ABSTRACT
High-risk neuroblastoma continues to have a poor prognosis, stimulating the ongoing development of alternative immune-therapy approaches. Despite exciting preclinical results, clinical outcomes have been less clear. Recently we identified that neuroblastoma expresses Arginase II, depleting local and systemic arginine concentrations, and suppression of autologous and engineered T cell immunity.
The interplay between cancer and the immune system is a corner-stone of cancer pathogenesis. T cells play a key role in co-ordinating the anticancer immune response and by directly targeting tumor cells. However, cancer cells employ a number of mechanisms to escape from T cell immunity such as downregulation of antigen presentation, secretion of immunomodulatory cytokines, and induction of immunosuppressive cells.Citation1
Arginine is a semi-essential amino acid that plays a key role in both normal and cancer cell physiology. Free arginine is maintained by the diet, turnover of proteins and endogenous synthesis through an “intestinal-renal axis”. Citation2 At the cellular level arginine is metabolized and re-synthesized through an arginine-citrulline pathway as part of the urea cycle. Under physiological states of high demand such as inflammation, organ dysfunction or pregnancy, arginine can become limited in availability. T cells are dependent on arginine for proliferation and expression of the CD3ζ chain of the Tell Cell Receptor (TCR) complex, and thus during these conditions T cell function can be impaired.Citation3,4 It is well established that a number of cancers can induce a population of immature myeloid cells, Myeloid Derived Suppressor Cells (MDSCs), which indirectly inhibit T cell immunity through arginine depletion.Citation5 However, the ability of tumor cells to directly suppress T cell responses through modulation of arginine in the microenvironment has not previously been studied.
Neuroblastoma is the most common extra-cranial solid malignancy of childhood and is associated with a number of immune interactions including paraneoplastic syndromes, spontaneous tumor regressions, and lymphopenia at diagnosis. Recently we identified that neuroblastoma cells can directly inhibit T cell proliferation due to a high-arginase activity.Citation6 Unlike MDSCs, which express the Arginase I enzyme isoform, neuroblastoma tumor cells from TH-MYCN transgenic mice and patients, express predominantly Arginase II. Both Arginase I and Arginase II catabolize arginine into ornithine and urea, but are localized in the cytosolic and mitochondrial compartments respectively, and the genes are expressed on different chromosomes. While the function of Arginase I in cell biology and immunity has been well studied, the role of Arginase II in modulating the immune response has received only limited attention to date.
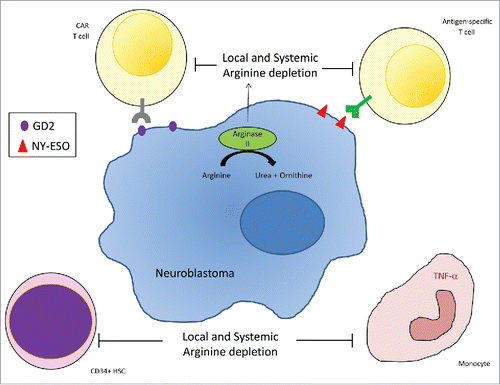
Neuroblastoma tumor arginase activity leads to both a local depletion of arginine within the microenvironment but also systemically, leading to low numbers of T cells within the tumor tissue and spleens of tumor-bearing mice, and T cell suppression in the blood of patients (). Tumor arginine depletion further modulates surrounding and circulating monocytic cells to a more immunosuppressive phenotype, and CD34+ haematopoietic cell division is impaired. Unlike in Acute Myeloid Leukemia, these systemic effects appear to be driven by the arginase activity of the tumor mass, and not by release of free arginase enzyme into the circulation.Citation7
Figure 1. Neuroblastoma Arginase II activity, leads to local microenvironment and systemic depletion of arginine concentrations. Arginine depletion suppresses T cell proliferation, NY-ESO antigen-specific T cell immunity, and anti-GD2 CAR T cell cytotoxicity. Surrounding CD14+ monocytes are also inhibited and CD34+ haematopoietic stem cell (HSC) proliferation is impaired.
High-risk neuroblastoma remains a disease of poor prognosis, with the majority of patients succumbing to disease despite intensive multi-drug chemotherapy, surgery, and radiotherapy. As a result, novel approaches are being developed of which the most promising are immunotherapies against the cancer germline protein NY-ESO and the surface molecule GD2-disialoganglioside. These targets are almost (but not entirely) restricted to neuroblastoma tumor cells. In both cases, immunotherapy approaches using engineered T cells or dendritic vaccine strategies have shown significant efficacy in vitro and in murine models against neuroblastoma cells. However, in human trials results have been less successful, with a suggestion that the best responses are seen in patients with low tumor burden at the time of administration.Citation8 One leading study of anti-GD2 Chimeric Antigen Receptor (CAR) T cells for neuroblastoma made the key observations that despite infusion of large numbers of these cells, CAR T cell numbers become low or undetectable within weeks, and that the majority of patients with active disease did not achieve a complete remission.Citation9 The finding suggests that the local and systemic tumor microenvironment can impair immunotherapeutic approaches, despite the presence of large target antigenic load on residual neuroblastoma tumors. Indeed patients who had detectable low-level persistence of CAR T cells had a longer survival.
We examined the potential of neuroblastoma arginase activity to account for limitations in T cell immunotherapy approaches. We demonstrated that the proliferation of engineered NY-ESO antigen specific T cells and anti-GD2 CAR T cells were both suppressed. The cytotoxic activity of anti-GD2 CAR T cells against neuroblastoma was impaired in an arginase dependent manner. With the growing development of immune therapies, these findings have significant translational consequences. Firstly high Arginase II expression correlates with a poor overall survival for neuroblastoma patients, suggesting a key role in neuroblastoma pathogenesis. This leads to the second implication that new drugs which block arginase expression or activity could be an adjunct to current therapy. Arginase-pathway modulating drugs may reawaken patients' immune responses to target neuroblastoma or could be administered with immunotherapy to enhance its effect. Further work is required to identify non-toxic arginase inhibitors for use in human clinical trials.Citation10
In summary, arginine depletion by neuroblastoma, through Arginase II expression, provides a key mechanism of escape from both autologous and engineered immunity.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- Pistoia V, Morandi F, Bianchi G, Pezzolo A, Prigione I, Raffaghelo L. Immunosuppressive microenvironment in neuroblastoma. Front Oncol 2013; 3:167; PMID:23805414; http://dx.doi.org/10.3389/fonc.2013.00167
- Morris SM Jr. Recent advances in arginine metabolism: roles and regulation of the arginases. Br J Pharm 2009; 15:922-30; PMID:19508396; http://dx.doi.org/10.1111/j.1476-5381.2009.00278.x
- Rodriguez PC, Quiceno DG, Ochoa AC. L-arginine availability regulates T lymphocyte cell-cycle progression. Blood 2007; 109:1568-73; PMID:17023580; http://dx.doi.org/10.1182/blood-2006-06-031856
- Zea AH, Rodriguez PC, Culotta KS, Hernandez CP, DeSalvo J, Ochoa JB, Park HJ, Zabaleta J, Ochoa AC. L-arginine modulates CD3zeta expression and T cell function in activated human T lymphocytes. Cell Immunol 2004; 232:21-31; PMID:15922712; http://dx.doi.org/10.1016/j.cellimm.2005.01.004
- Mussai F, De Santo C, Cerundolo V. Interaction between iNKT cells and MDSCs in cancer patients: Evidence and Therapeutic Opportunities. J Immunother 2012; 35:449-59; PMID:22735803; http://dx.doi.org/10.1097/CJI.0b013e31825be926
- Mussai F, Egan S, Hunter S, Webber H, Fisher J, Wheat R, McConville C, Sbirkov Y, Wheeler K, Bendle G et al. Neuroblastoma arginase activity creates an immunosuppressive microenvironment that impairs autologous and engineered immunity. Cancer Res 2015; 75(15):3043-53; PMID:26054597; http://dx.doi.org/10.1158/0008-5472.CAN-14-3443
- Mussai F, De Santo C, Abu-Dayyeh I, Booth S, Quek L, McEwen-Smith RM, Qureshi A, Dazzi F, Vyas P, Cerundolo V. Acute Myeloid leukaemia creates an arginase dependent immuno-suppressive microenvironment. Blood 2013; 122:749-58; PMID:23733335; http://dx.doi.org/10.1182/blood-2013-01-480129
- Krishnadas DK, Shusterman S, Bai F, Diller L, Sullivan JE, Cheerva AC, George RE, Lucas KG. A Phase I trial combining decitabine/dendritic cll vaccine targeting MAGE-A!, MAGE-A3 and NY-ESO for children with relapsed or therapy refractory neuroblastoma and sarcoma. Cancer Immunol Immunother 2015; 64(10):1251-60; PMID:26105625; http://dx.doi.org/10.1007/s00262-015-1731-3
- Louis CU, Savoldo B, Dotti G, Pule M, Yvon E, Myers GD, Rossig C, Russell HV, Diouf O, Liu E et al. Antitumor activity and long-term fate of chimeric antigen receptor-positive T cells in patients with neuroblastoma. Blood 2011; 118(23):6050-6; PMID:21984804; http://dx.doi.org/10.1182/blood-2011-05-354449
- Ivanenkov YA, Chufarova NV. Small-molecule arginase inhibitors. Pharm Pat Anal 2014; 3(1):65-85; PMID:24354980; http://dx.doi.org/10.4155/ppa.13.75
