ABSTRACT
Background & Aims: There is no generally accepted adjuvant therapy for hepatocellular carcinoma (HCC) after curative resection. Autologous cytokine-induced killer (CIK) cells therapy has been reported to improve outcomes of patients with HCC, but its role as an adjuvant therapy remains unclear. This study aimed to evaluate the efficacy and safety of CIK as an adjuvant therapy for HCC after curative resection.
Methods: This is a single center, phase 3, open label, randomized controlled trial (RCT). Two hundred patients who were initially diagnosed with HCC of Barcelona Clinic Liver Cancer (BCLC) stage A or B, and underwent curative hepatectomy were randomly assigned to receive four cycles of CIK treatment (the CIK group, n = 100) or no treatment (the control group, n = 100). The primary outcome was time to recurrence. The secondary outcomes included disease-free survival (DFS), overall survival (OS) and adverse events.
Results: All patients in the CIK group finished the treatment by protocol. The median time to recurrence (TTR) was 13.6 (IQR 6.5–25.2) mo in the CIK group and 7.8 (IQR 2.7–17.0) mo in the control group (p = 0.01). There were no significant differences between the groups in DFS and OS. All adverse events were grade 1 or 2. There were no significant differences in incidence between the two groups.
Conclusions: Four cycles of CIK therapy were safe and effective to prolong the median TTR in patients with HCC after curative resection, but the treatment did not improve the DFS and OS.
Abbreviations
| AFP | = | Alpha fetoprotein |
| BCLC | = | Barcelona Clinic Liver Cancer |
| CD3 | = | cluster of differentiation 3 |
| CI | = | confidence intervals |
| CIK | = | cytokine-induced killer cells |
| CT | = | computed tomography |
| DFS | = | disease free survival |
| EBL | = | estimated blood loss |
| ECOG PS | = | Eastern Cooperative Oncology Group performance status |
| ETV | = | Entecavir |
| GCP | = | Good Clinical Practice |
| HCC | = | hepatocellular carcinoma |
| IFN-c | = | interferon-c |
| IL | = | interleukin |
| IQR | = | Interquartile range |
| ITT | = | intention-to-treat |
| LAM | = | Lamivudine |
| LOS | = | Length of hospital stay |
| KM | = | Kaplan-Meier |
| MRI | = | magnetic resonance imaging |
| NK | = | natural killer |
| NLR | = | neutrophils to lymphocytes ratio |
| OS | = | overall survival |
| PBMC | = | peripheral blood mononuclear cells |
| Post/Pre-op AFP | = | Post-operative to pre-operative α fetoprotein ratio |
| RCT | = | randomized controlled trials |
| RECIST | = | Response Evaluation Criteria in Solid Tumors |
| rhIL-2 | = | recombinant Interleukin-2 |
| SYSU | = | Sun Yat-sen University |
| Tα1 | = | Thymosin α1 for injection |
| TP5 | = | Thymopentin for injection |
| TTR | = | time to recurrence |
| WBC | = | white blood cells |
Introduction
HCC is the fifth most common cancer and the third most common cause of cancer-related mortality in the world.Citation1 Resection is still the main choice of treatment for patients with early stage HCC and well-compensated liver function.Citation2 Unfortunately, recurrence after resection occurs in up to 80% of patients within five years following surgery, which together with concomitant hepatic decompensation, are the main causes of death.Citation3 Several adjuvant treatments including arterial infusional chemotherapy,Citation4 I131 lipiodol,Citation5,6 and interferonCitation7,8 have been used. Unfortunately, the clinical use of these adjuvant therapies is either controversial or requires further evaluation.Citation9,10 Antiviral therapy has been demonstrated to reduce late HCC recurrence and improve post-operative survival, but it has little effect on early recurrence.Citation11 Given that no antitumor therapies have been accepted as the standard of care in HCC after potentially curative treatment, further studies need to be explored.
CIK are a type of antitumor T cells characterized by the coexpression of T cell marker CD3 and natural killer (NK) cell marker CD56 molecules, which can easily be generated by expanding human peripheral blood mononuclear cells (PBMC) in the presence of interferon-c (IFN-c), anti-CD3 antibody and interleukin (IL)-2. Many attributes and rationales for the clinical use of CIK have been developed in the past two decades, and CIK as an adoptive immunotherapy should play an important role in cancer treatment.Citation12–14 As an immunotherapeutic modality, infusion of CIK is more likely to show efficacy in a relatively low tumor burden stage or in an adjuvant setting, than in high tumor burden diseases.Citation15 We hypothesized that CIK treatment is beneficial after an attempted curative resection of HCC, as residual tumor, if present, would be minimal. We conducted this study to assess the efficacy and safety of CIK as an adjuvant therapy for HCC after curative resection.
Results
Patients
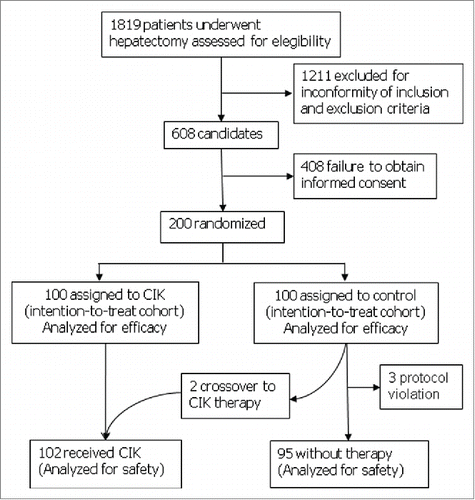
From May 2008 to Feb 2013, 1819 patients underwent hepatectomy in our department. Of the 608 patients who met the inclusion and exclusion criteria, written informed consent were obtained in 200 patients. The 200 patients were randomized to group A (the CIK group) and group B (the control group) with an allocation ratio of 1:1. All the patients in group A finished the four cycles of CIK infusion as scheduled by the protocol. The dose of CIK for each cycle ranged from 1.0 × 1010 to 1.5 × 1010 cells, and the total dose for each patient ranged from 4.0 × 1010 to 6.0 × 1010 cells. Three patients received additional CIK treatments after one year. Five patients in group B had protocol violation: two patients were crossed over to receive CIK treatment, two accepted adjuvant hepatic arterial che-moembolization, and one was later diagnosed histologically as having a mixed HCC and cholangiocarcinoma. All the 200 randomized patients were included in the efficacy analysis ().
All these patients were Chinese. Hepatitis B virus infection was the main cause of HCC (85.5%). Patients in group A were younger (median 43, IQR 38–56 y) when compared with group B (median 52, IQR 43–60 y, p = 0.001). Otherwise, patients in the two groups were balanced. There were no significant differences between the two groups in any baseline demographic and prognostic characteristics for surgery. Most (75.5%) patients received non-anatomic hepatectomy. There were also no significant differences between the length of hospital stay and the operation-related factors, and the post-operative use of anti-viral drugs and immunomodulators were comparable between the two groups. The baseline characteristics, operative and post-operative factors of these patients are shown in .
Table 1. Baseline and surgical prognostic characteristics of the patients (Intention-to-Treat Population).
Follow-up
As the actual accrual time was longer than we expected, we extended the study by 16 mo to maintain the power of 0.8. The follow up ended at the end of September 2014. The median follow-up was 38.2 mo (range 3.7–73.0 mo). No patients were lost to follow up in their first post-operative year. One patient was lost to follow-up after 14 mo of surgery, three patients after two years, four patients after three years, and three patients after four years of surgery, respectively. For those who were lost to follow-up, the date of the last assessment and the date of the last contact were used to calculate recurrence and death, respectively.
Time to recurrence
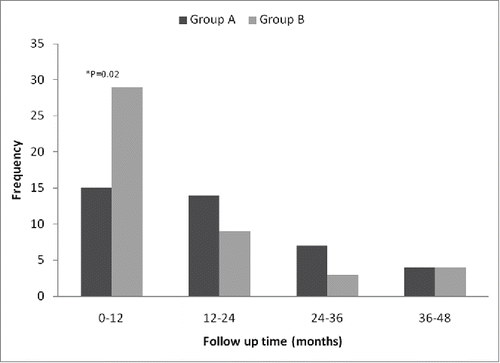
No patients were detected to have any residual tumor at the post-operative 4th-week assessment. By the date this study was censored on June 30, 2014, recurrence was diagnosed in 85 patients, with 40 patients in the CIK group and 45 patients in the control group, respectively (40% vs. 45%, p = 0.474). The median TTR of the CIK group was 13.6 mo (IQR 6.5–25.2 mo) vs. 7.8 mo (IQR 2.7–17.0 mo) of the control group. There was a 74% improvement in median TTP for the patients in the CIK group (p = 0.011). The recurrence events in each of the post-operative years are shown in . The one-year recurrence rate was significantly lower in the CIK group compared with the control group (16% vs. 29%, p = 0.028). There was no significant difference in the recurrence rate in the other post-operative years.
Figure 2. Recurrence distribution among different post-operative years between the CIK group and the control group.
Pre-specified subgroup analyses by age, pre-operative AFP, tumor capsulation, tumor differentiation and size of tumor were performed for TTR. In the subgroups with age <45 y, pre-operative AFP ≥25 ng/mL, tumor size >5 cm, and moderate/poor differentiated tumor, patients in the CIK group had significant prolonged TTR than the control group. (, p < 0.05)
Table 2. Subgroup analysis of time to recurrence (TTR) between the CIK group and the control group.
Disease-free survival and overall survival
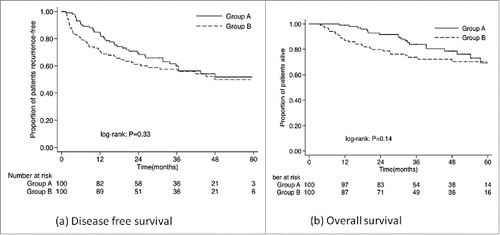
By the date of censor on September 30, 2014, 48 patients had died (20 in the CIK group and 28 in the control group). Except for one patient who died before the development of tumor recurrence, 47 patients died from HCC progression. The one-, three-, and five- year DFS were 83.8%, 59.9%, and 51.8% in the CIK group, and 69.9%, 55.9%, and 44.9% in the control group, respectively (p = 0.334). The one-, three-, and five- year OS were 91.7%, 82.2%, and 69.3% in the CIK group, and 87.0%, 76.3%, and 56.2% in the control group, respectively (p = 0.141). ()
Time-dependent effects of CIK on overall survival
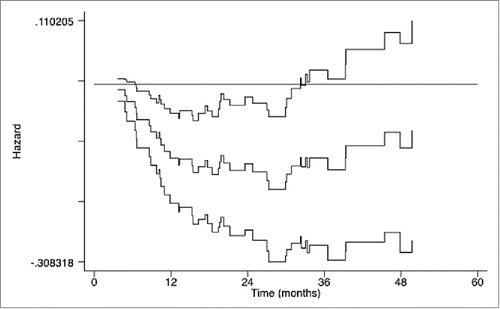
showed the trend of the hazard ratios (HR) over time for OS () of the patients in the CIK group compared to patients in the control group using the Aalen's linear hazard models. The curves of the HR and the 95% CI for OS showed a significantly lower risk of death in patients in the CIK group during the first 30 mo of surgery. Although the curves of the HR for OS were always less than 1 over time, the confidence intervals crossed the horizontal reference line in the later period, implying that there was no significant difference of mortality risk between the two groups in the later years of surgery.
Figure 4. Curves of Aalen's linear hazard model for overall survival (OS) of patients in the CIK group and the control group. The middle curve presents the hazard ratio (HR) for the CIK group compared to the control group, and the upper and lower curves present the corresponding 95% confidence intervals (CIs) of HRs over time; the horizontal reference line presents a coefficient equal to 0 and a HR equal to 1.
Safety
Neither hospital death nor serious adverse events occurred in the whole cohort. The overall incidence of treatment-related complications was 11.8% in the CIK group and 14.7% in the control group. All adverse events were of grade 1 or 2, without any significant difference between the two groups (). Fever was reported in eight (7.9%) patients who received CIK, and five (4.9%) of them had transient recurring fever after each cycle of CIK infusion. The fever was self-limiting. No infection or allergic reaction was observed in the patients who received CIK.
Table 3. Adverse events in patients who received CIK treatment or not (per treatment population).
Discussion
In this study, the patients who received adjuvant CIK therapy after curative resection of HCC had a 74% improvement in the median TTR when compared to those who did not receive CIK infusion. The benefit of CIK to prolong the TTR was more significant in patients with tumor >5 cm, complete tumor encapsulation, moderate/poor differentiated tumor, and a pre-operative AFP ≥25 ng/mL. Patients in the CIK group had significant lower recurrence rate in the first post-operative year, although the overall recurrence rates were not different between the two groups.
Adoptive immunotherapy is an old treatment strategy even though it has not been widely accepted. It has been shown to be useful in melanoma, lymphoma and renal cancer.Citation16,17 With rapid advances in technology in molecular biology, adoptive cancer immunotherapy using CIK cells is receiving attention. Citation13,18 This technique may have a renew interests in light of the expression of programmed cell death 1 (PD1) pathway in HCC and recent encouraging outcome demonstrated in patients treated with Nivolumab.Citation19,20 An international registry of clinical trials on CIK was established in 2010 by Schmidt-Wolf et al., with an aim to collect clinical data and standardize treatment of patients with cancer using CIK cells.Citation21 In previously published studies, CIK treatment has been shown to significantly improve patient survival, especially when combined with minimally invasive treatments to give a synergistic effect.Citation22 However, these studies were not RCT, and the study populations were heterogeneous and the sample sizes were relatively small.Citation21,23 The present trial was conducted to better evaluate the role of CIK as an adjuvant therapy after curative resection for HCC.
The recurrence rate in the CIK group was obviously lower than the control group within the first post-operative year. Using the Aalen's linear hazard model, we also observed a significantly lower risk of death in the first 30 post-operative months in patients who received CIK when compared to the control group. This significant suppression of early recurrence by CIK is consistent with our hypothesis and with the results of Takayama' s study.Citation24 CIK treatment has not been shown to improve OS of patients with HCC after curative resection in several other studies.Citation24,25 Our results suggested that CIK therapy reduced recurrence and death in the earlier post-operative period, but its efficacy failed to achieve any benefit in long-term survival. Recently, Lee et al. reported a multicenter trial using CIK as an adjuvant therapy for early stage HCC after curative treatments (which included surgical resection, radiofrequency ablation, and percutaneous ethanol injection). The results showed both recurrence-free survival and OS were improved in patients who received adjuvant CIK.Citation26 Similar results were obtained from a retrospective study on HCC after surgery which showed a significant survival benefit using CIK therapy.Citation27 A possible explanation on the inconsistency of CIK in providing any survival benefit in the different studies is the differences on the cycles of infusions and the durations of maintenance CIK treatment used. Another possibility is in the two studies with a positive impact of CIK on survival, higher portion of patients with very early stage HCC after curative treatment were included than our study, as well as patients treated with repeated ablation were included and CIK could give more favorable survivals in these patients.26,27
Currently, there are no standard dose which has been established for CIK therapy. In the previous studies, the number of CIK cells used per infusion ranged from 7.2 × 106 to 2.1 × 1010, and some patients were treated with up to 40 infusions of CIK.Citation21 In our study, we used four cycles of treatment which were completed within 3 mo of surgery, and the dose of each cycle ranged from 1.0 × 1010 to 1.5 × 1010 CIK cells. This strategy of “high dose, short term, less cycles” was selected based on a cost-effect consideration. We hypothesized that a patient's inherent immunologic function is most impaired by the trauma of the surgery during the early post-operative period, and any residual tumor is at its minimum at that time. This strategy seems to be successful in decreasing early recurrence in the first post-operative year, but it failed to maintain any long-term effect. In another RCT for HCC after curative resection, no significant difference was found in the DFS of HCC between patients who received three courses of CIK and those who received six courses.Citation25 On the other hand, Pan et al. reported patients who received more than eight cycles of CIK transfusion exhibited significantly better survival than those patients who received <8 cycles.Citation27 In a recent RCT, patients who received 16 cycles of CIK infusion in 60 weeks after curative treatments had significantly better OS and RFS.Citation26 In our study, three patients received additional CIK infusions in the later post-operative years, and all of them are still alive and without any recurrence. All these data provide us with clues that more infusions and a longer maintenance duration of CIK treatment might result in better survival. The existing evidences are not sturdy enough to advocate CIK to be used as an adjuvant therapy for HCC after curative resection. However, future studies should be conducted focusing on longer treatment duration, and on the cost effectiveness for CIK infusion.
A major limitation of this trial is that it was conducted without using a blind control. As the interventions were open to both the patients and investigators, patients in the control group were less compliant. Some patients decided to withdraw from the study to receive some form of adjuvant therapy, thus resulting in a bias in the two groups of patients. Moreover, the unblinded design made the patients of the CIK group feeling they were luckier and would have better chance to be cured, which might lead underreporting of their AEs. Another limitation is that the age distribution was imbalanced between the two groups. Patients in the CIK group were significantly younger than the control group. Although younger patients are more common to have more aggressive tumor factors, a large-scale propensity score matching analysis has shown age not to be a risk factor for long-term survival for patients with HCC who underwent curative resection.Citation28 Furthermore, the absence of flow cytometry determination of injected immune cells did not allow us to identify the correlation between quality and quantity of immune cells and patients‘ outcome. A study about how long the CIK cells will be detectable in the peripheral blood and whether long-lasting immune changes affect patients’ outcome would be of great interest, and is our future plan.
In summary, this study showed that CIK as an adjuvant treatment prolonged the TTR in patients with HCC, but CIK failed to result in better DFS and OS. CIK was safe, with no significant toxicity. More large-scale multicenter (RTC) are needed to define the role of CIK as an adjuvant therapy.
Patients and methods
Study population
Consecutive patients who underwent liver resection with curative intention for HCC at the Department of Hepatobiliary Surgery, Sun Yat-sen University (SYSU) Cancer Center between May 2008 and Feb 2013 were eligible for this study. The inclusion criteria were: (1) age older than 18 y; (2) no prior anticancer therapy; (3) HCC with histological diagnosis; (4) stage BCLC A or B with tumor number ≤3; (5) underwent curatively resection (defined as histologically negative margin, R0 resection); (6) Eastern Cooperative Oncology Group Performance status (ECOG PS) 0 or 1; (7) liver function status of Child-Pugh A; (8) adequate organ functions prior to study entry (platelet count ≥70 × 109/L, hemoglobin ≥85 g/L, prothrombin time ≤3 sec above control, albumin ≥35g/L, total bilirubin ≤25 μmol/L, alanine aminotransferase and aspartate aminotransferase ≤2.5 times the upper limit of normal, and serum creatinine ≤1.5 times the upper limit of normal; (9) written informed consent was obtained. The exclusion criteria were: (1) previous or concurrent cancer; (2) history of cardiac disease; (3) known central nervous system disease; (4) active infections other than chronic viral hepatitis; (5) history of organ allograft; (6) patients with clinically significant gastrointestinal bleeding within 30 d; (7) pregnant or breast-feeding women. The study was approved by the Ethics Committee of SYSU Cancer Center, and complied with the provisions of the Good Clinical Practice (GCP) guidelines and Declaration of Helsinki. The study was monitored by the GCP center of SYSU Cancer Center, and audited by the Department of Medical Sciences of SYSU. This trial has been registered and a copy of the brief protocol and inclusion and exclusion criteria are available at ClinicalTrials.gov (ClinicalTrials.gov registration number, NCT00769106).
Study design
This prospective randomized, open label, blank controlled, phase 3, parallel trial was conducted at SYSU Cancer Center. All eligible patients were randomly assigned in a 1:1 ratio to receive either intravenous autologous CIK infusion versus no treatment. Randomization with a block size of 20 was applied. The random allocation sequence was generated by a statistician. A non-research staff then made cards with the assigned groups, and sealed each of the cards into numbered and opaque envelopes. The envelopes were stored by block. After signing an informed consent with the investigator, each enrolled patient was assigned by a study nurse to a study group by opening a sequential envelope. Both the investigators and the patients were aware of the intervention assignments. Physicians and radiologists who assessed the outcomes, as well as statisticians were blinded of the group assignments.
Crossover of patients between the groups was not permitted. For patients who developed tumor recurrence within four weeks after operation, or received other anticancer treatment before recurrence, they were classified as protocol violation. On the basis of the intention-to-treat (ITT) principle, all randomized patients including the violation ones were included in the analysis for efficacy in this study.
Interventions
Patients in the CIK group were scheduled to receive four cycles of autologous CIK infusions within 3 mo after liver resection. The first CIK infusion was performed within six weeks after surgery. The patients received CIK infusions intravenously in an upper limb at each cycle. The interval between cycles was about two weeks. Treatment interruptions or discontinuation were permitted when patients experienced intolerable adverse effects.
The CIK infusions were prepared under conditions in accordance with current good manufacturing practices in the Biotherapy Center at SYSU cancer center as described in our previous report.Citation27 Briefly, 50 mL of heparinized peripheral blood were obtained from the patients after two weeks of surgery. The PBMCs were separated by Ficoll-Hypaque density centrifugation, resuspended in fresh serum-free X-VIVO 15 medium containing 1,000 U/mL interferonγ (IFNγ, ShangClone) at 2 × 106 cells/mL and incubated at 37°C in a humidified atmosphere containing 5% CO2 for 24 h. Then, 100 ng/mL mouse anti-human CD3 monoclonal antibody (R & D Systems), 100 U/mL Interleukin-1α (IL-1α, Life Technologies) and 1,000 U/mL human recombinant IL-2 (rhIL-2, Beijing Sihuan) were added. Cell growth was observed daily. Fresh medium containing IL-2 was added every two days to maintain a cell concentration of 2 to 4 × 106 cells/mL. On day 14, cells were harvested, washed, and resuspended with 100 mL of normal saline containing 1% of human serum albumin. The cell dose was based on the total viable cell number, as determined by manual hemacytometer cell counts. Before administration, samples of CIK were assessed for viability using the dye exclusion test and checked twice to exclude contamination by bacteria, fungi and endotoxins. Then, the autologous CIK was transfused through a vein within 60 min after harvest.
Patients in the control group were regularly followed up in the clinic without any anticancer therapy. Anti-viral treatment and other supportive medicine were allowed in the two groups. Any other anticancer therapy or medication was forbidden during the study period. After tumor recurrence was confirmed, the patients were treated actively with the best available clinical practice.
Outcomes and assessment
The primary outcome was TTR. The TTR was measured from the date of surgery to the date when intrahepatic or/and extrahepatic recurrence was first diagnosed. The diagnosis of recurrence was based on the Response Evaluation Criteria in Solid Tumors (RECIST). Once there was any suspicious finding by ultrasound or X-ray, an enhanced contrast computed tomography (CT) or magnetic resonance imaging (MRI) was performed to confirm the diagnosis of recurrence. When the recurrence was confirmed, the date of the first ultrasound or radiography which detected abnormality was used to calculate the TTR. A contrast abdominal CT or MRI was performed at four weeks (±1 week) after hepatectomy to assess any residual lesions. The patients were then assessed once every 3 mo. The assessment included physical examination, clinical laboratory tests (hematologic and biochemical parameters, α fetoprotein), and abdominal Doppler ultrasonography. Additionally, an abdominal CT or MRI with contrast and chest X-ray were conducted every 6 mo in the first two years of surgery, then every 12 mo for the subsequent three years. Patients who had no recurrence within five years of surgery were assessed every 6 mo from the 6th year onwards.
The secondary outcomes included DFS, OS, and safety. DFS was measured from the date of surgery to the date when recurrence was first diagnosed or to the date of death, whichever occurred earlier. OS was measured from the date of surgery to the date of death from any reason or to the date of the last follow-up. Safety was assessed using version 3.0 of the National Cancer Institute's Common Terminology Criteria for adverse events (CTCAE 3.0).Citation29
Statistical analysis
The sample size was calculated based on the primary outcome of TTR. Assuming a two-sided type I error of 0.05, a randomization ratio of 1:1 between the two groups, a median TTR of 8 mo in the control group, and an expected accrual time of 18 mo and additional follow-up time of 42 mo (total study duration of five years), we estimated that with 99 patients in each group, the study would have a power of 80% to detect a 4 mo (50%) prolongation in TTR for the CIK group. An interim analysis was planned for safety and compliance. A single final analysis was planned for the primary outcome. Subgroup analysis were planned to explore the potential benefit of CIK for patients with different characteristics.
The efficacy outcomes (TTR, DFS, and OS) were analyzed on the basis of the ITT principle. Safety outcomes were assessed based on the per protocol principle. Continuous variables were described as medians with interquartile range (IQR). Categorical variables were described as totals and frequencies. The differences between the groups were assessed by the Chi-square, student's t-test and Wilcoxon rank-sum test, as appropriate. TTP was assessed using the Wilcoxon rank-sum test. OS and DFS were estimated using the Kaplan–Meier (KM) method, and the differences in OS and DFS were compared using the log-rank test. Aalen's linear hazard models were used to analyze the time-dependent effects of CIK therapy on DFS and OS. All analyses were carried out with the STATA version 12.0 (StataCorp, College Station, TX) and a p value of <0 .05 (two tailed) was considered statistically significant.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Previous Presentation
Part of this work has been presented at the 3rd Cancer Immunotherapy and Immunomonitoring Conference (CITIM), April 2013, Krakow, Poland, and the Annual Global Academic Programs (GAP) Conference of MD Anderson Cancer Center, April 2015, Houston, Texas.
Registration Number
ClinicalTrials.gov number NCT00769106
Acknowledgments
The authors thank the following individuals (all affiliated with the Sun Yat-sen University Cancer Center) for their contributions to this project. We thank Jin-qing Li, MD, PhD; Ming Shi, MD; Xiao-jun Lin, MD, Bo-kang Cui, MD; Rong-ping Guo, MD; Yun-fei Yuan, MD; Wei Wei, MD; Ya-qi Zhang, MD; Guo-he Lin, MD; Yi-ze Mao; Bin-kui Li, MD; Yun Zheng, MD; Zhong-guo Zhou; Shi-ping Chen; Li-xi Huang; Miao-la Ke for their assistance in the trial implementation. We thank Qing Liu, PhD for his statistical advice and Yan-yan Li and Yan-shan Huang for their assistance with database management.
Funding
The study is supported by the 5010 Foundation of Sun Yat-sen University (Grant No. 2007043), the Science and Technology Program of Guangzhou (No. 201400000001-3), the National Natural Science Foundation of China (No. 81171890), and the National Basic Research Program of China (973 Program, No. 2013CB910304).
References
- de Lope CR, Tremosini S, Forner A, Reig M, Bruix J. Management of HCC. J Hepatol 2012; 56 Suppl 1:S75–87; PMID:22300468; http://dx.doi.org/10.1016/S0168-8278(12)60009-9
- Page AJ, Cosgrove DC, Philosophe B, Pawlik TM. Hepatocellular carcinoma: diagnosis, management, and prognosis. Surg Oncol Clin N Am 2014; 23:289–311; PMID:24560111; http://dx.doi.org/10.1016/j.soc.2013.10.006
- Poon RT, Fan ST, Lo CM, Liu CL, Wong J. Long-term survival and pattern of recurrence after resection of small hepatocellular carcinoma in patients with preserved liver function: implications for a strategy of salvage transplantation. Ann Surg 2002; 235:373–82; PMID:11882759; http://dx.doi.org/10.1097/00000658-200203000-00009
- Kim do Y, Ahn SH, Kim SU, Choi SB, Lee KH, Park MS, Park JY, Lee do Y, Han KH, Kim KS. Adjuvant hepatic arterial infusional chemotherapy with 5-fluorouracil and cisplatin after curative resection of hepatocellular carcinoma. Oncology 2011; 81:184–91; PMID:22067673; http://dx.doi.org/10.1159/000333827
- Furtado R, Crawford M, Sandroussi C. Systematic review and meta-analysis of adjuvant i(131) lipiodol after excision of hepatocellular carcinoma. Ann Surg Oncol 2014; 21:2700–7; PMID:24743904; http://dx.doi.org/10.1245/s10434-014-3511-2
- Dumortier J, Decullier E, Hilleret MN, Bin-Dorel S, Valette PJ, Boillot O, Partensky C, Letoublon C, Ducerf C, Leroy V et al. Adjuvant intraarterial lipiodol or 131I-lipiodol after curative treatment of hepatocellular carcinoma: a prospective randomized trial. J Nucl Med 2014; 55:877–83; PMID:24722530; http://dx.doi.org/10.2967/jnumed.113.131367
- Zhang W, Song TQ, Zhang T, Wu Q, Kong DL, Li Q, Sun HC. Adjuvant interferon for early or late recurrence of hepatocellular carcinoma and mortality from hepatocellular carcinoma following curative treatment: A meta-analysis with comparison of different types of hepatitis. Mol Clin Oncol 2014; 2:1125–34; PMID:25279210; http://dx.doi.org/10.3892/mco.2014.386
- Chen LT, Chen MF, Li LA, Lee PH, Jeng LB, Lin DY, Wu CC, Mok KT, Chen CL, Lee WC et al. Long-term results of a randomized, observation-controlled, phase III trial of adjuvant interferon Alfa-2b in hepatocellular carcinoma after curative resection. Ann Surg 2012; 255:8–17; PMID:22104564; http://dx.doi.org/10.1097/SLA.0b013e31-82363ff9
- Llovet JM, Bruix J. Novel advancements in the management of hepatocellular carcinoma in 2008. J Hepatol 2008; 48 Suppl 1:S20–37; PMID:18304676; http://dx.doi.org/10.1016/j.jhep.2008.01.022
- He VJ. Professor pierce chow: neo-adjuvant and adjuvant therapy for hepatocellular carcinoma-current evidence. Hepatobiliary Surg Nutr 2013; 2:239–41; PMID:24570951; http://dx.doi.org/10.3978/j.issn.2304-3881.2013.07.07
- Huang G, Lau WY, Wang ZG, Pan ZY, Yuan SX, Shen F, Zhou WP, Wu MC. Antiviral therapy improves postoperative survival in patients with hepatocellular carcinoma: a randomized controlled trial. Ann Surg 2015; 261:56–66; PMID:25072444; http://dx.doi.org/10.1097/SLA.0000000000000858
- Sangiolo D. Cytokine induced killer cells as promising immunotherapy for solid tumors. J Cancer 2011; 2:363–8; PMID:21716717; http://dx.doi.org/10.7150/jca.2.363
- Thanendrarajan S, Kim Y, Schmidt-Wolf I. New adoptive immunotherapy strategies for solid tumours with CIK cells. Expert Opin Biol Ther 2012; 12:565–72; PMID:22444075; http://dx.doi.org/10.1517/14712598.2012.668879
- Guo Y, Han W. Cytokine-induced killer (CIK) cells: from basic research to clinical translation. Chin J Cancer 2015; 34:6; PMID:25962508; http://dx.doi.org/10.1186/s40880-015-0002-1
- Hui KM. CIK cells–current status, clinical perspectives and future prospects–the good news. Expert Opin Biol Ther 2012; 12:659–61; PMID:22500927; http://dx.doi.org/10.1517/14712598.2012.676037
- Rosenberg SA, Lotze MT, Muul LM, Chang AE, Avis FP, Leitman S, Linehan WM, Robertson CN, Lee RE, Rubin JT et al. A progress report on the treatment of 157 patients with advanced cancer using lymphokine-activated killer cells and interleukin-2 or high-dose interleukin-2 alone. N Engl J Med 1987; 316:889–97; PMID:3493432; http://dx.doi.org/10.1056/NEJM198704093161501
- Osband ME, Lavin PT, Babayan RK, Graham S, Lamm DL, Parker B, Sawczuk I, Ross S, Krane RJ. Effect of autolymphocyte therapy on survival and quality of life in patients with metastatic renal-cell carcinoma. Lancet 1990; 335:994–8; PMID:1970108; http://dx.doi.org/10.1016/0140-6736(90)91064-H
- Morse MA, Clay TM, Lyerly HK. Current status of adoptive immunotherapy of malignancies. Expert Opin Biol Ther 2002; 2:237–47; PMID:11890864; http://dx.doi.org/10.1517/14712598.2.3.237
- Gao Q, Wang XY, Qiu SJ, Yamato I, Sho M, Nakajima Y, Zhou J, Li BZ, Shi YH, Xiao YS et al. Overexpression of PD-L1 significantly associates with tumor aggressiveness and postoperative recurrence in human hepatocellular carcinoma. Clin Cancer Res 2009; 15:971–9; PMID:19188168; http://dx.doi.org/10.1158/1078-0432.CCR-08-1608
- Shi F, Shi M, Zeng Z, Qi RZ, Liu ZW, Zhang JY, Yang YP, Tien P, Wang FS. PD-1 and PD-L1 upregulation promotes CD8(+) T-cell apoptosis and postoperative recurrence in hepatocellular carcinoma patients. Int J Cancer 2011; 128:887–96; PMID:20473887; http://dx.doi.org/10.1002/ijc.25397
- Hontscha C, Borck Y, Zhou H, Messmer D, Schmidt-Wolf IG. Clinical trials on CIK cells: first report of the international registry on CIK cells (IRCC). J Cancer Res Clin Oncol 2011; 137:305–10; PMID:20407789; http://dx.doi.org/10.1007/s00432-010-0887-7
- Li X, Dai D, Song X, Liu J, Zhu L, Xu W. A meta-analysis of cytokine-induced killer cells therapy in combination with minimally invasive treatment for hepatocellular carcinoma. Clin Res Hepatol Gastroenterol 2014; 38:583–91; PMID:24924902; http://dx.doi.org/10.1016/j.clinre.2014.04.010
- Ma Y, Xu YC, Tang L, Zhang Z, Wang J, Wang HX. Cytokine-induced killer (CIK) cell therapy for patients with hepatocellular carcinoma: efficacy and safety. Exp Hematol Oncol 2012; 1:11; PMID:23210562; http://dx.doi.org/10.1186/2162-3619-1-11
- Takayama T, Sekine T, Makuuchi M, Yamasaki S, Kosuge T, Yamamoto J, Shimada K, Sakamoto M, Hirohashi S, Ohashi Y et al. Adoptive immunotherapy to lower postsurgical recurrence rates of hepatocellular carcinoma: a randomised trial. Lancet 2000; 356:802–7; PMID:11022927; http://dx.doi.org/10.1016/S0140-6736(00)02654-4
- Hui D, Qiang L, Jian W, Ti Z, Da-Lu K. A randomized, controlled trial of postoperative adjuvant cytokine-induced killer cells immunotherapy after radical resection of hepatocellular carcinoma. Dig Liver Dis 2009; 41:36–41; PMID:18818130; http://dx.doi.org/10.1016/j.dld.2008.04.007
- Lee JH, Lee JH, Lim YS, Yeon JE, Song TJ, Yu SJ, Gwak GY, Kim KM, Kim YJ, Lee JW et al. Adjuvant immunotherapy with autologous cytokine-induced killer cells for hepatocellular carcinoma. Gastroenterology 2015; 148:1383–91 e6; PMID:25747273; http://dx.doi.org/10.1053/j.gastro.2015.02.055
- Pan K, Li YQ, Wang W, Xu L, Zhang YJ, Zheng HX, Zhao JJ, Qiu HJ, Weng DS, Li JJ et al. The efficacy of cytokine-induced killer cell infusion as an adjuvant therapy for postoperative hepatocellular carcinoma patients. Ann Surg Oncol 2013; 20:4305–11; PMID:23892527; http://dx.doi.org/10.1245/s10434-013-3144-x
- Su CW, Lei HJ, Chau GY, Hung HH, Wu JC, Hsia CY, Lui WY, Su YH, Wu CW, Lee SD. The effect of age on the long-term prognosis of patients with hepatocellular carcinoma after resection surgery: a propensity score matching analysis. Arch Surg 2012; 147:137–44; PMID:22006855; http://dx.doi.org/10.1001/archsurg.2011.288
- National Cancer Institute Common Toxicity Criteria. Available from URL: http://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm. [accessed Jun 15, 2015]