ABSTRACT
Mesothelioma is an almost invariably fatal tumor with chemotherapy extending survival by a few months. One immunotherapeutic strategy is to target dendritic cells (DCs), key antigen-presenting cells involved in antigen presentation, to induce antigen-specific T cell responses. However, DC-targeting will only be effective if DCs are fit-for-purpose, and the functional status of DCs in mesothelioma patients was not clear. We found that mesothelioma patients have significantly decreased numbers of circulating myeloid (m)DC1 cells, mDC2 cells and plasmacytoid (p)DCs relative to healthy age and gender-matched controls. Blood monocytes from patients could not differentiate into immature monocyte-derived DCs (MoDCs), indicated by a significantly reduced ability to process antigen and reduced expression of costimulatory (CD40, CD80 and CD86) and MHC (HLA-DR) molecules, relative to controls. Activation of mesothelioma-derived MoDCs with LPS+/-IFNγ generated partially mature MoDCs, evident by limited upregulation of the maturation marker, CD83, and the costimulatory markers. Attempts to rescue mesothelioma-derived DC function using CD40Ligand(L) also failed, indicated by maintenance of antigen-processing capacity and limited upregulation of CD40, CD83, CD86 and HLA-DR. These data suggest that mesothelioma patients have significant numerical and functional DC defects and that their reduced capacity to process antigen and reduced expression of costimulatory molecules could induce anergized/tolerized T cells. Nonetheless, survival analyses revealed that individuals with mesothelioma and higher than median levels of mDC1s and/or whose MoDCs matured in response to LPS, IFNγ or CD40L lived longer, implying their selection for DC-targeting therapy could be promising especially if combined with another treatment modality.
Introduction
Mesothelioma is an aggressive cancer of the mesothelium with a known carcinogen, asbestos, and a mean survival from diagnosis of only 9 mo.Citation1 However, some patients live substantially longer, including some who do not receive active therapy. Several prognostic markers have been identified and include epithelioid histology, normal hemoglobin, neutrophil-to-lymphocyte ratioCitation2 and white cell counts, as well as younger age,Citation3 with the latter two points implying a cancer-controlling role for the immune system. There is a lengthy latency period between asbestos exposure and detectable diseaseCitation4 which may reflect not only the time for a critical mutational load to develop, but also increasing age-relatedCitation5,6 and tumor-induced immune dysfunction,Citation7,8 eventually leading to tumor escape. Current treatment options including surgery and/or chemotherapy extend survival by only a few months.Citation1 Therefore, research into other treatment strategies is warranted and immunotherapy has shown promise in mesothelioma.Citation9,10
One immunotherapeutic strategy is to target DC. DCs are key antigen-presenting cells that, in their immature state, take up and process antigens including tumor antigens, and upon appropriate maturation present them to naive T cells to induce specific immune responses.Citation11 DCs consist of a number of different yet functionally related subsets. Circulating DCs comprise myeloid and plasmacytoid (p)DCs. Myeloid (mDC) or conventional DCs are made up of at least two subsets and originate from CD34+ progenitors or CD14+ monocytes.Citation12 The more common mDC1 cell is a major stimulator of T cells, and secretes IL-12 to induce polarization of naive CD4+ T cells into Th1 cells.Citation13 The rarely occurring mDC2 cell type plays a role in cross presentation and shares many genetic similarities to cross-presenting CD8+ DCs in mice.Citation14 Both mDC subpopulations are efficient in uptake, processing and presentation of antigens. Plasmacytoid DCs are generated from either CD34+ progenitors or CD11c- blood precursors and rapidly secrete large amounts of interferon α (IFNα) in response to viral challenge.Citation15-17 MoDCs represent DCs that are rapidly generated in response to inflammation.Citation18 Thus, dysfunction of one or more DC subsets could result in aberrant immune responses.
Studies in other human cancers such as squamous cell carcinoma of the head and neck, prostate cancer, pancreatic cancer, breast cancer, Kaposi sarcoma and multiple myeloma have shown significant decreases in the numbers of circulating mDCs,Citation19-21 pDCs,Citation22 or mDCs and pDCs relative to healthy age-matched controls.Citation23-26 Moreover, DCs extracted from tumors expressed an immature phenotype and were poor stimulators of T cell proliferation.Citation27 This may be explained by observations that blood DCs and MoDCs generated from patients with cancers such as breast cancer, hepatocellular carcinoma and squamous cell carcinomas of the head and neck expressed significantly lower levels of the antigen-presenting molecule, HLA-DR, and/or low levels of the CD80 and CD86 co-stimulatory moleculesCitation20,21,Citation25,28 relative to DCs from healthy volunteers; these data imply an inability to appropriately activate T cells in cancer patients. Mouse models of mesothelioma have shown that even though tumor antigen presentation to CD8+ T cells can be detected in draining lymph nodes, a function likely performed by DCs, the consequent T cell response was weak and unable to prevent tumor growth, implying inadequate co-stimulation.Citation29
There are no published data on the numbers and function of DCs in mesothelioma patients. We aimed to determine the effect of mesothelioma on pDC, mDC1 and mDC2 numbers in peripheral blood of patients compared to healthy aged and gender-matched controls. The study also assessed the capacity for monocytes from both cohorts to differentiate into MoDCs, as well as their ability to process and present antigen and respond to microbial, cytokine and CD40 ligand (CD40L) stimulation. We identified profound deficits in DC numbers and function that may be important in the context of immunotherapy strategies. However, our data also suggests that functional DCs may contribute to survival in people with mesothelioma and that an immunotherapy tailored to improve DC numbers and function could improve patient outcomes as increased levels of mDC1s and surface CD80 in individual patients were associated with prolonged survival.
Results
Patient characteristics
48 people with mesothelioma and 40 age-matched healthy volunteers were recruited to the study. Greater than 90% of participants with mesothelioma were newly diagnosed. The mean age of participants with mesothelioma was 66.9 y (range 47–84; SD 8.4) and for healthy volunteers was 67.5 y (range 48–84, SD 8.3, p = 0.7). 10 people with mesothelioma (21%) and 15 healthy volunteers (37%) were female (p = 0.17).
Mesothelioma patients have decreased numbers of blood DC subsets
Circulating DC subsets in mesothelioma patients vs. age-matched controls were examined using flow cytometry. Gating eliminated debris, red blood cells, B cells, monocytes and granulocytes (). DC subpopulations were identified by high expression of BDCA-1 (CD1c; mDC1s, ), very high expression of BDCA-3 (CD141; mDC2s, ) and high expression of BDCA-2 (CD303; pDCs, ).
Figure 1. Mesothelioma patients have decreased numbers of blood DC subsets. Whole blood was stained and analyzed by flow cytometry. Representative dot plot (a) showing gating of leukocytes by size (FSC) and granularity (SSC). CD14+ monocytes, granulocytes and CD19+ B cells were further excluded by gating (b). Blood DC subsets were identified by high expression of BDCA-1 (c: mDC1), BDCA-3 (d: mDC2 and BDCA2 (c: pDC). Circulating mDC1 (e), and mDC2 (f) and pDCs (g) are shown as the number of DCs per mL of blood. Each dot represents an individual volunteer (mesothelioma: n = 48, age-matched controls: n = 36). ***p < 0.0001.
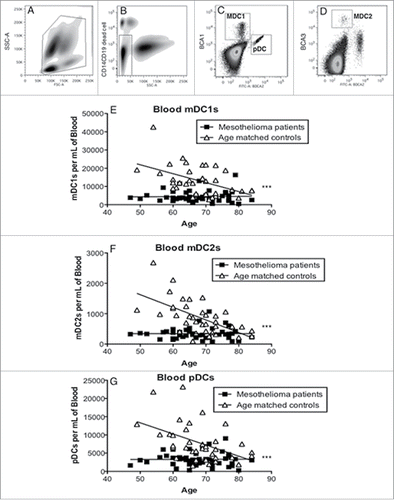
Myeloid DC1, mDC2 and pDC subsets in healthy individuals showed an age-related decrease in numbers, with pDCs demonstrating a statistically significant decrease (p < 0.0001, ). The numbers of all three DC subsets were even further reduced in mesothelioma patients relative to the age-matched controls with differences between patients and controls being statistically significant (p < 0.0001). These data suggest that the immune system in mesothelioma patients may be impaired relative to healthy age-matched controls.
Mesothelioma-derived monocytes differentiate into immature CD14- MoDCs
We next examined whether reduced numbers of circulating DCs in mesothelioma patients were associated with changes in DC function. As direct functional analysis of blood DC subsets is difficult due to low numbers this series of experiments involved the generation of MoDCs from monocyte precursors in vitro. Monocytes from mesothelioma patients and healthy controls were exposed to GM-CSF and IL-4 () and their ability to differentiate into immature (i)MoDCs investigated by examining expression of key DC surface markers on gated large, CD14- cells. No differences were observed between mesothelioma patients and age-matched controls in the percentage of CD14- cells that differentiated into classical CD11c+ DCs, or in CD11c surface expression levels (MFIs) ().
Figure 2. Mesothelioma-derived monocytes differentiate into iMoDCs. Blood monocytes from mesothelioma patients and age-matched volunteers were differentiated into iMoDCs using GM-CSF and IL-4 (a) and stained for analysis by flow cytometry. Representative plot (b) showing gating of large CD14- cells which were analyzed for expression of CD11c (c), CD40 (f), CD86 (i) and CD83 (l) with positive stained cells (gray filled) and unstained cells (unfilled). The percentages of iMoDCs positive for CD11c (d), CD40 (g), CD86 (j) and CD83 (m) and surface expression levels shown as MFIs of CD11c (e), CD40 (h), CD86 (k) and CD83 (n) in individual mesothelioma patients (n = 46) and age-matched controls (n = 27) is shown. *p < 0.05.
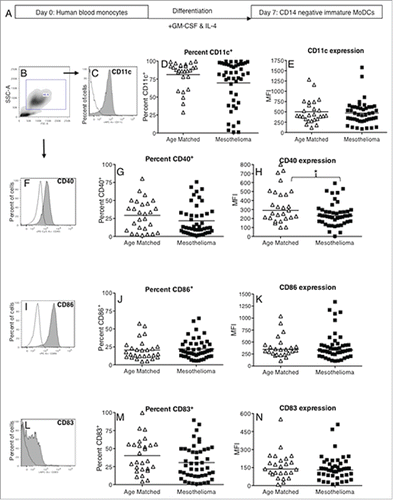
CD40 and CD86 are important co-stimulatory molecules and CD83 is a key maturation marker. No differences were seen in the percentage of cells expressing CD40, CD86, or CD83 (). No differences were seen for surface expression levels (MFI) of CD86 and CD83 () in mesothelioma vs. healthy controls. However, a statistically significant decrease was observed in mesothelioma patients for CD40 expression levels (: p = 0.04). Overall, these data imply that mesothelioma-derived monocytes can differentiate into immature CD14- MoDCs however, lower CD40 expression may interfere with their ability to be fully activated.
Mesothelioma-derived monocytes can differentiate into immature CD11c+CD1a+ DCs
CD11c+ DCs can differentiate into CD11c+CD1a+ DCs or CD11c+CD1a- DCs that play a pro-inflammatory or anti-inflammatory role respectivelyCitation30; the latter may prevent T cell activation. Therefore, iMoDCs were further gated as CD11c+ cells (Figs. S1a and b) then as CD1a+CD11c+ DCs (Fig. S1c). No differences were observed between MoDCs from mesothelioma patients and controls in either the percentage of CD11c+ cells expressing CD1a or CD1a expression levels (Figs. S1d and e). CD1a+CD11c+ DCs were further examined for expression of HLA-DR (a MHC class II molecule involved in antigen presentation to CD4+ T cells) and CD80 (a co-stimulatory molecule). Again, no differences were seen for the percentage of cells expressing HLA-DR (>99 % were HLA-DR+), CD80 (Fig. S1f), or for expression levels of HLA-DR (Fig. S1h) and CD80 (Fig. S1g). These data suggest that mesothelioma DCs maintain their ability to develop into pro-inflammatory CD11c+CD1a+ DCs.
Immature MoDCs from mesothelioma patients cannot process antigen
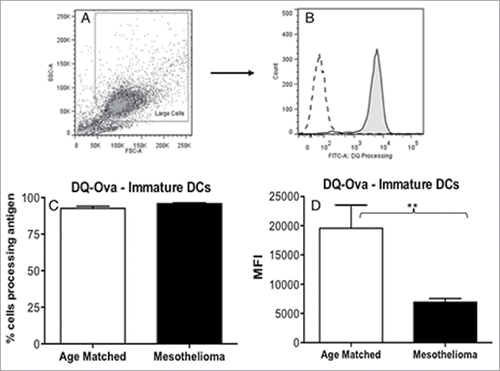
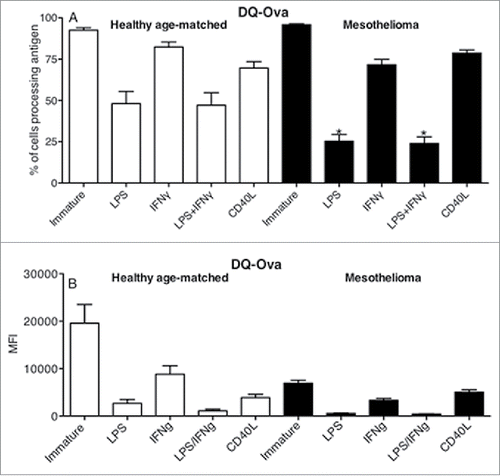
The primary role of immature DCs is to take up and process antigen. The DQ-OVA assay was used to compare the antigen-processing ability of MoDCs from mesothelioma patients vs. healthy volunteers. While no differences were observed for the percentage of MoDCs able to process antigen (), mesothelioma patients demonstrated significantly lower levels of antigen processing (MFIs) relative to healthy controls (: p = 0.004). These data reveal an important defect in antigen-processing ability in DCs from mesothelioma patients.
Figure 3. iMoDCs from mesothelioma patients have a reduced capacity to process antigen. Immature MoDCs from mesothelioma patients and age-matched controls were incubated for 1 h with DQ-Ovalbumin. Representative dot plot (a) showing gating of MoDCs based on size and granularity. The capacity to process antigen was determined by emission of a signal in the FITC channel (b) and measured by flow cytometry; gray histogram represents cells incubated with DQ-OVA, white histogram represents control cells that did not receive DQ-OVA. Pooled data (c) of the mean fluorescent intensity (MFI) indicating relative antigen-processing capacity of mesothelioma (n = 42) vs. age matched (n = 29) MoDCs is shown as mean ± SE. **p < 0.005.
MoDCs from mesothelioma patients cannot fully upregulate CD40 and CD86 following activation
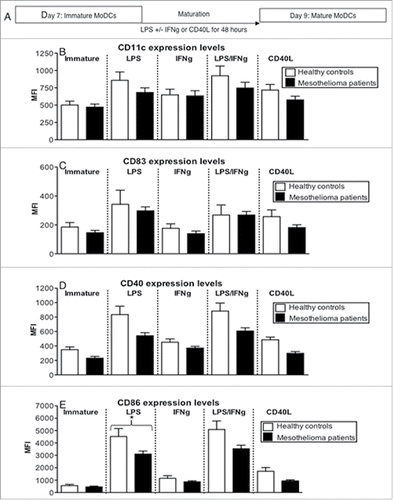
DCs need to be appropriately matured and express key antigen-presenting and co-stimulatory molecules before they can induce functional T cell responses. Their ability to respond to factors that activate DCs may also reveal a potential therapeutic approach. Therefore, we compared the maturational response of iMoDCs from mesothelioma patients and healthy volunteers to the microbial component lipopolysaccharide (LPS) with or without IFNγ, or to CD40L for 48 h. CD40L was included as CD40-targeting strategies are already available for cancer patients. No differences were seen between the percentage of MoDCs from mesothelioma patients and controls expressing CD11c, CD83, CD40 or CD86 after activation (Figs. S2a-d). With the exception of response to IFNγ maturation, there was a trend toward decreased expression levels of CD11c (), CD83 (), CD40 () in MoDCs from mesothelioma patients in response to each maturation stimulus. The only statistically significant decrease was seen for CD86 on mesothelioma MoDCs activated with LPS relative to age-matched controls (: p = 0.04).
Figure 4. MoDCs from mesothelioma patients do not fully upregulate CD83, CD40 and CD86 in response to maturation stimuli. Immature MoDCs generated from mesothelioma patients and age-matched volunteers were stimulated with LPS (a) and cell surface molecules analyzed by flow cytometry. Pooled data of the percentages of cells positive for CD11c (b), CD40 (d), CD86 (f) and CD83 (h). Surface expression levels were measured and shown as MFIs of CD11c (c), CD40 (e), CD86 (g) and CD83 (i) in mesothelioma patients (n = 46) vs. age matched (n = 27) iMoDCs. Pooled data is shown as mean ± SE.
There were no differences in the percentage of CD11c+ cells co-expressing CD1a (Fig. S3a) or CD1a expression levels (Fig. S4a). No differences between patients and controls were noted in the percentage of CD1a+CD11c+ DCs expressing CD80 or HLA-DR (Figs. S3b and c), although again, a trend toward decreased expression levels of CD80 and HLA-DR (Figs. S4b and c) was seen.
In general, LPS, with or without IFNγ, was the best inducer of likely beneficial phenotypic changes, while IFNγ and CD40L were the weakest. Taken together, these data imply that mesothelioma MoDCs do not achieve full activation relative to MoDCs from healthy controls.
MoDCs from mesothelioma patients lose antigen-processing function after activation
We further examined whether MoDCs from mesothelioma patients appropriately matured in response to stimuli by losing their capacity to process antigen. The healthy, age-matched (elderly) controls did not fully lose their antigen-processing ability after LPS+/-IFNγ stimulation with > 45% of matured MoDCs retaining their antigen-processing ability (), indicating an age-related defect (manuscript in preparation). In contrast, >75% of mesothelioma-derived LPS +/- IFNγ-matured MoDCs lost their ability to process antigen, this was matched by lower expression levels of degraded FITC-DQ-OVA indicating a low capacity to process antigen (). These data suggest that mesothelioma-derived MoDCs mature by loss of antigen processing better than their healthy counterparts. However, it should be noted that immature DCs from mesothelioma patients had a weaker baseline antigen-processing capacity ().
Figure 5. LPS-matured MoDCs from mesothelioma patients lose their capacity to process antigen. Immature and LPS+/-IFNγ or CD40L activated MoDCs from mesothelioma patients and healthy age-matched volunteers were incubated for 1 h with FITC-DQ-OVA. Pooled data of the percentage of DCs still processing antigen and MFIs indicating relative antigen-processing capacity is shown as mean ± SE from age-matched volunteers (n = 29) and mesothelioma patients (n = 42). *p < 0.05.
Mesothelioma MoDCs maintain their ability to induce T cell proliferation
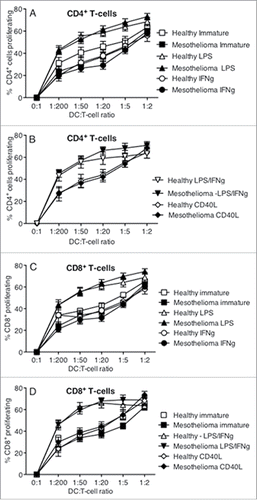
The ability of DCs to present antigen to T cells increases with activation/maturation. Therefore, activated matured MoDCs from mesothelioma patients and healthy controls were examined for their ability to induce proliferation of CFSE-labeled T cells from a universal healthy, male donor aged 34 y CD4 and CD8 T cells were identified by size and surface marker expression. No differences were noted in either CD4+ or CD8+ T cell proliferation induced by MoDCs from mesothelioma patients compared to healthy controls (). LPS, with or without IFNγ, proved to be the most potent stimulus, and CD40L the weakest, in terms of inducing T cell proliferation. These data show that MoDCs from mesothelioma patients are not defective in their ability to induce T cell proliferation.
Figure 6. LPS-matured DCs mesothelioma induce T cell proliferation. Immature and LPS+/-IFNγ or CD40L-activated MoDCs were co-cultured with allogeneic CFSE-labeled lymphocytes for 7 d. Cells were collected, stained for CD4+ and CD8+ expression and analyzed by flow cytometry. Lymphocytes were gated by size and CD4+ or CD8+ expression and the percentage of proliferating cells of total cells determined. Pooled data from healthy age-matched (n = 19) vs. mesothelioma patients (n = 23) MoDCs is shown as mean ± SE.
Mesothelioma MoDCs secrete similar levels of cytokines in response to activation
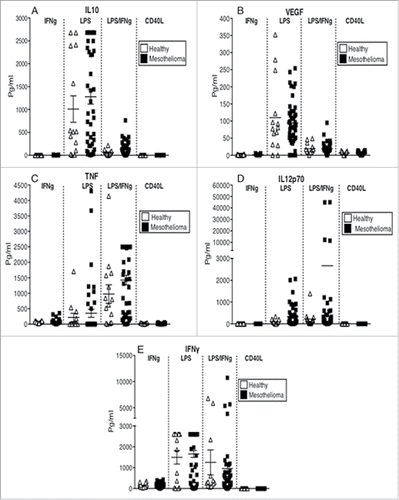
Cytokines produced by DCs after activation determine the immune response that will be ultimately generated, therefore culture media from activated MoDCs was analyzed by CBA. Regardless of the stimulus used, MoDCs from mesothelioma patients secreted similar levels of the suppressive cytokines IL-10 and VEGF (), as well as the pro-inflammatory cytokines TNF, IL-12p70 and IFNγ to their healthy counterparts (). These data show that MoDCs from mesothelioma patients are not defective in their cytokine responses. The dominating cytokines were IFNγ and IL-10 in response to LPS; IFNγ, IL-12p70 and TNF in response to LPS/IFNγ. CD40L and IFNγ were poor inducers of cytokine secretion.
Figure 7. MoDCs from mesothelioma patients secrete the same or higher levels of cytokines in response to stimulation. Culture media from LPS+/-IFNγ or CD40L-stimulated MoDCs from mesothelioma patients and healthy age-matched controls were analyzed by CBA for cytokine production. Pooled data for IL-10 (a), VEGF (b), TNF (c), IL12p70 (d) and IFNγ (e) secreted by MoDCs from mesothelioma patients (n = 45) and age-matched controls (n = 14) is shown as mean ± SE.
Increased circulating mDC1 numbers are associated with prolonged survival in mesothelioma patients
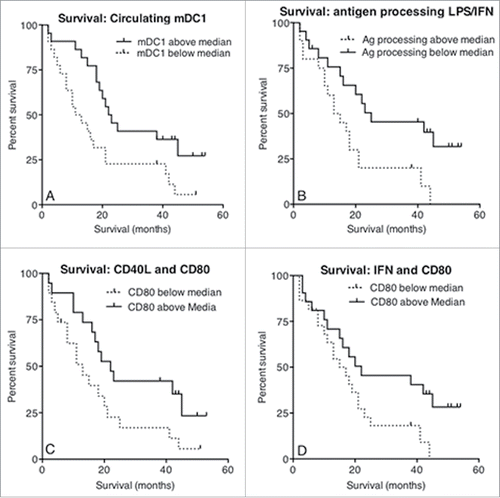
To investigate whether numbers of circulating DCs seen in people with mesothelioma reflected patient outcomes, patients were ranked and dichotomized into those above and below the median number of pDCs, mDC1s or mDC2s, and Kaplan–Meier plots generated. Patients with a higher than median number of circulating mDC1s demonstrated a significant increase in survival (; p = 0.02). In contrast, higher pDCs or mDC2 numbers did not predict for longer survival (data not shown).
Figure 8. Increased survival correlates with circulating mDC1s and MoDCs that respond appropriately to maturational stimuli. Whole blood from mesothelioma patients was analyzed for blood DC subpopulations and the number of circulating MDC1s plotted against survival from time of blood collection (a). The percentage of mesothelioma patient-derived LPS/IFNγ stimulated MoDCs able to process antigen measured using the DQ-OVA assay was plotted against survival (b). The percentage of cells expressing CD80 following stimulation with CD40L was plotted against survival (c). Similarly, CD80 expression levels (MFI) following stimulation with IFNγ were plotted against survival (d). All p values were determined using the Log-Rank Test.
Mesothelioma patients with MoDCs that maintain some ability to respond to maturation stimuli live longer
Loss of antigen-processing ability by immature DCs in response to activation stimuli is evidence of DC maturation, and patients with lower than median antigen-processing capacity after stimulation with LPS and IFNγ lived longer, with a median survival of 44 mo vs. > 55 mo. (; p = 0.01). Expression of key activation surface markers post stimulation with LPS+/-IFNγ or CD40L were also correlated against survival time. Only patients whose CD40L or IFNγ-matured MoDCs demonstrated a higher than median expression of CD80 experienced a significantly increased survival time (Fig. 8c; p = 0.04 and p = 0.045 respectively). Taken together, these data imply that patients with sufficient numbers of mDC1 and/or MoDCs that mature in response to stimuli experience a longer survival duration.
Discussion
Our study revealed that aging might impact on the immune system in people with mesothelioma, a cancer that is predominantly seen in the elderly due to the long latency between asbestos exposure and development of disease. Changes to circulating DC subsets and DCs derived from precursor monocyte cells were examined. We showed that pDC numbers were significantly reduced in the age group in which mesothelioma starts to emerge. However, we also showed that while the aging process likely contributes to reducing numbers of circulating pDCs, mesothelioma amplified this effect and also reduced the numbers of mDC1 and mDC2 cells. In regards to the latter finding, similar results have been reported in breast carcinoma, multiple myeloma, Kaposi's sarcoma and pancreatic adenocarcinoma.Citation19-21,Citation23-26 It is not yet clear if reduced circulating DC numbers reflect events occurring in tumors, regional lymph nodes or even in the bone marrow. One possibility is that tumor-associated factors interfere with the ability of bone marrow precursors to differentiate into DC subsets before they enter the bloodstream. Alternatively, tumor-associated DCs may lose their migratory capacity,Citation31,32 or undergo apoptosis.Citation33 Further studies are required, but candidate tumor-derived factors which may perturb DC differentiation or function include TGFβ, VEGF and IL-6, all of which are found in high concentration in pleural effusions associated with mesothelioma.Citation34 Loss of DC numbers and function will impair background antitumor immunity and the efficacy of subsequent antitumor immunotherapy, as shown by DC depletion studies in mice.Citation35
An important and novel observation found in these studies was that not only were numbers lower for all DC subsets in people with mesothelioma, but these lower numbers also predicted for shorter survival, at least for mDC1. These data raise the hypothesis that mDC1 cells contribute to patient survival likely via promoting protective immunity. This is supported by further data showing that patients whose MoDCs maintained their ability to increase CD80 expression in response to activation stimuli demonstrated increased survival. Interesting, others have shown that increased frequency of mDC1 after sunitinib treatment is predictive for tumor regression and improved progression-free survival in renal cancer patients.Citation36 This is important because of the increasing role of immunotherapy in the treatment of cancer, and the potential future application of this therapy to mesothelioma. Moreover, the data raise the possibility that targeting mDC1s or MoDCs using immunotherapy might prolong survival. These data also imply that there is a subgroup of mesothelioma patients whose DCs are not fully impaired and treating them with immunotherapy may extend survival duration. The use of immunotherapy is supported by murine studies that have demonstrated its potential for mesothelioma as a stand-alone therapy,Citation29,37-40 or in combination with chemotherapyCitation41-43 and/or surgical debulking of tumors.Citation44,45 The recent report of successful checkpoint blockade in mesothelioma suggests the possibility of combination of checkpoint blockade with strategies targeting impairment in DCs.Citation46
The most concerning effect of mesothelioma was loss of antigen-processing function in immature MoDCs. This is important as the data show that even in an environment removed from tumor-derived suppressive factors, iMoDCs from mesothelioma patients were unable to process antigen to the same levels as their healthy counterparts, implying irreversible mesothelioma tumor-induced dysfunction. Antigen processing is a key function of immature DCs, including those in the tumor microenvironment, as these cells are critically required to take up tumor antigen for presentation to T cells in draining lymph nodes. Loss of antigen-processing function was clearly mesothelioma-specific and independent of age, as iMoDCs from healthy aged-matched individuals maintained this ability. Again, this loss of function could have been driven by tumor-associated factors irreversibly affecting monocytic precursors. Similar results have been reported for late-stage breast cancer patients.Citation25 If this effect is exerted upon tissue and circulating DC subsets, low levels of antigen processing might prevent efficient antigen presentation to T cells following DC maturation. This is supported by murine studies showing defects in T cell function in mice with mesothelioma, wherein tumor antigen-specific T cells either accumulate in draining lymph nodes,Citation47 or leave lymph nodes but, without exogenous help, cannot prevent tumor progression.Citation29 These data suggest that identifying ways of rescuing antigen-processing function when tumor antigen-based vaccines are used might be an important immunotherapeutic strategy. Furthermore, given the clinical success of PD1 blockade and anti-CTLA4 blockade,Citation48,49,Citation50 rescuing DC function may be critical to the development of a CD8+ T cell response which can be released from inhibitory feedback
In healthy young adults, loss of antigen-processing function is associated with DC maturation as they transition to a cell that presents antigen in MHC molecules to T cells.Citation11 However, our data showed that healthy, elderly derived MoDCs retained some capacity to process antigen following activation with LPS and/or IFNγ implying an age-related inability to mature in a manner similar to young healthy controls. The mechanisms underlying this observation are unknown, but examining ROS within DCs may be useful, as studies of aged mice have shown that oxidative stress induces inadequate clearing of ROS (a hallmark of aging) leading to defects in antigen processing.Citation51 Mesothelioma-derived iMoDCs demonstrated lower baseline levels of antigen-processing ability which further reduced in response to LPS and/or IFNγ reduced implying some level of maturation, however this response did not correlate with an increase in the maturation marker, CD83. It is unclear whether this is due to the presence of ROS, or to defects in the mechanisms involved in downregulating antigen-processing function such as lower levels of TAP proteins, similar to that observed in cancer patients,Citation52 or if elderly derived MoDCs adopt a macrophage-like function and maintain their ability to process and destroy pathogens. Importantly, patients with a higher than median ability to downregulate antigen processing following activation with LPS and IFNγ lived significantly longer. This suggests that when the normal process of downregulation of antigen processing is retained in mesothelioma patient DCs, they mature appropriately and present tumor antigens to T cells to induce activated T cells that may slow tumor progression. Furthermore, patients maintaining DCs with an ability to mature once they have processed antigen are more likely to respond to vaccine-based immunotherapy. Therefore, screening maturation responses may be a potential predictive tool for this approach.
While mesothelioma-derived monocyte precursors responded to GM-CSF and IL-4 by differentiating into iMoDCs, including pro-inflammatory CD11c+CD1a+ DCs, there was evidence of functional impairment as they expressed lower baseline levels of co-stimulatory molecules, in particular CD40, which may interfere with their ability to be activated by therapeutic CD40-targetting strategies. This is supported by experiments attempting to activate mesothelioma-derived iMoDCs with LPS+/-IFNγ, or CD40L which revealed an inability to achieve full maturation relative to their healthy age-matched controls. A trend toward decreased expression of CD11c, CD83, CD40, CD80 and HLA-DR, with a significant decrease of CD86, was seen; all of which interfere with their ability to activate T cells. Similar deficiencies in expression of molecules involved in antigen presentation have been reported in late-stage breast cancer, squamous cell carcinomas and hepatocellular carcinoma.Citation20,21,Citation25,28,Citation53 Nonetheless, MoDCs from mesothelioma patients could induce allogeneic T cells to proliferate; it is possible that testing with antigen-specific T cells, such as those specific to tetanus toxoid, may have revealed greater differences between controls and patients. Our data contrasts with breast cancer studies showing that patient MoDCs had a decreased ability to induce allogeneic T cell proliferation.Citation25,54 These data imply differences between cancer types. The type of T cell generated is crucial as immunotherapeutic strategies aim to induce effector and not regulatory T cells. Further studies are required to examine the type of T cell generated by mesothelioma-derived DCs.
DCs respond to activation by secreting cytokines.Citation55 Our data show that MoDCs from mesothelioma patients are not defective in their cytokine responses. The cytokine response was determined by the stimulus used, with LPS/IFNγ inducing the strongest cytokine response from patients and controls alike. The dominating cytokines were IFNγ and TNF in response to LPS/IFNγ. Increased IFNγ and TNF indicate potential induction of a strong cell-mediated anti-mesothelioma immune response. However, while DCs from mesothelioma patients trended toward higher levels of secretion of three immune-stimulatory cytokines (including IL-12p70) in response to LPS/IFNγ, this coincided with a trend toward lower surface CD40 levels relative to healthy controls. One possible explanation is that as patient DCs also trended toward secreting higher levels of IL-10 and VEGF in response to LPS/IFNγ, these factors may reduce the impact of the immune-stimulatory cytokines on CD40 expression. Indeed, CD40 expression is reported to be lower on blood DCs from cancer patients and blocking all isoforms of VEGF in these patients restored CD40 expression.Citation56 LPS alone induced a mixed pro-inflammatory (IFNγ) and anti-inflammatory (IL-10 and VEGF) response which may thwart an antitumor immune response. These data are similar to studies in patients with Kaposi's sarcoma, a cancer associated with immune suppression, in which LPS induced a decrease in DC-derived IL-12 alongside an increase in IL-10 indicating a failure in the immune system's ability to keep the sarcoma in check.Citation23 Both CD40L and IFNγ were poor inducers of cytokine secretion. This cytokine milieu may be critical in driving T cell responses, even with lower expression of co-stimulatory molecules. Thus, choice of stimulus may be critical when designing a DC-targeting immunotherapy for mesothelioma. Our data show that the combination of LPS and IFNγ may be sufficient to induce a strong antitumor response while use of LPS or IFNγ alone, or CD40L, may only offer a limited benefit unless combined with another treatment modality. Interestingly, patients with a higher than median percentage of MoDCs expressing CD80 following CD40L activation lived longer. These data suggest that the co-stimulatory molecule CD80 may play an important role in increasing survival, and that therapies aiming to stimulate increased expression of CD80 should be further investigated.
Strengths of this study include use of age and gender-matched controls, immediate processing of fresh samples to avoid confounding factors introduced by freezing, plus use of a comprehensive panel to test DC attributes. The study had a small sample size however was adequately powered a priori to identify the primary endpoint of difference in DC numbers between patients and healthy controls. Differences in survival were identified on univariate analysis, and are provocative and hypothesis generating. We acknowledge that multivariate analysis incorporating other important prognostic factors such as histology and clinical status may be important, and that there may be confounding factors such as time from diagnosis. However, our sample size was not sufficient to perform multivariate analysis. We also relied on iMoDCs given the extremely low DC numbers in mesothelioma patients. MoDCs are an increasingly controversial cell type in regards to DCs; however, recent studies have shown that murine bone marrow cells cultured with GM-CSF generate a heterogeneous group of CD11c+ MHC class II+ DCs that comprise conventional DCs and monocyte-derived macrophages, with both undergoing maturation upon LPS stimulation but responding differentially and remaining as separable entities.Citation57 This may also be true for GM-CSF/IL-4-driven blood-derived human cells, therefore CD14- MoDCs remain useful to reveal differences between DCs from healthy elderly controls and cancer patients, both of whom are difficult groups to collect large blood volumes or perform serial sampling. Taken together, these data imply that mesothelioma affects DC maturation, likely through secretion of soluble factors. However, CD40L proved to be a weak DC stimulator in healthy, elderly control DCs, as well as in mesothelioma-derived DCs, relative to stimulation with LPS/IFNγ suggesting an age-related defect as well as a mesothelioma-driven one. These findings are important as CD40 activating strategies are being clinically tested in mesothelioma and other cancersCitation58,59; low levels of CD40 expression and a poor response to CD40 ligation in people with mesothelioma may make CD40 activation a less-attractive immunotherapy intervention. Future studies should look for expression of checkpoint inhibitory molecules on DCs, as high expression levels of the ligand for the checkpoint inhibitor, programmed cell death-1 (PD-1), on DCs in elderly hosts has been reportedCitation60; if this is true for DCs from mesothelioma patients then checkpoint blockade, which is in current clinical use in other indicationsCitation48,49,Citation50 and is undergoing clinical trials in mesothelioma,Citation61 could prove to be beneficial.
In conclusion, these studies demonstrated that a greater understanding is required of the effects of age and mesothelioma on DC subsets. We found specific age-related changes, in particular decreased circulating pDC numbers with further defects seen in people with mesothelioma including decreased circulating mDCs, decreased antigen processing in immature DCs, reduced expression of surface CD40 and a subsequent poor response to CD40 activation. Importantly, mesothelioma patients with higher numbers of circulating mDC1s and/or DCs that maintained one or more response to maturation signals lived longer. These studies show the importance of incorporating age-matched controls in order to distinguish effects of cancer from effects of aging. These data also suggest that functional DCs may contribute to survival in people with mesothelioma and that an immunotherapy tailored to improve DC numbers and function, particularly antigen processing, prior to DC maturation, could improve patient outcomes and may be important to include in combination immunotherapies. Moreover, our data suggests that immunotherapies involving DCs, such as cancer vaccines, could utilize a screening protocol to select for patients with functional DC.
Patients/materials & methods
Study subjects
Forty-eight people with mesothelioma were recruited for this study by three clinicians, with three additional patients recruited through radio advertising. Patients were excluded from the study if they had undergone active anticancer treatment (chemotherapy, radiotherapy, surgery) in the previous 9 mo. Forty age-matched healthy volunteers were by recruited by (i) radio advertising, (ii) print advertising in an elderly demographic newspaper, (iii) poster advertising and (iv) recruitment from laboratory volunteers. Healthy volunteers were excluded from the study if they currently had cancer, autoimmune disorders or other severe immune disorders. Written informed consent was obtained prior to blood collection. Health status was determined using a study-specific survey. This study was approved by the Human Ethics Committees for Sir Charles Gairdner Hospital (#2008-041); the Mount Hospital (#EC50.1), and Curtin University (#HR68/2008).
Collection of blood samples
Fifty mL of whole anti-coagulated blood was collected via mid-arm venepuncture into five 10-mL K2EDTA vacutainer tubes (BD PharMingen, USA) and transported to the laboratory for immediate processing.
Enumeration of DC subsets from whole blood
DC subsets were quantified using a Blood Dendritic Cell Enumeration Kit (Miltenyi-Biotec, Germany) as per manufacturer's instructions. Briefly, 300 μL of whole blood was incubated with 10 μL of an antibody cocktail containing BDCA1-PE (anti-human CD1c, clone AD5-8E7), BDCA2-FITC (anti-human CD303, clone AC144), BDCA3-APC (anti-human CD141, clone AD5-14H12), CD19-PeCy5 (B cells) and CD14-PeCy5 (monocytes) plus 5 μL of a dead cell discriminator (a fluorescent photolytic dye). The isotype control antibody cocktail containing mouse IgG2a-PE, IgG1-FITC, IgG1-APC, CD19-PeCy5 and CD14-PeCy5 and the dead cell discriminator were added to a control tube. All tubes were incubated horizontally for 10 min on ice under a 60 W globe to illuminate the dead cell discriminator with visible light which binds covalently and irreversibly to nucleic acids of dead cells. Red blood cells were lysed (1.55 M NH4CL, 0.1 M KHCO3, 1 mM EDTA, ph 7.4) at room temperature for 10 min in the dark and washing twice in FACS Buffer (1x PBS containing 1 % BSA (Sigma-Aldrich, USA), 2% FCS (Thermo Fisher Scientific, USA) and 0.01% sodium azide); the cell pellet was resuspended in 300 μL of FACS buffer, 150 μL of Fix Solution (3.7 % formaldehyde in PBS) and 5 μL Discriminator Stop Reagent (DNA in 10 mM TRIS, 10 mM NaCL, 1 mM EDTA, pH 8). Samples were analyzed on a FACSCantoII using Diva software (BD Biosciences). Absolute cell counts were determined by multiplying the number of DCs in the leukocyte gate () by the total number of PBMC in 50 mL determined by staining with trypan blue solution using a haemocytometer.
Isolation of peripheral blood mononuclear cells (PBMCs)
15 to 20 mL blood was aliquoted into 50 mL tubes and PBS (Invitrogen, USA) containing 2 mM EDTA (Sigma-Aldrich, USA) added to make a total volume of 35 mL per tube. The diluted blood was layered over 15 mL of Ficoll-paque PLUS (GE Healthcare, Sweden) and centrifuged at 400 g for 40 min at 20°C with the brake off. The interphase containing lymphocytes, monocytes and thrombocytes was removed and resuspended in 50 mL of PBS/2 mM EDTA. After centrifugation at 300 g for 10 min, the cells were resuspended in 2 mM EDTA and centrifuged at 200 g for 10 min at 20°C twice to remove platelets.
In vitro generation of monocyte-derived DCs
DCs were prepared using a modified procedure.Citation62 Briefly, 1 × 108 PBMCs were allowed to adhere to a 75 cm2 tissue culture flask in RPMI (Invitrogen, USA) media containing 10 % FCS, 50 μM 2ME (Sigma-Aldrich, USA), 100 U/mL Penicillin and 50 mg/mL Gentamycin. Following a 2 h incubation at 37°C in 5 % CO2, non-adherent cells were removed and the remaining adherent cells (monocytes) cultured for 7 d in 80 ng/mL GM-CSF (Shenandoah, USA) and 10 ng/mL IL-4 (R&D Systems, USA) added on day 0 and supplemented on day 4. The MoDC cultures continuously contained 10 μg/mL Polymixin-B (Sigma-Aldrich, USA) to inactivate LPS.
Lymphocyte isolation
Peripheral blood mononuclear cells (1 × 108 cells) were left to adhere to a 75 cm2 tissue culture flask in RPMI media containing 10 % FCS, 50 μM 2ME, 100 U/mL Penicillin and 50 mg/mL Gentamycin. Following a 2 h incubation at 37°C, 5 % CO2, the non-adherent cells predominantly lymphocyte population were collected for use as responder lymphocytes in the mixed lymphocyte reaction (MLR).
MoDC stimulation
On day 7, non-adherent immature MoDCs were washed twice by centrifugation at 1200 rpm for 5 min, placed into 75 cm2 flasks, topped with 15 mL culture media, 80 ng/mL GM-CSF and 10 ng/mL IL-4. MoDCs were stimulated for 2 d with either 10 μg/mL LPS (Sigma-Aldrich, USA), 20 ng/mL IFNγ (Sigma-Aldrich, USA), 10 μg/mL LPS and 20 ng/mL IFNγ, or 0.66 μg/mL CD40L (Genscript, USA). One flask was used as a no-stimuli control containing 10 μg/mL Polymixin-B (Sigma-Aldrich, USA).
DC phenotyping
Cells were stained for CD1a-PeCy5 (clone HI149), CD11c-APC (clone B-ly6), CD14-FITC (clone M5E2), CD40-PeCy5 (clone 5C3), CD80-PE (clone 2D10), CD83-APC (clone HB15E), CD86-PE (clone 2331 (FUN-1)) and HLA-DR-APC-Cy7 (clone L242), all purchased from BD PharMingen, USA. Cells were incubated for 30 min at 4°C in the dark, washed in PBS and cell surface expression measured by flow cytometry using a BD FACSCanto II and Diva or Flow Jo (Tree Star Inc.) software.
Antigen uptake and processing assay
MoDCs (5 × 104 cells) in 100 µL of media were incubated with 1 µL of 1 mg/mL DQ-conjugated ovalbumin (DQ-OVA; Invitrogen) for 1 h at 37°C. Controls included cells incubated with DQ-OVA at 4°C and without DQ-OVA at 37°C and 4°C. Following washing, cells were analyzed by flow cytometry. MoDCs were gated for by size then for degradation of FITC-labeled DQ-Ovalbumin (DQ-OVA), which indicates antigen processing. Antigen uptake and processing was calculated as follows: [(Median Fluorescence intensity (MFI) of 37°C with DQ-OVA) – (MFI of 37°C without DQ-OVA)] – [(MFI of 4° C with DQ-OVA) – (MFI of 4°C without DQ-OVA)]. An increase in the calculated MFI corresponds to an increase in antigen uptake and processing ability.
CFSE labeling T cells
Following incubation of PBMCs for 2 h, the non-adherent population containing monocyte-depleted T-cell-enriched cells was collected, washed in PBS and resuspended at 2 × 107 cells/mL in RPMI media containing 3.5 μM carboxyfluorescein diacetate succinimidyl ester (CFSE; Sigma-Aldrich, USA), a fluorescent dye that binds to cell membranes.Citation63 Cells were incubated at room temperature for 10 min, washed three times with RPMI and used as responder T cells for the MLR.
The mixed lymphocyte reaction (MLR)
MoDCs were seeded in duplicate into a 96-well plate at concentrations ranging from 1 × 103 to 1 × 105 cells/mL and 2 × 105 CFSE-labeled allogeneic T cells, from a universal, 34 y old male donor, added to each well. Control wells contained MoDCs alone, T cells alone and T cells stimulated with Concanavalin A (Con A; Sigma-Aldrich, USA). Plates were incubated in the dark at 37°C 5% CO2 for 8 d The cells were then washed and stained for CD4 and CD8 expression using CD4-PE (clone RPA-T4) and CD8-APC (clone RPA-T8; BD Pharmingen, USA), for 30 min at 4°C in the dark. Cells were washed for analysis by flow cytometry. The parent T cell population was identified based on the CFSE staining intensity of the T cells alone control. As T cells proliferate, each new daughter generation contains half the CFSE of the previous generation resulting in a sequential halving of CFSE staining intensity, represented as individual peaks during flow cytometry, as we have published.Citation63 The percentage of T cells proliferating was calculated by gating all CFSE+ cells and excluding the non-proliferating parent peak.
Cytokine Bead Array (CBA)
The cytokines TNFα, IL-10, IL-12(p70), VEGF and IFNγ were measured simultaneously by CBA (BD PharMingen, USA) as per the manufacturer's protocol.
Statistics
A sample size of 40 patients and 40 healthy controls was planned, as this was sufficient to detect a mean difference in DC cell count between patients and healthy controls of 20%, with a standard deviation of 25% of baseline and setting α = 0.0125 (due to multiple comparisons) and β = 0.80, allowing for 10% loss to follow up.
Statistical analyses were conducted using GraphPad Prism v4.03 (USA). Statistical differences were determined by a two-tailed Mann–Witney t-test and linear regression of continuous data. p values less than 0.05 were considered statistically significant. Overall survival was considered from the date of study entry to the date of death. Participants who were still alive were censored at the date of their last confirmed clinical appointment. Survival analyses were performed using the Log-Rank test and displayed on a Kaplan Meier plot.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
1082028_supplemental_figures.pdf
Download PDF (286.6 KB)Acknowledgments
We thank the Slater and Gordon Asbestos Research Fund for supporting this project. The authors acknowledge the provision of research facilities and the scientific and technical assistance of the staff of CHIRI Biosciences Research Precinct core facility, Curtin University.
References
- Robinson BW, Lake RA. Advances in malignant mesothelioma. N Engl J Med 2005; 353:1591-603; PMID:16221782; http://dx.doi.org/10.1056/NEJMra050152
- Kao SC, Pavlakis N, Harvie R, Vardy JL, Boyer MJ, van Zandwijk N, Clarke SJ. High blood neutrophil-to-lymphocyte ratio is an indicator of poor prognosis in malignant mesothelioma patients undergoing systemic therapy. Clin Cancer Res 2010; 16:5805-13; PMID:20956618; http://dx.doi.org/10.1158/1078-0432.CCR-10-2245
- Linton A, Pavlakis N, O'Connell R, Soeberg M, Kao S, Clarke S, Vardy J, van Zandwijk N. Factors associated with survival in a large series of patients with malignant pleural mesothelioma in New South Wales. Br J Cancer 2014; 111:1860-9; PMID:25188323; http://dx.doi.org/10.1038/bjc.2014.478
- Bianchi C, Giarelli L, Grandi G, Brollo A, Ramani L, Zuch C. Latency periods in asbestos-related mesothelioma of the pleura. Eur J Cancer Prev 1997; 6:162-6; PMID:9237066; http://dx.doi.org/10.1159/000343599
- Fulop T, Larbi A, Kotb R, Pawelec G. Immunology of aging and cancer development. Interdiscip Top Gerontol 2013; 38:38-48; PMID:23503514; http://dx.doi.org/10.1159/000343599
- Gravekamp C, Jahangir A. Is cancer vaccination feasible at older age? Exp Gerontol 2014; 54:138-44; PMID:24509231; http://dx.doi.org/10.1016/j.exger.2014.01.025
- Izzi V, Masuelli L, Tresoldi I, Foti C, Modesti A, Bei R. Immunity and malignant mesothelioma: from mesothelial cell damage to tumor development and immune response-based therapies. Cancer Lett 2012; 322:18-34; PMID:22394996; http://dx.doi.org/10.1016/j.canlet.2012.02.034
- Jackaman C, Cornwall S, Lew AM, Zhan Y, Robinson BW, Nelson DJ. Local effector failure in mesothelioma is not mediated by CD4+ CD25+ T-regulator cells. Eur Respir J 2009; 34:162-75; PMID:19251786; http://dx.doi.org/10.1183/09031936.00101008
- Hassan R, Miller AC, Sharon E, Thomas A, Reynolds JC, Ling A, Kreitman RJ, Miettinen MM, Steinberg SM, Fowler DH et al. Major cancer regressions in mesothelioma after treatment with an anti-mesothelin immunotoxin and immune suppression. Sci Transl Med 2013; 5:208ra147; PMID:24154601; http://dx.doi.org/10.1126/scitranslmed.3006941
- Calabro L, Morra A, Fonsatti E, Cutaia O, Fazio C, Annesi D, Lenoci M, Amato G, Danielli R, Altomonte M et al. Efficacy and safety of an intensified schedule of tremelimumab for chemotherapy-resistant malignant mesothelioma: an open-label, single-arm, phase 2 study. Lancet Respir Med 2015; 3(4):301-9; PMID:25819643; http://dx.doi.org/10.1016/S2213-2600(15)00092-2
- Banchereau J, Briere F, Caux C, Davoust J, Lebecque S, Liu YJ, Pulendran B, Palucka K. Immunobiology of dendritic cells. Annu Rev Immunol 2000; 18:767-811; PMID:10837075; http://dx.doi.org/10.1146/annurev.immunol.18.1.767
- Caux C, Vanbervliet B, Massacrier C, Dezutter-Dambuyant C, de Saint-Vis B, Jacquet C, Yoneda K, Imamura S, Schmitt D, Banchereau J. CD34+ hematopoietic progenitors from human cord blood differentiate along two independent dendritic cell pathways in response to GM-CSF+TNF α. J Exp Med 1996; 184:695-706; PMID:8760823; http://dx.doi.org/10.1084/jem.184.2.695
- Rissoan MC, Soumelis V, Kadowaki N, Grouard G, Briere F, de Waal Malefyt R, Liu YJ. Reciprocal control of T helper cell and dendritic cell differentiation. Science 1999; 283:1183-6; PMID:10024247; http://dx.doi.org/10.1126/science.283.5405.1183
- Robbins SH, Walzer T, Dembele D, Thibault C, Defays A, Bessou G, Xu H, Vivier E, Sellars M, Pierre P et al. Novel insights into the relationships between dendritic cell subsets in human and mouse revealed by genome-wide expression profiling. Genome Biol 2008; 9:R17; PMID:18218067; http://dx.doi.org/10.1186/gb-2008-9-1-r17
- Blom B, Ho S, Antonenko S, Liu YJ. Generation of interferon α-producing predendritic cell (Pre-DC)2 from human CD34(+) hematopoietic stem cells. J Exp Med 2000; 192:1785-96; PMID:11120775; http://dx.doi.org/10.1084/jem.192.12.1785
- Grouard G, Rissoan MC, Filgueira L, Durand I, Banchereau J, Liu YJ. The enigmatic plasmacytoid T cells develop into dendritic cells with interleukin (IL)-3 and CD40-ligand. J Exp Med 1997; 185:1101-11; PMID:9091583; http://dx.doi.org/10.1084/jem.185.6.1101
- Soumelis V, Liu YJ. From plasmacytoid to dendritic cell: morphological and functional switches during plasmacytoid pre-dendritic cell differentiation. Eur J Immunol 2006; 36:2286-92; PMID:16892183; http://dx.doi.org/10.1002/eji.200636026
- Hart DN. Dendritic cells: unique leukocyte populations which control the primary immune response. Blood 1997; 90:3245-87; PMID:9345009
- Hoffmann TK, Muller-Berghaus J, Ferris RL, Johnson JT, Storkus WJ, Whiteside TL. Alterations in the frequency of dendritic cell subsets in the peripheral circulation of patients with squamous cell carcinomas of the head and neck. Clin Cancer Res 2002; 8:1787-93; PMID:12060618
- Ma XJ, Pan XL, Lv ZH, Xu FL, Liu da Y, Lei da P, Xia M, Luan XY. Therapeutic influence on circulating and monocyte-derived dendritic cells in laryngeal squamous cell carcinoma patients. Acta Otolaryngol 2009; 129:84-91; PMID:18607895; http://dx.doi.org/10.1080/00016480802020459
- Sakakura K, Chikamatsu K, Takahashi K, Whiteside TL, Furuya N. Maturation of circulating dendritic cells and imbalance of T-cell subsets in patients with squamous cell carcinoma of the head and neck. Cancer Immunol Immunother 2006; 55:151-9; PMID:15889251; http://dx.doi.org/10.1007/s00262-005-0697-y
- Sciarra A, Lichtner M, Autran GA, Mastroianni C, Rossi R, Mengoni F, Cristini C, Gentilucci A, Vullo V, Di Silverio F. Characterization of circulating blood dendritic cell subsets DC123+ (lymphoid) and DC11C+ (myeloid) in prostate adenocarcinoma patients. Prostate 2007; 67:1-7; PMID:17075798; http://dx.doi.org/10.1002/pros.20431
- Della Bella S, Nicola S, Brambilla L, Riva A, Ferrucci S, Presicce P, Boneschi V, Berti E, Villa ML. Quantitative and functional defects of dendritic cells in classic Kaposi's sarcoma. Clin Immunol 2006; 119:317-29; PMID:16527545; http://dx.doi.org/10.1016/j.clim.2006.01.011
- Harrison SJ, Franklin IM, Campbell JD. Enumeration of blood dendritic cells in patients with multiple myeloma at presentation and through therapy. Leuk Lymphoma 2008; 49:2272-83; PMID:19052974; http://dx.doi.org/10.1080/10428190802464729
- Pinzon-Charry A, Ho CS, Maxwell T, McGuckin MA, Schmidt C, Furnival C, Pyke CM, López JA. Numerical and functional defects of blood dendritic cells in early- and late-stage breast cancer. Br J Cancer 2007; 97:1251-9; PMID:17923873; http://dx.doi.org/10.1038/sj.bjc.6604018
- Tjomsland V, Sandstrom P, Spangeus A, Messmer D, Emilsson J, Falkmer U, Falkmer S, Magnusson KE, Borch K, Larsson M. Pancreatic adenocarcinoma exerts systemic effects on the peripheral blood myeloid and plasmacytoid dendritic cells: an indicator of disease severity? BMC Cancer 2010; 10:87; PMID:20214814; http://dx.doi.org/10.1186/1471-2407-10-87
- Troy A, Davidson P, Atkinson C, Hart D. Phenotypic characterisation of the dendritic cell infiltrate in prostate cancer. J Urol 1998; 160:214-9; PMID:9628653; http://dx.doi.org/10.1016/S0022-5347(01)63093-3
- Ninomiya T, Akbar SM, Masumoto T, Horiike N, Onji M. Dendritic cells with immature phenotype and defective function in the peripheral blood from patients with hepatocellular carcinoma. J Hepatol 1999; 31:323-31; PMID:10453947; http://dx.doi.org/10.1016/S0168-8278(99)80231-1
- Jackaman C, Bundell CS, Kinnear BF, Smith AM, Filion P, van Hagen D, Robinson BW, Nelson DJ. IL-2 intratumoral immunotherapy enhances CD8+ T cells that mediate destruction of tumor cells and tumor-associated vasculature: a novel mechanism for IL-2. J Immunol 2003; 171:5051-63; PMID:14607902; http://dx.doi.org/10.4049/jimmunol.171.10.5051
- Cernadas M, Lu J, Watts G, Brenner MB. CD1a expression defines an interleukin-12 producing population of human dendritic cells. Clin Exp Immunol 2009; 155:523-33; PMID:19220838; http://dx.doi.org/10.1111/j.1365-2249.2008.03853.x
- Zeng Z, Xu X, Zhang Y, Xing J, Long J, Gu L, Wang X, Sun D, Ka W, Yao W et al. Tumor-derived factors impaired motility and immune functions of dendritic cells through derangement of biophysical characteristics and reorganization of cytoskeleton. Cell Motil Cytoskeleton 2007; 64:186-98; PMID:17183544; http://dx.doi.org/10.1002/cm.20175
- Imai K, Minamiya Y, Koyota S, Ito M, Saito H, Sato Y, Motoyama S, Sugiyama T, Ogawa J. Inhibition of dendritic cell migration by transforming growth factor-beta1 increases tumor-draining lymph node metastasis. J Exp Clin Cancer Res 2012; 31:3; http://dx.doi.org/10.1186/1756-9966-31-3
- Esche C, Gambotto A, Satoh Y, Gerein V, Robbins PD, Watkins SC, Lotze MT, Shurin MR. CD154 inhibits tumor-induced apoptosis in dendritic cells and tumor growth. Eur J Immunol 1999; 29:2148-55; PMID:10427977; http://dx.doi.org/10.1002/(SICI)1521-4141(199907)29:07%3c2148::AID-IMMU2148%3e3.0.CO;2-F
- DeLong P, Carroll RG, Henry AC, Tanaka T, Ahmad S, Leibowitz MS, Sterman DH, June CH, Albelda SM, Vonderheide RH. Regulatory T cells and cytokines in malignant pleural effusions secondary to mesothelioma and carcinoma. Cancer Biol Ther 2005; 4:342-6; PMID:15846066; http://dx.doi.org/10.4161/cbt.4.3.1644
- Murillo O, Dubrot J, Palazon A, Arina A, Azpilikueta A, Alfaro C, Solano S, Ochoa MC, Berasain C, Gabari I et al. In vivo depletion of DC impairs the anti-tumor effect of agonistic anti-CD137 mAb. Eur J Immunol 2009; 39:2424-36; PMID:19662633; http://dx.doi.org/10.1002/eji.200838958
- van Cruijsen H, van der Veldt AA, Vroling L, Oosterhoff D, Broxterman HJ, Scheper RJ, Giaccone G, Haanen JB, van den Eertwegh AJ, Boven E et al. Sunitinib-induced myeloid lineage redistribution in renal cell cancer patients: CD1c+ dendritic cell frequency predicts progression-free survival. Clin Cancer Res 2008; 14:5884-92; PMID:18794101; http://dx.doi.org/10.1158/1078-0432.CCR-08-0656
- Jackaman C, Cornwall S, Graham PT, Nelson DJ. CD40-activated B cells contribute to mesothelioma tumor regression. Immunol Cell Biol 2011; 89:255-67; PMID:20628372; http://dx.doi.org/10.1038/icb.2010.88
- Jackaman C, Lew AM, Zhan Y, Allan JE, Koloska B, Graham PT, Robinson BW, Nelson DJ. Deliberately provoking local inflammation drives tumors to become their own protective vaccine site. Int Immunol 2008; 20:1467-79; PMID:18824504; http://dx.doi.org/10.1093/intimm/dxn104
- Jackaman C, Lansley S, Allan JE, Robinson BW, Nelson DJ. IL-2/CD40-driven NK cells install and maintain potency in the anti-mesothelioma effector/memory phase. Int Immunol 2012; 24:357-68; PMID:22354912; http://dx.doi.org/10.1093/intimm/dxs005
- Broomfield SA, van der Most RG, Prosser AC, Mahendran S, Tovey MG, Smyth MJ, Robinson BW, Currie AJ. Locally administered TLR7 agonists drive systemic antitumor immune responses that are enhanced by anti-CD40 immunotherapy. J Immunol 2009; 182:5217-24; PMID:19380767; http://dx.doi.org/10.4049/jimmunol.0803826
- McDonnell AM, Lesterhuis WJ, Khong A, Nowak AK, Lake RA, Currie AJ, Robinson BW. Tumor-infiltrating dendritic cells exhibit defective cross-presentation of tumor antigens, but is reversed by chemotherapy. Eur J Immunol 2015; 45:49-59; PMID:25316312; http://dx.doi.org/10.1002/eji.201444722
- Lesterhuis WJ, Salmons J, Nowak AK, Rozali EN, Khong A, Dick IM, Harken JA, Robinson BW, Lake RA. Synergistic effect of CTLA-4 blockade and cancer chemotherapy in the induction of anti-tumor immunity. PloS one 2013; 8:e61895; PMID:23626745; http://dx.doi.org/10.1371/journal.pone.0061895
- Nowak AK, Robinson BW, Lake RA. Synergy between chemotherapy and immunotherapy in the treatment of established murine solid tumors. Cancer Res 2003; 63:4490-6; PMID:12907622
- Broomfield S, Currie A, van der Most RG, Brown M, van Bruggen I, Robinson BW, Lake RA. Partial, but not complete, tumor-debulking surgery promotes protective antitumor memory when combined with chemotherapy and adjuvant immunotherapy. Cancer Res 2005; 65:7580-4; PMID:16140921; http://dx.doi.org/10.1158/0008-5472.CAN-05-0328
- Khong A, Cleaver AL, Fahmi Alatas M, Wylie BC, Connor T, Fisher SA, Broomfield S, Lesterhuis WJ, Currie AJ, Lake RA et al. The efficacy of tumor debulking surgery is improved by adjuvant immunotherapy using imiquimod and anti-CD40. BMC Cancer 2014; 14:969; PMID:25518732; http://dx.doi.org/10.1186/1471-2407-14-969
- Alley EW, Molife LR, Santoro A, Beckey K, Yuan S, Cheng JD, Piperdi B, Schellens JHM. Clinical safety and efficacy of pembrolizumab (MK-3475) in patients with malignant pleural mesothelioma: Preliminary results from KEYNOTE-028 American Association for Cancer Research conference abstract number CT103 2015
- Stumbles PA, Himbeck R, Frelinger JA, Collins EJ, Lake RA, Robinson BW. Cutting edge: tumor-specific CTL are constitutively cross-armed in draining lymph nodes and transiently disseminate to mediate tumor regression following systemic CD40 activation. J Immunol 2004; 173:5923-8; PMID:15528325; http://dx.doi.org/10.4049/jimmunol.173.10.5923
- Homet Moreno B, Parisi G, Robert L, Ribas A. Anti-PD-1 Therapy in Melanoma. Semin Oncol 2015; 42:466-73; PMID:25965365; http://dx.doi.org/10.1053/j.seminoncol.2015.02.008
- Di Giacomo AM, Danielli R, Guidoboni M, Calabro L, Carlucci D, Miracco C, Volterrani L, Mazzei MA, Biagioli M, Altomonte M et al. Therapeutic efficacy of ipilimumab, an anti-CTLA-4 monoclonal antibody, in patients with metastatic melanoma unresponsive to prior systemic treatments: clinical and immunological evidence from three patient cases. Cancer Immunol Immunother 2009; 58:1297-306; PMID:19139884; http://dx.doi.org/10.1007/s00262-008-0642-y
- Ott PA, Hodi FS, Robert C. CTLA-4 and PD-1/PD-L1 blockade: new immunotherapeutic modalities with durable clinical benefit in melanoma patients. Clin Cancer Res 2013; 19:5300-9; PMID:24089443; http://dx.doi.org/10.1158/1078-0432.CCR-13-0143
- Cannizzo ES, Clement CC, Morozova K, Valdor R, Kaushik S, Almeida LN, Follo C, Sahu R, Cuervo AM, Macian F et al. Age-related oxidative stress compromises endosomal proteostasis. Cell Rep 2012; 2:136-49; PMID:22840404; http://dx.doi.org/10.1016/j.celrep.2012.06.005
- Whiteside TL, Stanson J, Shurin MR, Ferrone S. Antigen-processing machinery in human dendritic cells: up-regulation by maturation and down-regulation by tumor cells. J Immunol 2004; 173:1526-34; PMID:15265880; http://dx.doi.org/10.4049/jimmunol.173.3.1526
- Onishi H, Morisaki T, Baba E, Kuga H, Kuroki H, Matsumoto K, Tanaka M, Katano M. Dysfunctional and short-lived subsets in monocyte-derived dendritic cells from patients with advanced cancer. Clin Immunol 2002; 105:286-95; PMID:12498810; http://dx.doi.org/10.1006/clim.2002.5293
- Gabrilovich DI, Corak J, Ciernik IF, Kavanaugh D, Carbone DP. Decreased antigen presentation by dendritic cells in patients with breast cancer. Clin Cancer Res 1997; 3:483-90; PMID:9815709.
- Shortman K, Liu YJ. Mouse and human dendritic cell subtypes. Nat Rev Immunol 2002; 2:151-61; PMID:11913066; http://dx.doi.org/10.1038/nri746
- Fricke I, Mirza N, Dupont J, Lockhart C, Jackson A, Lee JH, Sosman JA, Gabrilovich DI. Vascular endothelial growth factor-trap overcomes defects in dendritic cell differentiation but does not improve antigen-specific immune responses. Clin Cancer Res 2007; 13:4840-8; PMID:17699863; http://dx.doi.org/10.1158/1078-0432.CCR-07-0409
- Helft J, Bottcher J, Chakravarty P, Zelenay S, Huotari J, Schraml BU, Goubau D, Reis E Sousa C. GM-CSF Mouse Bone Marrow Cultures Comprise a Heterogeneous Population of CD11c(+)MHCII(+) Macrophages and Dendritic Cells. Immunity 2015; 42:1197-211; PMID:26084029; http://dx.doi.org/10.1016/j.immuni.2015.05.018
- Sterman DH, Recio A, Carroll RG, Gillespie CT, Haas A, Vachani A, Kapoor V, Sun J, Hodinka R, Brown JL et al. A phase I clinical trial of single-dose intrapleural IFN-beta gene transfer for malignant pleural mesothelioma and metastatic pleural effusions: high rate of antitumor immune responses. Clin Cancer Res 2007; 13:4456-66; PMID:17671130; http://dx.doi.org/10.1158/1078-0432.CCR-07-0403
- Khong A, Nelson DJ, Nowak AK, Lake RA, Robinson BW. The use of agonistic anti-CD40 therapy in treatments for cancer. Int Rev Immunol 2012; 31:246-66; PMID:22804570; http://dx.doi.org/10.3109/08830185.2012.698338
- Lages CS, Lewkowich I, Sproles A, Wills-Karp M, Chougnet C. Partial restoration of T-cell function in aged mice by in vitro blockade of the PD-1/ PD-L1 pathway. Aging cell 2010; 9:785-98; PMID:20653631; http://dx.doi.org/10.1111/j.1474-9726.2010.00611.x
- Calabro L, Morra A, Fonsatti E, Cutaia O, Amato G, Giannarelli D, Di Giacomo AM, Danielli R, Altomonte M, Mutti L et al. Tremelimumab for patients with chemotherapy-resistant advanced malignant mesothelioma: an open-label, single-arm, phase 2 trial. Lancet Oncol 2013; 14:1104-11; PMID:24035405; http://dx.doi.org/10.1016/S1470-2045(13)70381-4
- Romani N, Gruner S, Brang D, Kampgen E, Lenz A, Trockenbacher B, Konwalinka G, Fritsch PO, Steinman RM, Schuler G. Proliferating dendritic cell progenitors in human blood. J Exp Med 1994; 180:83-93; PMID:8006603; http://dx.doi.org/10.1084/jem.180.1.83
- Nelson D, Bundell C, Robinson B. In vivo cross-presentation of a soluble protein antigen: kinetics, distribution, and generation of effector CTL recognizing dominant and subdominant epitopes. J Immunol 2000; 165:6123-32; PMID:11086045; http://dx.doi.org/10.4049/jimmunol.165.11.6123
