ABSTRACT
Glioblastoma multiforme (GBM) is a highly malignant tumor with a poor outcome that is often positive for human cytomegalovirus (HCMV). GBM patients often have excessive numbers of neutrophils and macrophages near and within the tumor. Here, we characterized the cytokine patterns in the blood of GBM patients with and without Valganciclovir treatment. Furthermore, we determined whether neutrophil activation is related to HCMV status and patient outcome. Blood samples for analyses of cytokines and growth factors were collected from 42 GBM patients at the time of diagnosis (n = 42) and at weeks 12 and 24 after surgery. Blood neutrophils of 28 GBM patients were examined for CD11b expression. The levels of pro- and anti-inflammatory cytokines and chemokines—including interleukin (IL)-1β, IL-2, IL-6, IL-8, IL-10, IL-12p70, IL-17A, transforming growth factor (TGF)-β1, interferon-γ, interferon-α, tumor necrosis factor α, and monocyte chemoattractant protein (MCP)-1were analyzed with a bead-based flow cytometry assay. During the first six months after surgery, neutrophil activity was increased in 12 patients and was unchanged or decreased in 16. Patients with increased neutrophil activity had enhanced IL-12p70, high grade HCMV and a shorter time to tumor progression (TTP) than patients without or decreased neutrophil activity (median TTP; 5.4 vs. 12 months, 95% confidence interval; 1.6–10 vs. 0.1–0.6, hazard ratio = 3 vs. 0.4, p = 0.004). The levels of IL-12p70 were significantly decreased in Valganciclovir treated patients (n = 22, T 12W vs. T 24W, p = 0.03). In conclusion, our findings suggest that neutrophil activation is an early sign of tumor progression in GBM patients.
Abbreviations
| GBM | = | glioblastoma |
| HCMV | = | human cytomegalovirus |
| IFN | = | interferon |
| IL | = | interleukin |
| MCP-1 | = | monocyte chemoattractant protein 1 |
| NK | = | natural killer |
| OS | = | overall survival |
| TGF | = | transforming growth factor |
| TTP | = | time to tumor progression |
| VIGAS | = | Efficacy and Safety of Valcyte as an Add-on Therapy in Patients with Malignant Glioblastoma and Cytomegalovirus Infection |
Introduction
Glioblastomas multiforme (GBM) is the most frequent and malignant brain tumor in adults. In Sweden, GBM is diagnosed in 300–500 people annually. Despite a tremendous increase in knowledge concerning the molecular and biological characteristics of GBM, the prognosis remains dismal.Citation1 The median survival time is 12–15 months and less than 3% of patients survive more than 5 years.Citation2-4 Numerous studies have reported that GBM patients are immunosuppressed, as exemplified by decreased function of natural killer (NK) cells and T cells and high peripheral release of both TGF-β and prostaglandins.Citation5-8 GBMs produce high levels of TGF-β, a potent immunosuppressive cytokine that may inhibit the infiltration of immune cells and prevent an efficient antitumor response.
Despite their immunosuppressive state, patients with GBM often have an increase in circulating neutrophils.Citation9 An increased number of neutrophils in the margins of these tumors has been linked to increased malignancy.Citation9,10 Infiltrated neutrophils can release elastase, which can damage surrounding tissue and may promote tumor invasion.Citation10 In addition, glioma cells have been reported to produce survival factors for neutrophils, such as interleukin (IL)-6 and IL-8, resulting in a delay in their spontaneous apoptosis.Citation11
Increasing evidence suggests that HCMV is linked to several forms of cancer.Citation12,13 An active HCMV infection has been reported in 90–100% of GBM,Citation14-16 medulloblastomas,Citation17 neuroblastomas,Citation18 colon cancers,Citation19 breast cancers,Citation20-23 and prostatic carcinomas.Citation24 HCMV is a common virus that causes life-long latency after a primary infection and is carried by 60–90% of the world's population.Citation25 The role of HCMV in cancer development is unclear and under debate. HCMV is considered to be an onco-modulatory rather than an oncogenic virus. It encodes numerous viral proteins that can dysregulate pivotal cellular functions that are important in oncogenesis, including cellular differentiation, cell cycle control and proliferation, induced oncogene expression, epigenetic functions, induced migration and angiogenesis, DNA repair mechanisms, and inhibition of apoptosis.Citation13,26-30 Through HCMV's unique ability to avoid detection and elimination by the immune system, virus-infected tumor cells may be difficult to eliminate. We and others have reported that 90–100% of GBMs are infected by HCMV, and we found that the level of HCMV infection in these tumors is a prognostic factor for patient survival.Citation15
In a randomized, double-blind study aiming to evaluate the safety and efficacy of antiviral treatment of HCMV in GBM patients (the VIGAS study), we found that the median overall survival (OS) was similar in Valganciclovir treated and placebo groups (17.9 vs. 17.4 months, p = 0.430). Patients could take Valganciclovir for compassionate use after the study phase and explorative analyses showed prolonged survival in patients who were treated with Valganciclovir for more than 6 months (OS 24.1 months).Citation31 Several GBM patients were thereafter prescribed Valganciclovir as add on to standard therapy at our hospital. In a retrospective study of the outcome of these patients, we found that GBM patients who received anti-HCMV treatment in addition to standard therapy had significantly prolonged survival, especially those who had received continuous Valganciclovir treatment (56.4months vs. 13.5 months, P < 0.001).Citation31,32 These observations suggest that HCMV may play an important role in the pathogenesis of GBM and that this virus may serve as a unique target for GBM therapy, which needs to be addressed in a randomized trial.
HCMV infection of neutrophils results in activation of these cells and increased resistance to apoptosis.Citation33 Cytokines such as IL-6, IL-8, interferon-γ, and TNF-α are induced by HCMV infection and may contribute to neutrophil activation and survival.Citation34 In this study, we assessed the activation state of peripheral blood neutrophils in HCMV-positive GBM patients included in the VIGAS trial. We measured cytokine and chemokine levels before and during antitumor treatment and antiviral treatment and searched for correlations between neutrophil activation and cytokine levels as well as to TTP and OS.
Results
Neutrophil activation is associated with shorter TTP. Activation and recruitment of neutrophils to the tumor site have been reported in patients with glioblastoma and may correlate with greater malignancy.Citation8 In addition; the number of circulating neutrophils is often increased in GBM patients.
We found that the number of neutrophils was significantly decreased over time in Valcyte treated patients (T0 vs. T12W; p = 0.01, T0 vs. T24W; p = 0.0004, T12W vs. T24W; p = 0.04). In the placebo cohort, the number of neutrophils was also significantly decreased from T0 to T24W (p = 0.0002). However, the decreased number of neutrophils in the placebo cohort was not statistically significant at T0 vs. T12W (p = 0.16) and T12 vs. T24W (p = 0.14) (Fig. S1). Information regarding neutrophil count was not available from four patients in the placebo cohort.
Previously, we showed that HCMV infection activates neutrophils.Citation32 Here, we characterized the activation status of peripheral neutrophils in 28 of 42 GBM patients in the VIGAS trial as measured by the induced surface expression of CD11b, at T = 0, 3, and 6 months as well as in healthy controls (n = 6). All GBM patients were diagnosed with an HCMV infection in their tumor, as required for inclusion in the VIGAS study. However, as previously reported, 29% (12/42) of them were HCMV IgG negative, as shown by enzyme-linked immunosorbent assay. Nevertheless, blood T cells from 85% of these patients were reactive against HCMV (immediate early and pp65 peptides), and their blood T cells were positive for HCMV DNA.Citation35
Over time, neutrophil activity was enhanced in 12 patients and was unchanged or decreased in 16 patients (Group I; GI with enhanced neutrophil activity: T0 vs. T24W: p < 0.0001 and T12W vs. T24W: p < 0.0001, Group II; GII with unchanged or decreased neutrophil activity: T12W vs. T24W: p = 0.014, ). Patients with enhanced neutrophil activity (Group I) had significantly shorter TTP than patients with unchanged or decreased neutrophil activity (Group II) (median TTP; 5.4 vs. 12 months, 95% confidence interval (CI); 1.6–10 vs. 0.1–0.6, hazard ratio= 3 vs. 0.4, p = 0.004) (). During the first 6 months after the first surgery, 10 (36%) of 28 patients had a tumor recurrence, and 7 (70%) had high neutrophil activity at the time of recurrence. OS tended to be shorter in patients with enhanced neutrophil activity but did not reach statistical significance in this small patient cohort (16.4 vs. 19.5 months, respectively; 95% CI; 0.9–5.6 vs. 0.2–1, hazard ratio = 2 vs. 0.4, p = 0.07) (). All patients received antiviral treatment (Valganciclovir) except for two patients in Group I and three patients in Group II who received placebo treatment. We noted a high grade of HCMV infection (grade 3 and 4) in brain tumor tissues from all patients in Group I but in only in 69% of patients in Group II (, p = 0.047). This finding suggested enhanced neutrophil activity in patients with higher HCMV activity. However, none of the neutrophils examined for HCMV infection expressed pp65, as shown by immunofluorescence staining (data not shown). Due to the small number of patients without antiviral treatment, we could not evaluate the effect of Valganciclovir treatment on neutrophil activity in this cohort.
Figure 1. Neutrophil activity was enhanced in 12 patients and was unchanged or decreased in 16 patients over time (Group I;GI with enhanced neutrophil activity: T0 vs. T24W: p < 0.0001 and T12W vs. T24W: p < 0.0001, Group II; GII with unchanged or decreased neutrophil activity: T12W vs. T24W: p = 0.014, ). Kaplan–Meier survival curves of GBM patients and grade of HCMV infection in their tumors. (B and C) GBM patients with high neutrophil activity (GI) had significantly shorter TTP (A) and shorter median OS (B) than patients with unchanged or decreased neutrophil activity (GII). (D) Significantly more patients in GI had high-grade HCMV infection (Grades 3+, 4+) in their tumor compared with GII (p = 0.047).
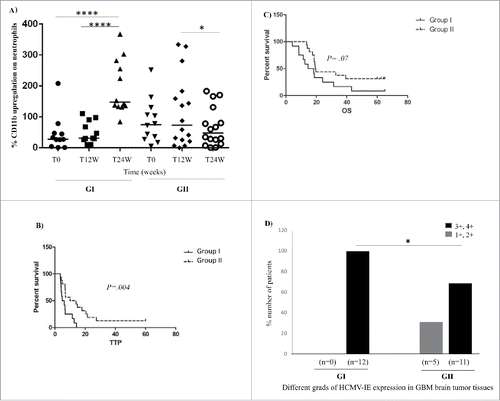
Despite their higher neutrophil activity, GBM patients are considered to suffer from a general immunosuppression characterized by high levels of the strong immunosuppressive cytokine TGF-β, among others. Interestingly, we found that the levels of IL-12p70, which has been shown to activate neutrophils,Citation35 was significantly increased only in Group I patients who had enhanced neutrophil activation (T0 vs. T24W, p = 0.002, ).
Figure 2. Levels of 12 cytokines, chemokines, and inflammatory factors in plasma samples from GBM patients in Group I (GI) and Group II (GII) and healthy controls (HC) (A–F).The levels of IL-12p70 was significantly increased over time (T0 vs. T24W, p = 0.002) only in Group I patients with significantly enhanced neutrophil activation (A). The levels of IL-8 and MCP-1 were significantly elevated overtime in both Group I and Group II compared with healthy controls (HC) (IL-8: GI: T0 vs. HC, p = 0.0009; T12W vs. HC, p = 0.008 and T24W vs. HC, p = 0.0009 and GII: T0 vs. HC, p <0.0001; T12W vs. HC, p = 0.0002 and T24W vs. HC, p = 0.0007) (MCP-1: GI: T0 vs. HC, p = 0.0002; T12W vs. HC, p = 0.0002; T24W vs. HC, p = 0.0002 and GII: To vs. HC, p = 0.0002; T12W vs. HC, p = 0.0002 and T24W vs. HC, p = 0.0005) (B, C). However the level of IL-8 was deceased at 12 weeks (p = 0.02) (D) only in Group II with decreased or unchanged neutrophil activity. The levels of MCP-1 and IL-8 did not differ between GI and GII (B–C). Other examined factors did not differ between the two groups (A–F).
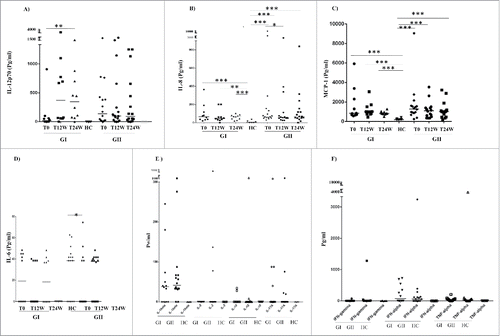
The levels of the neutrophil attractants IL-8 and MCP-1 were significantly elevated over time in patients in both Group I and Group II compared with healthy controls (HC) (IL-8: GI: T0 vs. HC, p = 0.0009; T12W vs. HC, p = 0.008 and T24W vs. HC, p = 0.0009 and GII: T0 vs. HC, p < 0.0001; T12W vs. HC, p = 0.0002 and T24W vs. HC, p = 0.0007) () (MCP-1, GI: T0 vs. HC, p = 0.0002; T12W vs. HC, p = 0.0002; T24W vs. HC, p = 0.0002 and GII: T0 vs. HC, p = 0.0002; T12W vs. HC, p = 0.0002 and T24W vs. HC, p = 0.0005) (). However, the level of IL-6 and IL-8 were deceased at 12 weeks (p = 0.02 and p = 0.04, respectively, ) only in Group II who had decreased or unchanged neutrophil activity (). The levels of MCP-1 did not differ between GI and GII groups ( and Fig. S2). Other examined factors did not differ between the two groups ( and Fig. S2).
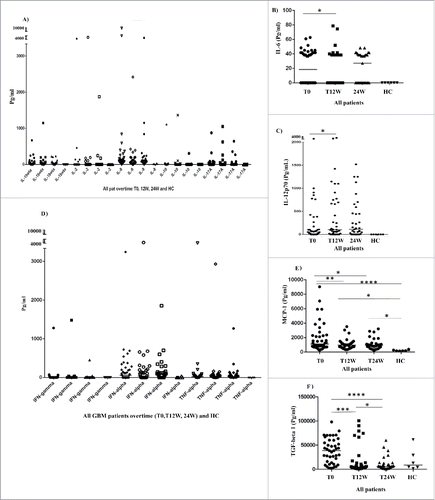
All 42 GBM patients in the VIGAS study were examined for plasma levels of cytokines and growth factors at baseline (T0, n = 42) and after antitumor treatment for 12 weeks (T12, n = 37) and 24 weeks (T24, n = 35). Specifically, we measured 12 pro- or anti-inflammatory cytokines that are vital in regulating the immune response: IL-1β, IL-2, IL-6, IL-8, IL-10, IL-12p70, IL-17A, interferon (IFN)-α, IFNγ, TNF-α, TGF-β, and MCP-1. The median levels of IFNγ IL-2, IL-10, and IL-17A were equal with zero both in GBM patients and HC (). The median levels of IL-1β, IL-6, IL-8, IL-12p70, IFN-α TNF-α, MCP-1, and TGF-β (but not IFNγ, IL-2, IL-10, and IL-17A) were higher at baseline in GBM patients than in healthy controls (). The median levels of all examined cytokines except for IL-8, MCP-1, TGF-β were below detection levels in healthy controls. During the 24 weeks of antitumor treatment, GBM patients had significantly decreased levels of MCP-1 (T0 vs. T12, p = 0.008; T0 vs. T24, p = 0.03), TGF-β (T0 vs. T12, p = 0.0006; T0 vs. T24, p < 0.0001, T12 vs. T24, p = 0.04), and IL-6 (T0 vs. T12, p = 0.04) and increased levels of IL-12p70 (T0 vs. T12, p = 0.04) (). The levels of IL-1β, IL-2, IL-8, IL-10, IL-17A, IFNγ, and TNF-α were unchanged during follow-up ().
Figure 3. Median levels of 12 cytokines/chemokines, and inflammatory factors in plasma of all GBM patients over time and in healthy controls (HC) (3A–F). The median levels of IL-1β, IL-6, IL-8, IL-12p70, IFN-α TNF-α, MCP-1, and TGF-β (but not IFNγ, IL-2, IL-10, and IL-17A) were higher at baseline in GBM patients than in healthy controls (). During the 24 weeks of antitumor treatment, GBM patients had significantly decreased levels of IL-6 (T0 vs. T12, p = 0.04) (B) and increased levels of IL-12p70 (T0 vs. T12, p = 0.04) (C), significantly decreased levels of MCP-1 (T0 vs. T12, p = 0.008; T0 vs. T24, p = 0.03) (E) and TGF-β (T0 vs. T12, p = 0.0006 and T0 vs. T24, p < 0.0001, T12W vs. T24W, p = 0.04) (F).
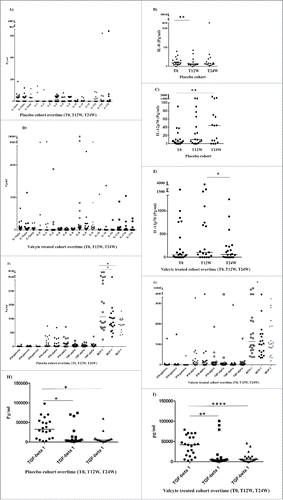
Among the VIGAS patients, 22 patients were randomized to receive antiviral treatment (Valganciclovir) and 20 patients to placebo (in the intention-to-treat protocol) during the 24-week study phase. The cytokine profiles in these two groups did not differ ( and Fig. S3), except for IL-12p70 and IL-8. IL-12p70 levels decreased significantly in Valganciclovir-treated patients (T12 vs. T24, p = 0.03, ) but increased significantly in placebo patients (T0 vs. T24, p = 0.004) (). The level of IL-8 decreased only in the placebo group at 12 weeks (T0 vs. T12, p = 0.008) (). All GBM patients had short term corticosteroid treatment, high dose pre- and directly post-surgery. The postoperative corticosteroid intake was thereafter rapidly decreased to a minimum effective dose to control cerebral edema. Cytokine data collected from placebo and Valganciclovir treated patients at base line, 12 weeks and 24 weeks post-surgery did not demonstrate any major differences over time except for what is described above, suggesting a limited effect by corticosteroid treatment (data not shown).
Figure 4. Median levels of 12 examined pro-inflammatory cytokines and chemokines in the plasma of GBM patients treated with valganciclovir (V) or placebo (P) over time (A–I). The cytokine profiles in these two groups did not differ (), except for IL-12p70 and IL-8. IL-12p70 levels decreased significantly in valganciclovir-treated patients (T12 vs. T24, p = 0.03 (E) but increased significantly in placebo patients (T0 vs. T24, p = 0.004) (C). The level of IL-8 decreased only in the placebo group at 12 weeks (T0 vs. T12, p = 0.008) (B).
Discussion
The results of this study further support the potential role of neutrophils in the pathogenesis of GBM. We found that activation of neutrophils was associated with higher plasma levels of IL12p70 and a shorter TTP; patients with high neutrophil activity had a significantly shorter TTP and also tended to have shorter OS. Of 10 patients who had a tumor recurrence during the first 6 months, 7 (70%) had high neutrophil activity at the time of recurrence. Thus, activation of peripheral neutrophils may be an early sign of tumor progression and may be related to a more aggressive tumor. In either case, activation of neutrophils may be a useful biomarker of disease progression and should be further investigated for potential clinical impact.
Despite the activation of neutrophils, GBM patients often have a sustained general immunosuppression characterized by a decreased function of cytotoxic T cells and NK cells.Citation5,6,8,37-39 These patients can also be lymphopenic, and their peripheral T cells may be reduced in number as a result of their disease and its treatment.Citation40 Tumor cells can also influence the immune system by secreting cytokines, such as TGF-β and IL-10, that inhibit T cells, dendritic cells and NK cells—thereby enhancing the ability of the virus to promote its own survival. In GBM patients, we assessed the levels of 12 pro-inflammatory and immunosuppressive cytokines and chemokines that can modulate the immune response, including factors that activate neutrophils. We found that GBM patients (n = 42) had elevated levels of IL-1β, IL-6, IL-8, IL-12 p70, TNF-α, IFN-α, TGF-β, and MCP-1. The levels of IFNγ, IL-2, IL-10, and IL-17A were similar in GBM patients and healthy controls. Several of these factors are affected by HCMV infection, and most GBMs are positive for HCMV.Citation14-16 Only levels of TGF-β1, MCP-1, and IL-6 were significantly decreased during antitumor treatment, and only IL12p70 during anti-viral treatment.
IL-1, IL-6, IL-8, IL-12p70, and MCP-1 can induce the migration, activation, and respiratory burst of neutrophils.Citation36,41,42 Among the examined cytokines in all GBM patients (n = 42) included in the VIGAS study, only IL-12p70 levels were increased in patients at 12 weeks after surgery. The level of IL-12p70 increased over time in the GI group, which may explain higher neutrophil activity in this group. Neutrophils have been described as an important source of different cytokines including IL-12 p70.Citation43 This cytokine can be released during infections previous studies have reported induction of IL-12 during Herpes Simplex virus infection.Citation43-46 In our study, all patients with high neutrophil activity (n = 12) had high-grade HCMV infection in their GBMs, while 69% of patients without high neutrophil activity had high grade infection.
In theory, higher HCMV activity can directly affect and activate neutrophils,Citation33 or induce neutrophil-activating factors such as IL-12p70. In support of this notion, IL-12p70 levels were lower in Valganciclovir-treated patients than in placebo-treated patients; Valganciclovir-treated patients would be expected to have less HCMV activity and subsequently less inflammation at the tumor site. Secretion of high levels of IL-12p70 by APCs and activated neutrophils may further increase neutrophil activity and migration to the tumor site and thereby promote tumor progression in the absence of an activation of an antitumor immune response. Elevated secretion of IL-12p70 from activated neutrophils may also create an autocrine loop contributing to enhanced neutrophil activation and release of endogenous enzymes such as Elastase in the tissue and further facilitate infiltration of tumor cells leading to tumor progression.Citation47 Interestingly, IL-12p70 levels were higher among patients with enhanced neutrophil activity who had shorter TTP. Unfortunately, we were not able to evaluate the effect of Valganciclovir treatment on neutrophil activity between GI (n = 12) and GII (n = 16), since nearly all patients examined for neutrophil activity received antiviral treatment (Valganciclovir); only two patients in Group I and three patients in Group II received placebo treatment. We noted that the number of circulating neutrophils were significantly decreased over time in both Valganciclovir treated and placebo cohort during the first 6 months after surgery, which may reflect the lower tumor burden in both groups at this time interval.
The levels of IL-8 and MCP-1 did not differ between GI and GII groups, but were significantly elevated in GBM patients compared with healthy controls. MCP-1 recruits monocytes, T cells, and dendritic cells to sites of infection and can enhance neutrophil and macrophage migration and infiltration. Enhanced inflammation would facilitate reactivation of latent HCMV and subsequent viral replication and spread, and the virus would inhibit the antiviral effects of neutrophils. Since monocytes and macrophages can harbor latent HCMV and contribute to viral spread during an active infection, increased MCP-1 secretion might be beneficial for the virus by enhancing viral activity, increasing neutrophil activity, however the levels of IL-8 and MCP-1 did not differ between the GI and GII cohorts.
In summary, our findings suggest that neutrophil activation is an early sign of tumor progression in GBM patients. Patients with enhanced neutrophil activity had worse prognosis and enhanced IL-12p70 levels were associated with neutrophil activation. Patients with high neutrophil activity had high grade HCMV infection, significantly shorter TTP and IL-12p70 levels were reduced in patients receiving anti-HCMV treatment. We conclude that altered levels of inflammatory mediators may contribute to immune abnormalities in GBM patients and may in part be affected by HCMV infection.
Materials and methods
Patient samples. Forty-two consecutive GBM patients were enrolled in a randomized, double-blind study (VIGAS) to evaluate the safety and efficacy of antiviral treatment of HCMV in HCMV-positive GBM patients (ethical permission 2006/755–31). The study was registered at the Swedish Medical Agency (Eudra number 2006–002022-29) and at ClincalTrials.Gov (Identifier NCT00400322). Inclusion criteria were age >18 years, diagnosis of GBM, HCMV infection in the tumor, and successful radical surgery (estimated tumor removal >90%). After surgery, patients received standard chemotherapy (temozolomide) and were randomized to receive oral anti-HCMV treatment (Valganciclovir) or placebo. Valganciclovir was given twice daily (900 mg/day) for 3 weeks and then twice daily (450 mg/day) for 21 weeks. Study patients in whom standard treatment failed were allowed to receive prescribed Valganciclovir. All patients were monitored for tumor progression and OS time.
Blood and plasma samples were collected once from six age matched healthy subjects and three times from patients: at the time of diagnosis (n = 42) and at 3 (n = 37) and 6 months (n = 35) after diagnosis. Plasma samples were stored at –85°C until further use. Neutrophils were purified from fresh blood collected once from six healthy subjects and three times from patients: at the time of diagnosis (n = 28) and at 12 (n = 28) and 24 (n = 26) weeks after diagnosis.
Serological screening for HCMV-IgG and HCMV-IgM. Plasma was examined for HCMV IgG and IgM with Enzygnost anti-HCMV-IgG and HCMV-IgM serological assay kit; OWBK15, Dade Behring, Germany) according to the manufacturer's instructions. IgG and IgM results have been reported elsewhere.Citation28
Isolation of human peripheral blood neutrophils. Neutrophils were isolated according to the Böyum method.Citation48 Briefly, 25 mL of whole blood was layered on 20 mL of Polymorphprep and 5 mL of Lymphoprep (both from Medinor, Stockholm, Sweden; Lymphoprep: Cat. 1114547 and Polymorphprep: Cat. 1114683) and centrifuged for 40 min at 480 g at room temperature. The polymorphonuclear lymphocytes (neutrophils) rich band was collected and contaminating erythrocytes were removed by hypotonic lysis. Cells were washed twice in Krebs-Ringer's phosphate buffer (KRG) without Ca2+ and Mg2+ and suspended in KRG.
Measurements of surface expression levels of CD11b on neutrophils. One million cells in 100 µL of KRG were incubated with or without N-formyL-methionyL-leucyL-phenylalanine (fmlp, final concentration, 0.1 M) for 10 min at 37° C and then with or without 5 µL of anti-CD11b antibodies, IgG1 isotype control (both from DakoCytomation, Denmark, anti-CD11b antibodies: Cat. R0841and IgG1 antibodies, Cat. X0928) for 30 min on ice. Cells were washed in KRG, suspended in 0.1% paraformaldehyde, and analyzed by flow cytometry (FACSCalibur, BD Pharmingen) and CellQuest software. Gates were set to exclude contaminating lymphocytes, monocytes, erythrocytes, and cellular debris. Percent CD11b up-regulation on blood neutrophils was calculated by subtraction of mean channel values for fmlp activated neutrophils from mean channel values for neutrophils without activation, divided with mean channel values for unactivated neutrophils.
Quantification of cytokines in plasma samples. Sera from all patients and six healthy controls were thawed on ice, and the levels of IL-1β, IL-2, IL-6, IL-8, IL-12, IL-17, IL-10, TNF-α, IFN-α IFNγ, MCP-1, and TGF-β were measured with FlowCytomix (Bender MedSystems), a custom bead-based flow cytometry assay (FACSCalibur, BD Pharmingen), and CellQuest software. The data were analyzed with FlowCytomixPro 1.0 Software (Bender MedSystems).
Antigenemia assay. Purified blood neutrophils were counted and spotted on slides with a Cytospin centrifuge. Cells were fixed in a solution of saccharose, formalin, and phosphate-buffered saline (Substrate Department, Karolinska University Hospital) for 10 min; permeablized in detergent solution consisting of saccarose, NP40, fetal calf serum, and phosphate-buffered saline (Substrate Department, Karolinska University Hospital) for 5 min; and washed in phosphate-buffered saline. The cells were incubated with antibodies against HCMV pp65 (Argene, France, Cat. 11-002). HCMV pp65 was detected with immunofluorescently labeled goat anti-mouse antibodies (DakoCytomation, Denmark, Cat. F0313). Human fibroblasts infected with HCMV strain AD169 were used as positive control. All slides were analyzed by fluorescence microscopy.
Immunohistochemical staining. Paraffin-embedded brain tumor tissues from all patients were stained for HCMV immediate-early protein (Millipore, Stockholm, Sweden, Cat. 810R) and graded (1+, 2+, 3+, 4+) according to the estimated number of tumor cells expressing immediate-early protein, as described.Citation13,20
Statistical analyses. OS and TTP were analyzed with Cox regression models; the covariates were enhanced neutrophil activity (Group I) or decreased/unchanged neutrophil activity (Group II). All patients who were alive or did not have progression at the time of investigation were censored in the analysis (August 23, 2012). The results are presented as hazard ratios with 95% confidence intervals. Survival graphs were created with the Kaplan–Meier method. The median times to end points were estimated from the Kaplan–Meier curves, with 95% CIs. Values are expressed as medians, and statistical significance was tested by one-way ANOVA, Wilcoxon matched pairs and Mann–Whitney U test and Kruskal–Wallis test. P < 0.05 was considered significant.
Disclosure of potential conflicts of interest
C.S.N. has received speaker's fees and sponsored travel costs to present data on the indirect molecular effects of HCMV at scientific meetings over 6 years ago.
Supplemental_Material.zip
Download Zip (898.3 KB)Acknowledgment
We thank Stephen Ordway for editing the manuscript.
Funding
This work was partly supported by independent grants from Hoffmann La Roche. This work was supported by BILTEMA Foundation, Nexttobe, Stichting af Jochnicks Foundation, Sten A Olssons Foundation for Research and Culture, Familjen Erling-Perssons Foundation, RATOS, independent grants from Hoffmann La Roche, Torsten and Ragnar Söderbergs Foundations, Dan och Jane Olssons Foundation, Swedish Cancer Foundation, Swedish Medical Research Council, Swedish Society for Medical Research (SLS), Goljes Memory Foundation, Magnus Bergvalls Foundation, Swedish Society for Medical Research (SSMF), IngaBritt och Arne Lundbergs Foundation and Tore Nilsons Foundation.
References
- Bergenheim T, Malmstrom A, Bolander H, Michanek A, Stragliotto G, Damber L, Björ O, Henriksson R. Registration on regional basis of patients with primary brain tumors. Regional differences disclosed. Lakartidningen 2007; 104:332-8, 340–1; PMID:17328357
- Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJB, Belanger K, Brandes AA, Marosi C, Bogdahn U et al. Radiotherapy plus Concomitant and Adjuvant Temozolomide for Glioblastoma. N Engl J Med 2005; 352:987-96; PMID:15758009; http://dx.doi.org/10.1056/NEJMoa043330
- Hart MG, Grant R, Garside R, Rogers G, Somerville M, Stein K. Temozolomide for high grade glioma. Cochrane Database Syst Rev 2008; 8(4): CD007415; PMID:18843749; http://dx.doi.org/10.1002/14651858.CD007415
- Affronti ML, Heery CR, Herndon JE, 2nd, Rich JN, Reardon DA, Desjardins A, Vredenburgh JJ, Friedman AH, Bigner DD, Friedman HS. Overall survival of newly diagnosed glioblastoma patients receiving carmustine wafers followed by radiation and concurrent temozolomide plus rotational multiagent chemotherapy. Cancer 2009; 115:3501-11; PMID:19514083; http://dx.doi.org/10.1002/cncr.24398
- Weller M, Fontana A. The failure of current immunotherapy for malignant glioma. Tumor-derived TGF-beta, T-cell apoptosis, and the immune privilege of the brain. Brain Res Brain Res Rev 1995; 21:128-51; PMID:8866671; http://dx.doi.org/10.1016/0165-0173(95)00010-0
- Waziri A. Glioblastoma-derived mechanisms of systemic immunosuppression. Neurosurg Clin North Am 2010; 21:31-42; http://dx.doi.org/10.1016/j.nec.2009.08.005
- Ooi YC, Tran P, Ung N, Thill K, Trang A, Fong BM, Nagasawa DT, Lim M, Yang I. The role of regulatory T-cells in glioma immunology. Clin Neurol Neurosurg 2014; 119:125-32; PMID:24582432; http://dx.doi.org/10.1016/j.clineuro.2013.12.004
- Kaminska B, Kocyk M, Kijewska M. TGF beta signaling and its role in glioma pathogenesis. Adv Exp Med Biol 2013; 986:171-87; PMID:22879069; http://dx.doi.org/10.1007/978-94-007-4719-7_9
- Fossati G, Ricevuti G, Edwards SW, Walker C, Dalton A, Rossi ML. Neutrophil infiltration into human gliomas. Acta Neuropathol 1999; 98:349-54; PMID:10502039; http://dx.doi.org/10.1007/s004010051093
- Iwatsuki K, Kumara E, Yoshimine T, Nakagawa H, Sato M, Hayakawa T. Elastase expression by infiltrating neutrophils in gliomas. Neurol Res 2000; 22:465-8; PMID:10935217
- Hor WS, Huang WL, Lin YS, Yang BC. Cross-talk between tumor cells and neutrophils through the Fas (APO-1, CD95)/FasL system: human glioma cells enhance cell viability and stimulate cytokine production in neutrophils. J Leukoc Biol 2003; 73:363-8; PMID:12629150; http://dx.doi.org/10.1189/jlb.0702375
- Soderberg-Naucler C. HCMV microinfections in inflammatory diseases and cancer. J Clin Virol 2008; 41:218-23; PMID:18164235; http://dx.doi.org/10.1016/j.jcv.2007.11.009
- Straat K, Liu C, Rahbar A, Zhu Q, Liu L, Wolmer-Solberg N, Lou F, Liu Z, Shen J, Jia J et al. Activation of telomerase by human cytomegalovirus. J Natl Cancer Inst 2009; 101:488-97; PMID:19318640; http://dx.doi.org/10.1093/jnci/djp031
- Rahbar A, Orrego A, Peredo I, Dzabic M, Wolmer-Solberg N, Straat K, Stragliotto G, Söderberg-Nauclér C. Human cytomegalovirus infection levels in glioblastoma multiforme are of prognostic value for survival. J Clin Virol 2013; 57:36-42; PMID:23391370; http://dx.doi.org/10.1016/j.jcv.2012.12.018
- Cobbs CS, Harkins L, Samanta M, Gillespie GY, Bharara S, King PH, Nabors LB, Cobbs CG, Britt WJ. Human cytomegalovirus infection and expression in human malignant glioma. Cancer Res 2002; 62:3347-50; PMID:12067971
- Lucas KG, Bao L, Bruggeman R, Dunham K, Specht C. The detection of CMV pp65 and IE1 in glioblastoma multiforme. J Neuro-oncol 2011; 103:231-8; http://dx.doi.org/10.1007/s11060-010-0383-6 ; PMID:20820869
- Baryawno N, Rahbar A, Wolmer-Solberg N, Taher C, Odeberg J, Darabi A, Khan Z, Sveinbjörnsson B, FuskevÅg OM, Segerström L et al. Detection of human cytomegalovirus in medulloblastomas reveals a potential therapeutic target. J Clin Invest 2011; 121:4043-55; PMID:21946257; http://dx.doi.org/10.1172/JCI57147
- Wolmer-Solberg N, Baryawno N, Rahbar A, Fuchs D, Odeberg J, Taher C, Wilhelmi V, Milosevic J, Mohammad AA, Martinsson T et al. Frequent detection of human cytomegalovirus in neuroblastoma: a novel therapeutic target? Int J Cancer 2013; 133:2351-61; PMID:23661597; http://dx.doi.org/10.1189/jlb.0702375
- Harkins L, Volk AL, Samanta M, Mikolaenko I, Britt WJ, Bland KI, Cobbs CS. Specific localisation of human cytomegalovirus nucleic acids and proteins in human colorectal cancer. Lancet 2002; 360:1557-63; PMID:12443594; http://dx.doi.org/10.1016/S0140-6736(02)11524-8
- Taher C, de Boniface J, Mohammad AA, Religa P, Hartman J, Yaiw KC, Frisell J, Rahbar A, Söderberg-Naucler C. High prevalence of human cytomegalovirus proteins and nucleic acids in primary breast cancer and metastatic sentinel lymph nodes. PloS One 2013; 8:e56795; PMID:23451089; http://dx.doi.org/10.1371/journal.pone.0056795
- Taher C, Frisk G, Fuentes S, Religa P, Costa H, Assinger A, Vetvik KK, Bukholm IR, Yaiw KC, Smedby KE et al. High prevalence of human cytomegalovirus in brain metastases of patients with primary breast and colorectal cancers. Transl Ooncol 2014; 7:732-40; PMID: 25500083; http://dx.doi.org/10.1016/j.tranon.2014.09.008
- Harkins LE, Matlaf LA, Soroceanu L, Klemm K, Britt WJ, Wang W, Bland KI, Cobbs CS. Detection of human cytomegalovirus in normal and neoplastic breast epithelium. Herpesviridae 2010; 1:8; PMID:21429243; http://dx.doi.org/10.1186/2042-4280-1-8
- El-Shinawi M, Mohamed HT, El-Ghonaimy EA, Tantawy M, Younis A, Schneider RJ, Mohamed MM. Human cytomegalovirus infection enhances NF-kappaB/p65 signaling in inflammatory breast cancer patients. PloS One 2013; 8:e55755; PMID:23418456; http://dx.doi.org/10.1371/journal.pone.0055755
- Samanta M, Harkins L, Klemm K, Britt WJ, Cobbs CS. High prevalence of human cytomegalovirus in prostatic intraepithelial neoplasia and prostatic carcinoma. J Urol 2003; 170:998-1002; PMID:12913758; http://dx.doi.org/10.1097/01.ju.0000080263.46164.97
- Forbes BA. Acquisition of cytomegalovirus infection: an update. Clin Microviol Rev 1989; 2:204-16
- Boldogh I, AbuBakar S, Deng CZ, Albrecht T. Transcriptional activation of cellular oncogenes fos, jun, and myc by human cytomegalovirus. J Virol 1991; 65:1568-71; PMID:1847472
- Boldogh I, AbuBakar S, Fons MP, Deng CZ, Albrecht T. Activation of cellular oncogenes by clinical isolates and laboratory strains of human cytomegalovirus. J Med Virol 1991; 34:241-7; PMID:1719130; http://dx.doi.org/10.1002/jmv.1890340409
- Cinatl J Jr, Vogel JU, Kotchetkov R, Wilhelm Doerr H. Oncomodulatory signals by regulatory proteins encoded by human cytomegalovirus: a novel role for viral infection in tumor progression. FEMS Microbiol Rev 2004; 28:59-77; PMID:14975530; http://dx.doi.org/10.1016/j.femsre.2003.07.005
- Graf L, Webel R, Wagner S, Hamilton ST, Rawlinson WD, Sticht H, Marschall M. The cyclin-dependent kinase ortholog pUL97 of human cytomegalovirus interacts with cyclins. Viruses 2013; 5:3213-30; PMID:24351800; http://dx.doi.org/10.3390/v5123213
- Skaletskaya A, Bartle LM, Chittenden T, McCormick AL, Mocarski ES, Goldmacher VS. A cytomegalovirus-encoded inhibitor of apoptosis that suppresses caspase-8 activation. Proc Natl Acad Sci USA 2001; 98:7829-34; PMID:11427719; http://dx.doi.org/10.1073/pnas.141108798
- Stragliotto G, Rahbar A, Solberg NW, Lilja A, Taher C, Orrego A, Bjurman B, Tammik C, Skarman P, Peredo I et al. Effects of valganciclovir as an add-on therapy in patients with cytomegalovirus-positive glioblastoma: a randomized, double-blind, hypothesis-generating study. Int J Cancer 2013; 133:1204-13; PMID:23404447; http://dx.doi.org/10.1002/ijc.28111
- Soderberg-Naucler C, Rahbar A, Stragliotto G, Soderberg-Naucler C. Survival in patients with glioblastoma receiving valganciclovir. N Engl J Med 2013; 369:985-86; PMID:24004141; http://dx.doi.org/10.1056/NEJMc1302145
- Skarman PJ, Rahbar A, Xie X, Soderberg-Naucler C. Induction of polymorphonuclear leukocyte response by human cytomegalovirus. Microbes Infect 2006; 8:1592-601; PMID:16702012; http://dx.doi.org/10.1016/j.micinf.2006.01.017
- Zheng Q, Tao R, Gao H, Xu J, Shang S, Zhao N. HCMV-encoded UL128 enhances TNF-alpha and IL-6 expression and promotes PBMC proliferation through the MAPK/ERK pathway in vitro. Viral Immunol 2012; 25:98-105; PMID:22486303; http://dx.doi.org/10.1089/vim.2011.0064
- Rahbar A, Peredo I, Solberg NW, Taher C, Dzabic M, Xu X, Skarman P, Fornara O, Tammik C, Yaiw K et al. Discordant humoral and cellular immune responses to Cytomegalovirus (CMV) in glioblastoma patients whose tumors are positive for CMV. OncoImmunology 2014; 4: e982391-1-e982391-8; PMID: 25949880; http://dx.doi.org/10.4161/2162402X.2014.982391
- Ferreira SH, Moreno SE, Alves-Filho JC, Alfaya TM, Da Silva JS, Liew FY. IL-12, but not IL-18, is critical to neutrophil activation and resistance to polymicrobial sepsis induced by cecal ligation and puncture. J Immunol 2006; 177:3218-24; PMID:16920961; http://dx.doi.org/10.4049/jimmunol.177.5.3218
- Eisele G, Wischhusen J, Mittelbronn M, Meyermann R, Waldhauer I, Steinle A, Weller M, Friese MA. TGF-beta and metalloproteinases differentially suppress NKG2D ligand surface expression on malignant glioma cells. Brain 2006; 129:2416-25; PMID:16891318; http://dx.doi.org/10.1093/brain/awl205
- Wischhusen J, Friese MA, Mittelbronn M, Meyermann R, Weller M. HLA-E protects glioma cells from NKG2D-mediated immune responses in vitro: implications for immune escape in vivo. J Neuropathol Exp Neurol 2005; 64:523-8; PMID:15977644
- Roszman T, Elliott L, Brooks W. Modulation of T-cell function by gliomas. Immunol Today 1991; 12:370-4; PMID:1958290; http://dx.doi.org/10.1016/0167-5699(91)90068-5
- Rapp M, Ozcan Z, Steiger HJ, Wernet P, Sabel MC, Sorg RV. Cellular immunity of patients with malignant glioma: prerequisites for dendritic cell vaccination immunotherapy. J Neuerosurg 2006; 105:41-50; PMID: 16874889; http://dx.doi.org/10.3171/jns.2006.105.1.41
- Fielding CA, McLoughlin RM, McLeod L, Colmont CS, Najdovska M, Grail D, Ernst M, Jones SA, Topley N, Jenkins BJ. IL-6 regulates neutrophil trafficking during acute inflammation via STAT3. J Immunol 2008; 181:2189-95; PMID:18641358; http://dx.doi.org/10.4049/jimmunol.181.3.2189
- Hammond ME, Lapointe GR, Feucht PH, Hilt S, Gallegos CA, Gordon CA, Giedlin MA, Mullenbach G, Tekamp-Olson P. IL-8 induces neutrophil chemotaxis predominantly via type I IL-8 receptors. J Immunol 1995; 155:1428-33; PMID:7636208
- Gately MK, Renzetti LM, Magram J, Stern AS, Adorini L, Gubler U, Presky DH. The interleukin-12/interleukin-12-receptor system: role in normal and pathologic immune responses. Annu Rev Immunol 1998; 16:495-521; PMID:9597139; http://dx.doi.org/10.1146/annurev.immunol.16.1.495
- Denkers EY, Del Rio L, Bennouna S. Neutrophil production of IL-12 and other cytokines during microbial infection. Chem Immunol Allergy 2003; 83:95-114; PMID:12947981; http://dx.doi.org/10.1159/000071557
- Daheshia M, Kanangat S, Rouse BT. Production of key molecules by ocular neutrophils early after herpetic infection of the cornea. Exp Eye Res 1998; 67:619-24; PMID:9990326; http://dx.doi.org/10.1006/exer.1998.0565
- Kanangat S, Thomas J, Gangappa S, Babu JS, Rouse BT. Herpes simplex virus type-1 mediated up-regulation of IL-12 (p40) mRNA expression. Implications in immnuopathogenesis and protection. J Immunol 1996; 156:1110-6; PMID:8557986
- Iwatsuki K1, Kumara E, Yoshimine T, Nakagawa H, Sato M, Hayakawa T. Elastase expression by infiltrating neutrophils in gliomas. Neurol Res. 2000; 22:465-8
- Boyum A. Isolation of mononuclear cells and granulocytes from human blood. Isolation of monuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand J Clin Lab Invest Suppl 1968; 97:77-89; PMID:4179068
