ABSTRACT
In order to enhance the STING dependent type I interferon (IFN) response, we formulated cyclic dinucleotides (CDN) with cancer vaccines to develop STINGVAX. Interestingly, tumors from STINGVAX treated mice demonstrated dramatic PD-L1 upregulation. When combined with PD-1 blockade, STINGVAX induced regression of established tumors that did not respond to PD-1 blockade alone.
Recent clinical trials with nivolumab, pembrolizumab, and PD-L1 blocking antibodies have all demonstrated durable responses in advanced cancer patients, and these studies demonstrated the tight correlation between clinical response and PD-L1 (B7-H1) expression in the tumor microenvironment, as well as neoantigens, tumor infiltrating T-cells, and IFNγ+. Towards the goal of safely improving the clinical efficacy of PD-1:PD-L1 blocking antibodies, we focused our attention on Stimulator of Interferon Gene (STING) signaling pathway, which can potentially increase PD-L1 expression in the tumor microenvironment.Citation2
Initially characterized as ubiquitous bacterial secondary messengers, CDN [(cyclic-di-GMP (CDG), cyclic-di-AMP (CDA), and cyclic GMP-AMP (cGAMP)] were shown to constitute a novel class of pathogen associated molecular pattern molecules (PAMP) that activate the TBK1/IRF3/type 1 IFN signaling axis via the cytoplasmic pattern recognition receptor, STING.Citation3,4 The STING signaling pathway has emerged as a central TLR-independent mediator of host innate defense to sensing cytosolic nucleic acids, either through direct binding of exogenous CDN from bacteria, or, shown recently, through synthesis of a structurally distinct CDN produced by a host cyclic GMP-AMP synthetase (cGAS) in response to cytosolic double-stranded DNA (dsDNA).Citation5 We hypothesized that STING agonists, either natural or chemically modified, would provide a completely novel class of molecules to enhance the potency of cancer vaccines.
We engineered STINGVAX, a novel combinatorial cancer vaccine that targets the STING pathway and co-localizes CDN with tumor antigens and GM-CSF to amplify tumor specific CTL in the context of established tumor.Citation6 Using multiple types of tumor treatment models of palpable established tumors, STINGVAX demonstrated better antitumor response than vaccines formulated with other adjuvants including a TLR4 agonist (MPL) as well as other well characterized adjuvants (p(I:C) and R848) formulated vaccines. Mechanistic studies showed that STINGVAX's in vivo efficacy was STING dependent, T-cell dependent, and IFNαR dependent.
To advance STINGVAX for clinical trials, we synthesized CDN derivative compounds that contained phosphodiesterase resistant di-thiol backbone and the same 2′-5′, 3′-5′ non-canonical (or mixed) linkage phosphate bridge as 2′-3′ cGAMP, the CDN produced by host cell cGAS in response to binding cytosolic ds-DNA. The non-canonical mixed linkage (ML) has been reported to increase the binding affinity for human STING by at least 10-fold.Citation7,8 Interestingly, our structural analysis also showed that the R,R diastereoisomer of the dithiol CDN was more active than the R,S isomer. Given these in vitro findings with our rationally designed CDN, we found that STINGVAX formulated with the synthetic ML R,R-S2 CDA was more potent than those formulated with the canonical CDA in their antitumor efficacy.
As a potent inducer of IFN dependent Th1 response against the tumor, we were concerned about the adaptive immune resistance mechanism within the tumor microenvironment, which can blunt tumor specific T cell responses. In the STINGVAX treated mice, we noted prominent upregulation of PD-L1 on the tumor cells, which correlated with CDN dependent CD8+IFNγ+ T cell infiltration. We reasoned, therefore, that the blockade of this PD-1:PD-L1 interaction would unleash the tumor specific CTL response held in check. When anti-PD-1 blocking antibody was combined with STINGVAX formulated with RR-S2 CDA, we noted regression of tumor that was not evident with STINGVAX alone treated mice.Citation6 When these mice were rechallenged with the tumor after the regression, the subsequent tumor injection was rejected, demonstrating immunological memory.
The antitumor activity of STING agonist was also found with intratumoral injection of CDN.Citation9 Corrales et.al. focused their attention on the STING signaling within the tumor microenvironment. Rather than formulating a cancer vaccine, another STING agonist, namely DMXAA, was injected directly into bulky B16 tumors resulted in its regression. More interestingly, this “in situ” vaccination method of intratumoral STING agonist injection also resulted in a profound abscopal effect.
Presumably, these antitumor responses elicited by STING agonists work through activating the antigen presenting cells (APC) to prime the T-cells. For STINGVAX, these dendritic cells (DC) circulate between the vaccine injection site and the draining lymph nodes where the T-cell priming can occur. For the intratumoral injection, it is unclear whether CDN is activating the tumor infiltrating DC or other myeloid tumor infiltrating cells to reverse the toleragenic tumor microenvironment. Early reports suggested that the CDN may induce toxicity of the tumor cells directly,Citation10 but our experiments to test this mechanism did not confirm this hypothesis in multiple tumor models. It should be noted that the CDN's direct tumor cell toxicity effect was found in doses over 100μg/injection, which is more than 5-fold higher than used in STINGVAX formulation and in the intratumoral CDN injections. Studies to understand the details of CDN's mechanism of action at the cellular level are currently underway.
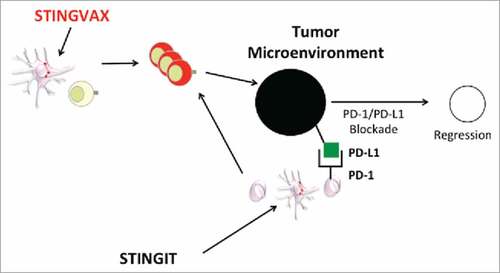
Both methods of activating the STING signaling, whether with co-administration with antigen based vaccine such as STINGVAX or direct STING agonist injection intratumorally, support the need to translate STING agonists into cancer patients, particularly synthetic CDN agonists that can stimulate the human STING molecule.Citation6 (). With multiple ongoing clinical trials with PD-1:PD-L1 blocking agents, CDN are attractive candidates to combine with these agents once the phase I studies are completed. In both the STINGVAX formulation as well as the STING intratumoral (STINGIT) injection studies completed independently in our laboratory, we found dramatic upregulation of PD-L1 expression in the tumor microenvironment. As a potent inducer of IFNγ-secreting T-cells and PD-L1 in the tumor microenvironment, both STINGVAX and STINGIT are well suited for clinical combination with immune checkpoint-blocking antibodies, particularly those cancers that are refractory to immune checkpoint inhibition.
Figure 1. CDN can STING the tumor microenvironment to induce regression when combined with PD-1:PD-L1 Blockade. STINGVAX can prime tumor specific T-cells at site of vaccine injection DLN. Alternatively, STING agonist intratumorally (STINGIT) can reverse toleragenic APC in the TME to prime T-cells. With increased TIL that express IFNg, tumor PD-L1 can potentially be induced to enhance the efficacy of PD-1:PD-L1 blocking antibody.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Additional information
Funding
References
- Rizvi NA, Hellmann MD, Snyder A, Kvistborg P, Makarov V, Havel JJ, Lee W, Yuan J, Wong P, Ho TS et al. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science 2015; 348(6230):124-8; PMID:25765070; https://doi.org/10.1126/science.aaa1348
- Taube JM, Anders RA, Young GD, Xu H, Sharma R, McMiller TL, Chen S, Klein AP, Pardoll DM, Topalian SL et al. Colocalization of inflammatory response with B7-h1 expression in human melanocytic lesions supports an adaptive resistance mechanism of immune escape. Sci Transl Med 2012; 4:127ra37; PMID:22461641; https://doi.org/10.1126/scitranslmed.3003689
- Burdette DL, Monroe KM, Sotelo-Troha K, Iwig JS, Eckert B, Hyodo M, Hayakawa Y, Vance RE. STING is a direct innate immune sensor of cyclic di-GMP. Nature 2011; 478:515–8; PMID:21947006; https://doi.org/10.1038/nature10429
- Barber GN. Cytoplasmic DNA innate immune pathways. Immunol Rev 2011; 243:99–108; PMID:21884170; https://doi.org/10.1111/j.1600-065X.2011.01051.x
- Sun L, Wu J, Du F, Chen X, Chen ZJ. Cyclic GMP-AMP synthase is a cytosolic DNA sensor that activates the type I interferon pathway. Science 2013; 339:786–91; PMID:23258413; https://doi.org/10.1126/science.1232458
- Fu J, Kanne DB, Leong M, Glickman LH, McWhirter SM, Lemmens E, Mechette K, Leong JJ, Lauer P, Liu W et al. STING agonist formulated cancer vaccines can cure established tumors resistant to PD-1 blockade. Sci Transl Med 2015; 7:283ra52; PMID:25877890; https://doi.org/10.1126/scitranslmed.aaa4306
- Gao P, Ascano M, Zillinger T, Wang W, Dai P, Serganov AA, Gaffney BL, Shuman S, Jones RA, Deng L et al. Structure-function analysis of STING activation by c[G(2',5')pA(3',5')p] and targeting by antiviral DMXAA. Cell 2013; 154:748–62; PMID:23910378; https://doi.org/10.1016/j.cell.2013.07.023
- Zhang X, Shi H, Wu J, Zhang X, Sun L, Chen C, Chen ZJ. Cyclic GMP-AMP containing mixed phosphodiester linkages is an endogenous high-affinity ligand for STING. Mol Cell 2013; 51:226–35; PMID:23747010; https://doi.org/10.1016/j.molcel.2013.05.022
- Corrales L, Glickman LH, McWhirter SM, Kanne DB, Sivick KE, Katibah GE, Woo SR, Lemmens E, Banda T, Leong JJ et al. Direct activation of STING in the tumor microenvironment leads to potent and systemic tumor regression and immunity. Cell Rep 2015; 11:1018–30; PMID:25959818; https://doi.org/10.1016/j.celrep.2015.04.031
- Chandra D, Quispe-Tintaya W, Jahangir A, Asafu-Adjei D, Ramos I, Sintim HO, Zhou J, Hayakawa Y, Karaolis DK, Gravekamp C. STING ligand c-di-GMP improves cancer vaccination against metastatic breast cancer. Cancer Immunol Res 2014; 2(9):901–10; PMID:24913717; https://doi.org/10.1158/2326-6066.CIR-13-0123
