1:1 fashionCitation10 and blocks its interaction with receptors expressed on multiple myeloid cell populations. The blockade may lead to depletion of Gr1−CD11b+ blood monocytes, which are one of the precursors of TAMs. In addition, the trafficking of Gr1+CD11b+ MDSCs to tumor sites is inhibited. Tasquinimod also induce M2 to M1 polarization of macrophages in the tumors, associated with downregulation of Arginase-1 and induction of iNOS expression in these cells. Thus, tasquinimod may impair the suppressive function of infiltrating SMCs through blockade of S100A9-TLR4-Arginase-1 signaling.
In summary, tasquinimod is an agent that has the potential of overcoming the suppressive tumor environment. Targeting the suppressive tumor microenvironment continues to be an active field of exploration to address management of metastatic cancers as part of immuno-oncology at large.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol 2009; 9:162-74; PMID:19197294; http://dx.doi.org/10.1038/nri2506
- Sica A, Bronte V. Altered macrophage differentiation and immune dysfunction in tumor development. J Clin Invest 2007; 117:1155-66; PMID:17476345; http://dx.doi.org/10.1172/JCI31422
- Solito S, Bronte V, Mandruzzato S. Antigen specificity of immune suppression by myeloid-derived suppressor cells. J Leukoc Biol 2011; PMID:21486906; http://dx.doi.org/10.1189/jlb.0111021
- Sinha P, Okoro C, Foell D, Freeze HH, Ostrand-Rosenberg S, Srikrishna G. Proinflammatory S100 proteins regulate the accumulation of myeloid-derived suppressor cells. J Immunol 2008; 181:4666-75; PMID:18802069; http://dx.doi.org/10.4049/jimmunol.181.7.4666
- Pili R, Haggman M, Stadler WM, Gingrich JR, Assikis VJ, Bjork A, Nordle O, Forsberg G, Carducci MA, Armstrong AJ. Phase II randomized, double-blind, placebo-controlled study of tasquinimod in men with minimally symptomatic metastatic castrate-resistant prostate cancer. J Clin Oncol 2011; 29:4022-8; PMID:21931019; http://dx.doi.org/10.1200/JCO.2011.35.6295
- Shen L, Sundstedt A, Ciesielski M, Miles KM, Celander M, Adelaiye R, Orillion A, Ciamporcero E, Ramakrishnan S, Ellis L et al. Tasquinimod modulates suppressive myeloid cells and enhances cancer immunotherapies in murine models. Cancer Immunol Res 2015; 3:136-48; PMID:25370534; http://dx.doi.org/10.1158/2326-6066.CIR-14-0036
- Corzo CA, Condamine T, Lu L, Cotter MJ, Youn JI, Cheng P, Cho HI, Celis E, Quiceno DG, Padhya T et al. HIF-1alpha regulates function and differentiation of myeloid-derived suppressor cells in the tumor microenvironment. J Exp Med 2010; 207:2439-53; PMID:20876310; http://dx.doi.org/10.1084/jem.20100587
- Bjork P, Bjork A, Vogl T, Stenstrom M, Liberg D, Olsson A, Roth J, Ivars F, Leanderson T. Identification of human S100A9 as a novel target for treatment of autoimmune disease via binding to quinoline-3-carboxamides. PLoS Biol 2009; 7:e97; PMID:19402754; http://dx.doi.org/10.1371/journal.pbio.1000097
- Cheng P, Corzo CA, Luetteke N, Yu B, Nagaraj S, Bui MM, Ortiz M, Nacken W, Sorg C, Vogl T et al. Inhibition of dendritic cell differentiation and accumulation of myeloid-derived suppressor cells in cancer is regulated by S100A9 protein. J Exp Med 2008; 205:2235-49; PMID:18809714; http://dx.doi.org/10.1084/jem.20080132
- Kallberg E, Vogl T, Liberg D, Olsson A, Bjork P, Wikstrom P, Bergh A, Roth J, Ivars F, Leanderson T. S100A9 interaction with TLR4 promotes tumor growth. PLoS One 2012; 7:e34207; PMID:22470535; http://dx.doi.org/10.1371/journal.pone.0034207
