ABSTRACT
The myeloid-derived suppressor cells (MDSCs) contribute to tumor immune evasion and still remain an elusive therapeutic target. Our study identified granulocytic MDSCs accumulating in prostate cancer patients during disease progression. We demonstrate the feasibility of using STAT3siRNA-based strategy for targeting MDSCs to alleviate arginase-dependent suppression of T cell activity.
Metastatic castration-resistant prostate cancers are known for being resistant to current standard therapies and even to the emerging immunotherapeutic regimens. Growing evidence suggests that advanced prostate cancers develop potently immunosuppressive microenvironment which partly relies on the heterogeneous population of MDSCs. The MDSCs play pivotal role in cancer progression and poor patients’ survival.Citation1 The MDSCs are immature myeloid cells which lack expression of multiple immune cell surface markers, including antigen-presenting molecules. Depending on the expression of myeloid lineage markers, human MDSCs can be divided into CD14+CD15LOCD33HI monocytic MDSCs (M-MDSCs) and CD14−CD15HICD33LO granulocytic MDSCs (G-MDSCs).Citation1 The M-MDSCs were previously found in prostate cancer patients and their levels correlated with levels of PSA (prostate-specific antigen) used as a marker of tumor burden.Citation2 Even though G-MDSCs were recently observed in other genitourinary tumors, the contribution of G-MDSCs to prostate cancer progression remained unknown.
Our recently published study focused on the phenotypic analysis of immune cell populations in blood derived from patients with localized or metastatic prostate cancers compared to healthy subjects.Citation3 In contrast to earlier studies, our flow cytometric analysis revealed significant accumulation of granulocytic (CD15HICD33LO) but not monocytic (CD15LOCD33HI) cell populations in patients’ circulation with progression of the disease. Further studies of both myeloid cell populations confirmed that only CD15HICD33LO myeloid cells were functionally MDSCs as indicated by their potent inhibitory effect on T cell proliferation and activity. Due to complex identification which relies on markers common to other immune cell lineages, eliminating MDSCs using monoclonal antibodies is challenging. Thus, it seems more practical to target signaling molecules which control MDSC function in the specific tumor microenvironment. Our analysis of cytokine and growth factor (GF) profiles revealed elevated levels of factors related to recruitment and expansion of myeloid cells, such as G-CSF and IL-8 among others. Given the critical role of STAT3 transcription factor in the downstream signaling of G-CSF as well as other GFs present in the tumor microenvironment,Citation3 we assessed levels of activated STAT3 (pSTAT3) in prostate cancer-associated G-MDSCs using flow cytometric and immunohistochemical analyses. We observed elevated pSTAT3 in circulating G-MDSCs and also in polymorphonuclear cells infiltrating prostate tissue biopsies from cancer patients. In addition, STAT3 activation clearly correlated with high levels of Arginase-1 (ARG1) expression in G-MDSCs compared to healthy granulocytes, and also with detectable ARG1 activity in blood samples from cancer patients. Highly elevated levels of ARG1 in G-MDSCs with weak induction of IDO and undetectable iNOS mRNA expression likely reflected different levels of STAT3 involvement in the regulation of these target genes. In G-MDSCs, STAT3 was shown to have potent and direct effect on ARG1, while it collaborates with noncanonical NF-κB signaling to promote IDO expression. In contrast, STAT3 activity is not required for iNOS expression which is driven by STAT1 and NF-κB signaling activated in monocytic MDSCs but not in G-MDSCs.Citation1 Altogether, these observations strengthened the evidence that G-MDSCs are commonly present in prostate cancer patients’ circulation and likely reach into the prostate tumor microenvironment.
TLR9 was recently found to be expressed by tumor-associated MDSCs in mice, with its highest levels observed in M-MDSCs.Citation4 However, the pattern of TLR9 expression differs dramatically between mouse and human immune systems. While in mice TLR9 is ubiquitous in myeloid cells, it is expressed specifically by human plasmacytoid DCs in healthy subjects.Citation5 Thus, the expression of TLR9 in prostate cancer patients’ MDSCs and specifically in G-MDSCs was a novel finding of our study. Whether downstream TLR9 signaling restricts or supports the immunosuppressive functions of G-MDSCs remains unclear. Treatments with synthetic TLR9 agonists, CpG oligodeoxynucleotides (ODN), can trigger MDSC differentiation and reduce their immunosuppressive potential in mice.Citation4 However, other studies suggested that activation of TLRs in myeloid cells can promote expression of molecules critical for MDSC function, such as ARG1, S100A8, IL-10 and PGE2.Citation1 The functional dichotomy of TLR9 effects results from crosstalk with other signaling pathways operating under normal or tumor-induced inflammatory conditions. The outcome of TLR9 signaling seems to be defined by a multilayered negative feedback regulation through STAT3 regardless whether it is triggered by synthetic ligands or release of natural agonists (e.g. mitochondrial DNA) from dying cells.Citation6,7
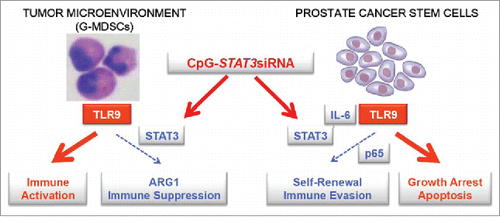
We previously demonstrated that TLR9 agonists, CpG ODN, can be harnessed for delivery of therapeutic siRNA molecules into TLR9+ cells in both mouse and human systems.Citation8,9 Targeting STAT3 with simultanous TLR9 activation using CpG-STAT3siRNA was shown to eliminate tumorigenic effects of TLR9 signaling in mouse tumor models.Citation7 Here, we demonstrated that CpG-STAT3siRNA allows for targeting of TLR9+ G-MDSCs to eliminate their immunosuppressive functions without myeloid cell depletion. Further studies should determine whether our approach only modulates G-MDSC activity or induces their differentiation into mature granulocytes or DCs/macrophages. In fact, we previously observed that CpG-STAT3siRNA induces activity of neutrophils against blood cancer xenotransplants in NSG mice.Citation9 In a parallel study, we recently identified that prostate cancer stem cells in human tumors rely on TLR9 signaling through NF-κB and STAT3 signaling crucial for their self-renewal and tumor-propagating potential. We demonstrated that it is feasible to target tumorigenic signaling in cancer stem cells using CpG-siRNA approach.Citation10 Taken together, our findings provide support for application of CpG-STAT3siRNA strategy to immunotherapy of advanced prostate cancers alone or in combination with immunotherapies, such as emerging T cell-based therapies (). Disruption of STAT3 signaling in the tumor microenvironment with concurrent TLR9 stimulation has potential to disrupt the central immunosuppressive circuit paving way to effective antitumor immune responses without toxicities associated with pharmacological agents.
Figure 1. TLR9-targeted STAT3 inhibition allows for 2-pronged therapeutic effect against prostate cancers. CpG-STAT3siRNA conjugates target STAT3 signaling in TLR9+ G-MDSCs, an immunosuppressive population of myeloid cells which accumulate during prostate cancer progression from localized to metastatic disease. STAT3 silencing reduces production of a potently immunosuppressive mediator, arginase-1 (ARG1), thereby restoring effector T cell proliferation and activity. As shown in a parallel study, CpG-siRNA strategy allows for delivery of therapeutic siRNAs to prostate cancer stem cells which express TLR9 and rely on NF-κB/STAT3 signaling for self-renewal and tumor-propagating potential. The combination of breaking immune suppression in the tumor microenvironment and decreasing cancer cell survival is likely to augment the overall therapeutic effect against prostate cancer.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- Gabrilovich DI, Ostrand-Rosenberg S, Bronte V. Coordinated regulation of myeloid cells by tumours. Nat Rev Immunol 2012; 12:253-68; PMID:22437938; http://dx.doi.org/10.1038/nri3175
- Vuk-Pavlovic S, Bulur PA, Lin Y, Qin R, Szumlanski CL, Zhao X, Dietz AB. Immunosuppressive CD14+HLA-DRlow/- monocytes in prostate cancer. Prostate 2010 Mar 1; 70(4):443-55; PMID:19902470; http://dx.doi.org/10.1002/pros.21078
- Hossain DM, Pal SK, Moreira DFD, Duttagupta P, Zhang Q, Won H, Jones J, D'Apuzzo M, Forman S, Kortylewski M. TLR9-Targeted STAT3 Silencing Abrogates Immunosuppressive Activity of Myeloid-Derived Suppressor Cells from Prostate Cancer Patients. Clin Cancer Res 2015; 21(16):3771-82; PMID:25967142; http://dx.doi.org/10.1158/1078-0432.CCR-14-3145
- Zoglmeier C, Bauer H, Norenberg D, Wedekind G, Bittner P, Sandholzer N, Rapp M, Anz D, Endres S, Bourquin C. CpG blocks immunosuppression by myeloid-derived suppressor cells in tumor-bearing mice. Clin Cancer Res 2011; 17:1765-75; PMID:21233400; http://dx.doi.org/10.1158/1078-0432.CCR-10-2672
- Krieg AM. CpG still rocks! Update on an accidental drug. Nucleic acid therapeutics 2012; 22:77-89; PMID:22352814; http://dx.doi.org/10.1089/nat.2012.0340
- Kortylewski M, Kujawski M, Herrmann A, Yang C, Wang L, Liu Y, Salcedo R, Yu H. Toll-like receptor 9 activation of signal transducer and activator of transcription 3 constrains its agonist-based immunotherapy. Cancer Res 2009; 69:2497-505; PMID:19258507; http://dx.doi.org/10.1158/0008-5472.CAN-08-3031
- Gao C, Kozlowska A, Nechaev S, Li H, Zhang Q, Hossain DM, Kowolik CM, Chu P, Swiderski P, Diamond DJ et al. TLR9 Signaling in the Tumor Microenvironment Initiates Cancer Recurrence after Radiotherapy. Cancer Res 2013; 73:7211-21; PMID:24154870; http://dx.doi.org/10.1158/0008-5472.CAN-13-1314
- Kortylewski M, Swiderski P, Herrmann A, Wang L, Kowolik C, Kujawski M, Lee H, Scuto A, Liu Y, Yang C et al. In vivo delivery of siRNA to immune cells by conjugation to a TLR9 agonist enhances antitumor immune responses. Nat Biotechnol 2009; 27:925-32; PMID:19749770; http://dx.doi.org/10.1038/nbt.1564
- Zhang Q, Hossain DM, Nechaev S, Kozlowska A, Zhang W, Liu Y, Kowolik CM, Swiderski P, Rossi JJ, Forman S et al. TLR9-mediated siRNA delivery for targeting of normal and malignant human hematopoietic cells in vivo. Blood 2013; 121:1304-15; PMID:23287859; http://dx.doi.org/10.1182/blood-2012-07-442590
- Moreira D, Zhang Q, Hossain DM, Nechaev S, Li H, Kowolik CM, D'Apuzzo M, Forman S, Jones J, Pal SK et al. TLR9 signaling through NF-kappaB/RELA and STAT3 promotes tumor-propagating potential of prostate cancer cells. Oncotarget 2015; 6(19):17302-13; PMID:26046794; http://dx.doi.org/10.18632/oncotarget.4029
