ABSTRACT
Recent studies have demonstrated that regulatory T cells (Tregs) are recruited to tumor sites where they can suppress antitumor immunity. The chemokine receptor CCR4 is expressed at high levels on functional CD4+CD25+FoxP3+ Tregs and production of the CCR4 ligand CCL22 by tumor cells and tumor-associated macrophages is associated with Treg recruitment to the tumor site. Here, we tested IgG1 and IgG4 isotypes of human anti-CCR4 mAb2-3 for their in vitro activity and in vivo capacity in a NSG mouse model bearing CCL22-secreting ovarian cancer (OvCA) xenograft to modulate Tregs and restore antitumor activity. Both mAb2-3 isotypes blocked in vitro chemoattraction of Tregs to CCL22-secreting OvCA cells. However, they differed in their in vivo mode of action with IgG1 causing Treg depletion and IgG4 blocking migration to the tumors. Primary T cells that were primed with OvCA-pulsed dendritic cells (DCs) demonstrated INFγ secretion that could be enhanced through Treg depletion by mAb2-3. Humanized mice reconstructed with allogeneic tumor-primed T cells (TP-T) were used to evaluate the restoration of OvCA immunity by depletion or blockade of Tregs with mAb2-3. We observed that IgG1 was more potent than IgG4 in inhibiting tumor growth. Mechanism studies demonstrated that mAb2-3 treatment lead to inhibition of IL-2 binding to its receptor. Further studies showed that mAb2-3 induced CD25 shedding (sCD25) from Tregs which lead to a decrease in IL-2-dependent survival. Together, the results demonstrate that mAb2-3 is an agonist antibody that can restore anti-OvCA immunity through modulation of Treg activity.
Introduction
OvCA is the fifth leading cause of cancer deaths in American women. Globally, there were 239,000 new cases in 2012 with 152,000 deaths worldwide.Citation1 Due to the absence of specific symptoms and the lack of trustworthy screening for early detection, the majority of women with OvCA (60–65%) are diagnosed at a late stage when the cancer has spread beyond the confines of the ovary. Currently, the standard treatment of OvCA is a combination of surgical intervention and platinum-containing chemotherapy, such as carboplatin plus paclitaxel.Citation2,3 Treatment for stage III or IV disease is rarely curative, with 5-y survival rates under 20%. No effective therapy is available for relapsed or metastatic disease that has failed platinum therapy.Citation4
Recent evidence indicates that OvCA are immunogenic tumors,Citation5 suggesting that restoration and augmentation of host antitumor immunity may provide a new mode of immunotherapy that would benefit patients. Several reports have demonstrated that PD-1/PD-L1 and CTLA-4 blockade improves immunity and clinical outcomes in mouseCitation6,7 and humanCitation8 melanoma, findings that are being extended to treat other cancers.Citation8-10 From the immunology viewpoint, immune manipulation is an attractive primary and/or adjuvant approach for cancer treatment because it has the specificity to discriminate between neoplastic and non-neoplastic cells.Citation11 The discovery of a therapeutic human monoclonal antibody (mAb) that could restore host immunity in OvCA patients would represent a significant advancement in cancer treatment.
In healthy individuals, CD4+CD25+ Tregs play an important role in the control of homeostatic tolerance by suppressing autoreactive T cells and preventing autoimmune diseases.Citation12,13 In contrast, in cancer patients the fine balance between Tregs and effector T cells (Teffs) is often impaired with the expansion of Tregs that may foster cancer progression.Citation14,15 Increasing evidence indicates that Tregs are recruited to the tumor site where they can suppress host antitumor immunity.Citation16,17 The recruitment of Tregs to the tumor is mediated through high-level secretion of the CCR4 receptor chemokine CCL22 by tumor cells and microenvironmental macrophages.Citation16 These CCR4+ Tregs create a favorable environment for dysregulation of local antitumor immunity and enhancement of tumor growth. Indeed, elevated levels of Tregs among tumor-infiltrating lymphocytes (TILs) have been described in many cancers, including OvCA.Citation18,19 In addition, results of several studies have shown that increased Treg infiltration in OvCA is associated with poor survival.Citation16,20 Therefore, blocking Treg migration and function(s) by depletion or CCL22/CCR4 blockade may lead to reversal of Treg immunosuppression, which, in turn, may have therapeutic value in cancer treatment, particularly in OvCA where tumor cell secretion of CCL22 and recruitment of Tregs is well documented.Citation16
In the field of cancer immunotherapy, reversing Treg suppression is believed to be one of the main obstacles that must be overcome to improve antitumor immunity. We and others have recently reported that anti-CCR4 mAb can reverse Treg suppression and restore Teff proliferation in vitro.Citation21,22 Anti-CCR4 depletion of Tregs has also been shown to augment the in vitro induction of peripheral blood NY-ESO-1 specific CD8+ T cells from melanoma patients.Citation22 In this study, we investigated two isotypes of anti-CCR4 mAb2-3 that have markedly different biological activities on Tregs in vivo.Citation21 In particular, mAb2-3 IgG1 induces a profound depletion of Tregs. In contrast, the non-depleting IgG4 isotype of mAb2-3 shows no killing of Tregs but retains the ability to block CCL22/CCR4 chemoattraction. Both isotypes of mAb2-3 specifically inhibited the tumor penetration of Tregs in CCL22-secreting OvCA xenografts. In addition, inhibition of OvCA tumor growth was more potent through Treg depletion by mAb2-3 IgG1 than by blockade of Treg migration by mAb2-3 IgG4 in OvCA xenograft-bearing mice reconstructed with allogeneic TP-T cells. Mechanistic studies further revealed that mAb2-3 treatment of Tregs leads to inhibition of interleukin (IL)-2 binding through induction of CD25 shedding which lead to a decrease in IL-2-dependent survival. These studies demonstrate that reversing Treg immunosuppression is possible and that restoration of antitumor immunity through Treg modulation by mAb2-3 may offer a promising addition to our anti-OvCA treatment armamentarium.
Results
CCR4 expression profiles on human T cell populations
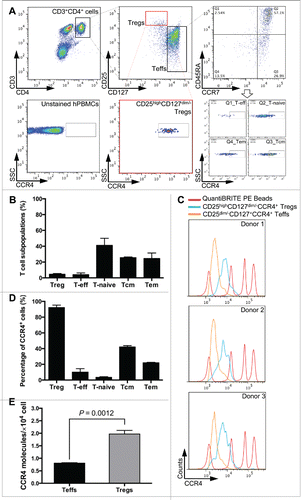
Human peripheral blood mononuclear cells (hPBMCs) were isolated from healthy donors and stained with multiple T cell surface markers to delineate the T cell subpopulations (). Cell markers (CD3, CD4, CD25, and CD127) were used to identify CD4+CD25highCD127dim/− Tregs and CD4+CD25−CD127+ Teffs. In addition, anti-CCR7 and anti-CD45RA antibodies were used to assign Teffs into four subsets, i.e. naive (Tnaive), central memory (Tcm), effector memory (Tem), and other Teffs. The percentage of each CD4+ T cell subset from hPBMCs was measured (). The CCR4 expression profiles of CD4+ T cell subsets were further screened and quantified by QuantiBRITE PE beads and PE-labeled anti-CCR4 antibody using flow cytometry (Fig. 1C, S1A, and S1B). The CCR4 molecules were uniformly expressed on Tregs () with a surface density (19,717 ± 1416, n = 3) that was circa 2.5-fold higher than on Teffs (8063 ± 165, n = 3) (). Although CCR4 expression on Teffs was variable (4–40%) (), similar numbers of CCR4 molecules were present on CCR4 positive cells (Fig. S1C).
Figure 1. Identification of CCR4 molecules on CD4+ T cell populations. (A) Gating strategy for identification of human effector (CD4+CD25−, Teffs) and regulatory (CD4+CD25+CD127dim/−, Tregs) T cell populations and the CCR4-expressing T cell subsets in both populations. Black and red boxes and dotted lines, and the arrow reflect the gated populations for further analysis. (B) The percentage of T cell subpopulations, including naïve (T-naive), central memory (Tcm), effector memory (Tem), regulatory (Treg), and other effector (T-eff), in total T cells. (C) Fluorescence histograms of QuantiBRITE PE beads (red line), CD25highCD127dim/−CCR4+ Tregs (blue line), and CD25dim/−CD127+CCR4+ T cells (orange line) were performed by flow cytometry in three independent healthy donors. PE beads showed the fluorochrome contained low level (474 PE molecules/bead), medium low level (5,359 PE molecules/bead), medium high level (23,843 PE molecules/bead), and high level (62,336 PE molecules/bead) of PE molecules. (D) The percentage of CCR4+ subsets in each T cell subpopulation. (E) The expression levels of CCR4 molecule on CD4+CD25−CD127+ Teffs and CD4+CD25highCD127dim/− Tregs. All experiments were performed in three independent donors and showed the means ±S.E.M.
The immunosuppression ability of CCR4+ Tregs on Teff cell proliferation
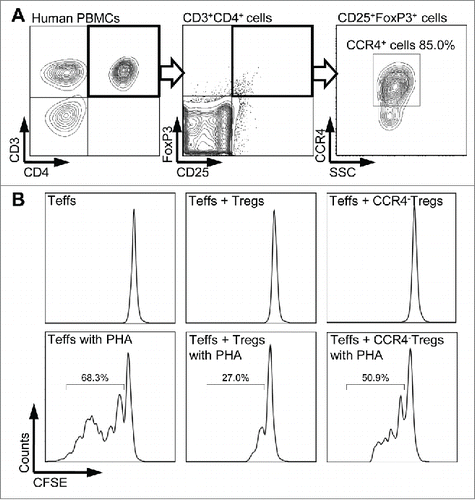
It is well known that FoxP3 is critically important for both the development of Tregs as well as their suppressor function.Citation23 To evaluate if the CCR4+ Tregs mediate immunosuppression, CCR4 staining was performed together with CD25 and FoxP3 co-staining. In , 85% of cells in the CD3+, CD4+, CD25+, and FoxP3+ T cell gate were found to co-express CCR4. Treg suppression assay was then performed to determine the biological function of CCR4+ Tregs. As shown in and S2, Teff proliferation was suppressed in the co-cultures with total Tregs but not in the co-culture with CCR4− Tregs, suggesting that the CCR4+ Treg subset plays an important role in suppressor activity.
Figure 2. CCR4+ Tregs-mediated immunosuppression. (A) Expression of CCR4 on CD4+CD25+FoxP3+ Tregs was assessed by flow cytometry. Representative fluorescence-activated cell sorter (FACS) plots of CD25 and FoxP3 expression gated on CD3+CD4+ lymphocytes (left two plots) and CCR4 staining gated on CD25+FoxP3+ lymphocytes (right plot) in healthy donor blood sample. Bold frames present the gated populations for further analyses. (B) CFSE proliferation profiles of CD4+ effector T cells cultured with or without 20 µg/mL PHA and Tregs or CCR4− Tregs (at the ratio of Teff:Treg = 10:1) were analyzed using flow cytometry by gating CFSE+ cells. CCR4− Tregs were separated by using mAb2-3-conjugated beads. Percentages represent the proportion of dividing CFSE-labeled CD4+ Teffs after 7 d in culture. Experiments were reproduced in three independent donors.
In vivo depletion of Tregs by mAb2-3 IgG1 in huPBL-NSG mice
To investigate whether mAb2-3 treatment could modulate the Treg population in vivo, we used two isotypes of mAb2-3 – IgG1 and IgG4 isotype, the latter has limited in vivo depletion activity due to its narrow range and low affinities for Fcγ receptors (FcγRs).Citation24 These antibodies were injected into human peripheral blood lymphocyte NSG mice (aka huPBL-NSG mice) and the Treg percentages in mouse blood were examined. As shown in Fig. S3A, at day 1 post treatment the CD4+CD25+CD127dim/− Treg population were markedly decreased in the mAb2-3 IgG1 group but as, expected, not in the mAb2-3 IgG4 or control mAb treated groups (Fig. S3B and S3C). At Day 7, there was <50% recovery of Tregs in mAb2-3-treated mice compared to mAb2-3 IgG4 and control mAb treated groups. Long-term effects of mAb2-3 IgG4 multiple dose treatments in hu-PBL-NSG mice were also investigated. Fig. S4 shows that the percentage of CD3+CD4+CD25+CD127− cells in human CD45+ lymphocytes in mouse blood, spleen, and bone marrow were not altered significantly over the three-week study. Interestingly, the total numbers of CD3+ T cells and CD8+ T cells increased in the mice treated with mAb2-3 IgG4 at the last time point (Fig. S4C and S4E, respectively). These results indicate that mAb2-3 IgG1, not IgG4, leads to in vivo depletion of Tregs.
Inhibition of OvCA-mediated Treg migration by mAb2-3 in vitro and in vivo
The CCL22 expression levels in three OvCA cell lines IGROV-1, OVCAR-5, and OVCAR-8 were examined. In agreement with transcriptional profiling studies (data not shown), CCL22 expression was highest in IGROV-1, modest in OVCAR-5, and undetectable in OVCAR-8 cells (). No CCR4 expression was detected on any of these cell lines (Fig. S5A). We further performed a chemotaxis assay using either culture supernatant from these cell lines or recombinant human CCL22. All three cultured mediums showed increased Treg migration compared to fresh medium. However, both mAb2-3 IgG1 and IgG4 were capable of inhibiting Treg chemotaxis induced by CCL22 containing IGROV-1 and OVCAR-5 supernatants but not by OVCAR-8 supernatants. (). Treg chemotaxis to CCL22 was also inhibited by mAb2-3 in a dose-dependent manner (Fig. S5B). These results indicate that both mAb2-3 IgG1 and IgG4 were able to inhibit the recruitment of Tregs to CCL22-secreting OvCA cells in vitro.
Figure 3. Inhibition of ovarian cancer-cells-mediated Treg chemotaxis by mAb2-3 in vitro and in vivo. (A) To facilitate intracellular chemokine CCL22 staining 3 µg/mL brefeldin A was added (blue lines) or not (red lines) to the overnight cultures of IGROV-1, OVCAR-5 and OVCAR-8 cell lines. (B) In vitro chemotaxis of CD4+CD25+ Tregs induced by CCL22-expressing ovarian cancer cell supernatant was performed using transwell assay. Treg recruitment was inhibited by mAb2-3 IgG1 and IgG4, but not by control antibodies. (C) The in vivo bioluminescence images of ovarian cancer xenograft mouse model at 18 h post-injection of luciferized CD4+ T cells and (D) CD4+CD25+CD127dim/− Tregs. The intensity of region of interest (ROI) (red circle, xenograted tumor) was further quantified in the left panel. Results were expressed as means ±S.D. “*” and “**” represent Students t-test p value < 0.05 and 0.01, respectively.
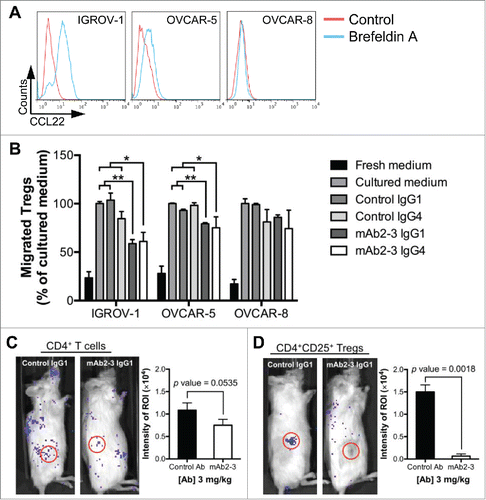
A recent report comparing the genomic profiles of OvCA cell lines and high-grade serous ovarian cancer (HGSOC) tumor samples indicated that IGROV-1 may originate from a different OvCA subtype.Citation25 Nevertheless, for our studies that rely on secretion of expression of CCL22, IGROV-1 is still suitable for our studies irrespective of its genomic profile. Next, to evaluate if mAb2-3 could block Treg recruitment in vivo, we injected luciferase-transduced CD4+ or CD4+CD25+ T cells in mice bearing IGROV-1 xenograft tumors followed by treatment with mAb2-3 or control antibody. After 18 h, bioluminescent imaging showed that the CCL22-secreting tumor recruited the CD4+ and CD4+CD25+ T cells in mice treated with control IgG1, but such recruitment was reduced by treatment with mAb2-3 IgG1 (, respectively). Furthermore, we investigated the recruitment CD4+CD25+CD127dim/− Tregs after 48 h of mAb2-3 treatment and found that (a) Tregs accumulated in tumor tissue in the control IgG1 group, (b) Tregs were depleted by mAb2-3 IgG1 and (c) Tregs were diffusely distributed in the mice treated with mAb2-3 IgG4 (Fig. S6A). Both mAb2-3 IgG1 and IgG4 treatment resulted in lower bioluminescent intensity in the tumor tissue (Fig. S6B) and lesser tumor-infiltrating Tregs (Fig. S6C) than control group. These results indicate that mAb2-3 IgG1 treatment resulted in Treg depletion while mAb2-3 IgG4 treatment lead to inhibition of tumor-infiltrating Treg recruitment in vivo.
Enhanced antitumor immunity mediated by mAb2-3 in vitro
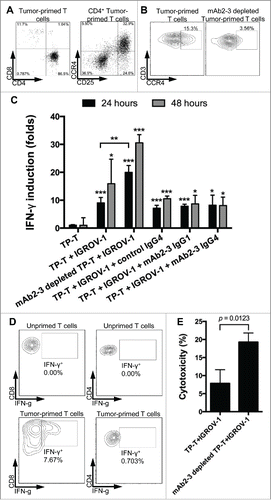
To establish an OvCA xenograft bearing humanized mouse model, we created IGROV-1-specific T cells in vitro that could be tested subsequently for in vivo immunotherapy. DCs were differentiated from monocytes harvested from hPBMCs (Fig. S7A), pulsed with IGROV-1 cell lysates, and co-cultured with autologous hPBMCs to generate TP-T cells. These TP-T cells were able to respond to tumor antigens, leading to production of IFNγ in a co-culture with IGROV-1-pulsed DCs (Fig S7B). Furthermore, as shown in the representative experiment in , TP-T cells consisted of circa 31.6±1.4% (n = 3) CD25+CCR4+ T cells among all CD4+ T cells. These CCR4+ TP-T cells could also be removed using mAb2-3-conjugated magnetic beads (). In addition, TP-T cells co-cultured with IGROV-1 cells exhibited an increased release of IFNγ compared to TP-T cells cultured alone (). Cell staining studies showed an increase in IFNγ expression for both CD8+ and CD4+ TP-T cells reacting to IGROV-1 cells compared to unprimed T cells from the same donor (). Additionally, the co-culture showed enhanced IFNγ activity when CCR4+ cells were depleted with mAb2-3 from the TP-T population (). mAb2-3-depleted TP-T cells also induced higher cellular cytotoxicity on IGROV-1 cells than non-depleted TP-T cell (). Surprisingly, there was no enhanced effect of soluble mAb2-3 IgG1 or IgG4 treatment compared to control IgG1 on IFNγ activity (). This lack of mAb2-3 enhancement suggests that Treg depletion is required in this in vitro system to achieve reversal of TP-T suppression possible because of the high percentage of Tregs in the co-cultures, their release of suppressive mediators and/or requirement for cell-to-cell contact. Collectively, these data indicate that the TP-T cells, especially mAb2-3-depleted CCR4− TP-T cells, could induce antitumor responses and mediate tumor cell death.
Figure 4. The activity of tumor-primed T cells on IGROV-1 cells. (A) Tumor-primed T cells were stained by anti-CD3, CD4+, CD8+, CD25 and (B) CCR4 antibodies. CCR4-depleted tumor-primed T cells by mAb2-3-conjugated beads and T cell subsets in tumor-primed T cells were analyzed by flow cytometry. (C) Tumor-primed T cells and mAb2-3-depleted tumor-primed T cells were incubated with IGROV-1 cells for 24 and 48 h and then the supernatant were harvested and detected the expression level of IFNγ. The IFNγ in the cocultured supernatant was measured by mesoscale discovery (MSD) and showed the folds of the IFNγ concentration in the tumor-primed T cells cultured supernatant. (D) Intracellular IFNγ staining of CD4+ and CD8+ T cells from coculture. Cells were harvested at 48 h post-coculture; incubated for 6 h in the presence of brefeldin A; stained for CD3, CD4+, and CD8+; fixed in paraformaldehyde; permeabilized; and stained for intracellular IFNγ. The cells were gated on lymphocytes by size and CD markers and analyzed by flow cytometry. (E) The cytotoxic activities of tumor-primed T cells and mAb2-3-depleted tumor-primed T cells were further detected by LDH ELISA assay. All experiments represented triplicates in each time point with the means ±S.D. and performed in two independent experiments. “*”, “**,” and “***” represents Students t-test p value< 0.05, 0.01 and 0.005, respectively.
Evaluation of mAb2-3 antitumor effect in vivo
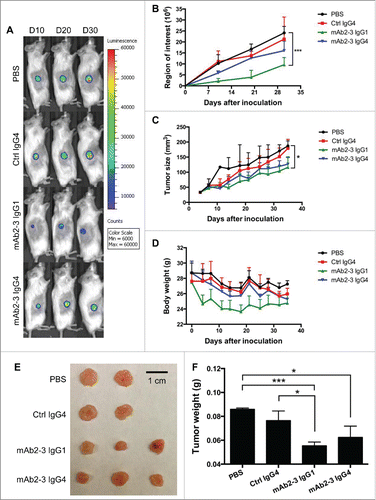
We next sought to confirm the functional and thus potential therapeutic relevance of our findings that reduced tumor-infiltrating Tregs by mAb2-3-mediated depletion or blockade could enhance antitumor activity. Mice bearing luciferase-expressing IGROV-1 xenografts received 4 × 106 TP-T cells and were treated with mAb2-3 twice a week for five weeks. Bioluminescent images were taken every 10 d to quantitate the tumor size (). Mice treated with mAb2-3 IgG1 and IgG4 showed lower relative low luminescence intensity compared to control groups, with mAb2-3 IgG1 treatment showing greater antitumor effects than mAb2-3 IgG4 (). The same observation was seen in tumor size measurement (). Interestingly, the greatest reduction in mouse body weight was seen in the group treated with mAb2-3 IgG1 (). The antitumor effect of mAb2-3 was also observed in tumor tissue () and tumor weight (). These results showed that mAb2-3 mediated TP-T cells against tumor inhibiting tumor growth in vivo.
Figure 5. mAb2-3 mediated the tumor growth inhibition in IGROV-1-xenografted mice reconstructed with IGROV-1-primed T cells. (A) NSG mice were inoculated with 2 × 106 luciferazed IGROV-1 tumor cells subcutaneously, injected 4 × 106 IGROV-1-primed T cells intravenously, and treated with anti-CCR4 antibodies. Tumor growth curves of luciferazed IGROV-1 human ovarian carcinoma tumor xenografts in NSG mice were measured. Mice were treated with three mg/kg of control IgG4 (n = 2), mAb2-3 IgG1 (n = 3), and mAb2-3 IgG4 (n = 3) and equal volume of PBS (n = 2). Antibodies were administered intravenously twice a week for five weeks. Mice were imaged using an IVIS imaging system every 10 d Color scale: blue, luminescent signal intensity; red, least intense signal; most intense signal. (B) Luciferase signals of tumor tissues in each group were quantified. (C) Tumor size and (D) body weight in mice treated with antibodies were measured twice a week. (E) Tumor tissue and (F) tumor weight were harvested and measured. Bar scale, 1 cm. *p < 0.05; ***p < 0.005; p value were calculated with two-way ANOVA. All data were shown the means ±S.E.M.
To confirm the potent therapeutic effect of mAb2-3, we increased by 2.5-fold the number of TP-T cells injected into mice bearing IGROV-1 xenografts. Under these experimental conditions, there was statistically significant inhibition of the tumor growth curves in mice treated with both mAb2-3 IgG1 and IgG4 (Fig. S8A). The body weight and tumor tissues showed similar results as (Fig. S8B and S8C). TP-T cells (CD3+) were found infiltrating the site of tumor engraftment for all treatment groups (Fig. S8D, upper panel). In addition, CD25+ TP-T cells were detectable and accumulated in xenografted tumor treated with PBS and control mAb, but the accumulation of CD25+ TP-T cells was reduced in tumor treated with mAb2-3 IgG1 and IgG4 (Fig. S8D, lower panel). The TP-T cells in mouse blood were further investigated by FACS and the results showed that there were no difference in CD4+ and CD8+ T cells among each treatment group but the Treg population was decreased only in mAb2-3 IgG1-treated group (Fig. S8E–G). Taken together, these data indicate that Treg depletion by mAb2-3 IgG1 and tumor-recruiting Treg blockade by mAb2-3 IgG4 could enhance antitumor immunity in vivo.
Modulation of Treg survival by disruption of IL-2 binding and induction of CD25 shedding by mAb2-3
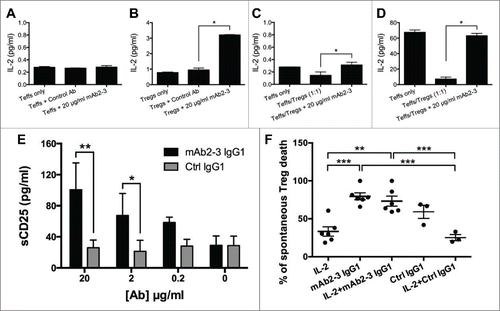
The suppressive mechanisms used by Tregs include releasing inhibitory cytokines and cytolytic enzymes, as well as mediating metabolic disruption by CD25/IL-2 and CD39/adenosine.Citation26 We first studied cytokine production by Tregs and found that mAb2-3 did not alter the levels of suppressive cytokines, i.e., IL-10 and TGF-β (Fig. S9). We next investigated if the binding of mAb2-3 to CCR4 on Teff and Treg could affect the interactions between CD25 (TAC), the α chain of IL-2 receptor (IL-2R), and IL-2. shows that endogenous IL-2 secretion from Teffs was not induced by mAb2-3 treatment. In contrast, when exogenous IL-2 was added into the Tregs culture, marked increase in IL-2 accumulation was detected in the supernatant with mAb2-3 only (). In addition, a Teff/Treg co-culture system was set up in which cells were treated with mAb2-3 or control mAb and also incubated without () or with () exogenous IL-2. The results showed that mAb2-3 but not control mAb treatment lead to an increase in IL-2 levels in both co-culture supernatants.
Figure 6. The intermediation of mAb2-3 in interaction between IL-2 and CD25. (A) In the absence of exogenous IL-2, endogenous IL-2 levels in 1 × 104 CD4+CD25− Teffs cultured supernatants incubated with or without mAb2-3 IgG1 were analyzed by ELISA. (B) CD4+CD127dimCD49d− Tregs (3000/reaction) were incubated with 0.25 IU/mL of exogenous IL-2 in the presence and absence of 20 μg/mL of mAbs and 0.5/1 μg/mL of plate-bound anti-CD3/28 antibodies. Bars represent means ±S.D. (C) In the absence of exogenous IL-2, endogenous IL-2 concentration was shown from 1 × 104 Teffs alone or with Tregs and treated with 20 μg/mL of mAb2–3. Bars represent means ±S.D. (D) The concentrations of IL-2 in supernatants from Teffs and Tregs coculture treated with mAb2-3 in the presence of 4 IU/mL of exogenously added IL-2. Bars represent means ±S.D. (E) The concentration of soluble CD25 in 48-h cultured supernatant of Tregs treated with 20 µg/mL mAb2-3 or control IgG1 was investigated with ELISA. Data was shown the average from three independent donors. Bars represent means ±S.E.M. “*” and “**” represent p value < 0.05 and 0.01, respectively, by using two-way ANOVA. (F) In vitro cell survival assay was performed by measuring the viability dye in cultured Tregs treated with the presence or absence of 0.5 IU/mL IL-2, 20 µg/mL mAb2-3 IgG1, and 20 µg/mL control IgG1 for 5 d. The normalized percentage of dead Tregs from different groups among spontaneous death Tregs was shown. Each dot indicates an individual donor in each group. Bars represent means ±S.E.M. “**” and “***” represent p value < 0.01 and 0.005, respectively, by using Students t-test.
Mac-1 cells expressing both CCR4 and IL-2Rs were then used to further examine if mAb2-3 affected the binding of IL-2 to IL-2R in a competition assay. The results showed that like the anti-TAC (IL-2Rα) mAb, mAb2-3 effectively inhibited the binding of biotinylated-IL-2 to Mac-1 cells when compared to treatment with the control mAb that does not block IL-2 binding (Fig. S10A and S10B).Citation27 In addition, the Mac-1 culture supernatants were harvested following treatment with exogenous IL-2 and different mAbs, and subjected to ELISA assay for soluble IL-2 detection. Treatment with both anti-TAC mAb and mAb2-3 lead to increased IL-2 level in the culture supernatant presumably due to inhibition of exogenous IL-2 binding to Mac-1 cells, but control mAb-treated or untreated groups did not (Fig. S10C).
It is known that IL-2Rs consists of three subunits, α, β, and γ chains, and the α chain markedly increases the affinity of the receptor to IL-2, from Kd = 1 nM (βγ chains) to Kd = 10 pM (αβγ chains). In control experiments with transiently transfected 293T cell, inhibition of IL-2 binding was found not to be due to direct binding of mAb2-3 to the individual α, β, and γ subunit chains or to the complex (data not shown). CD25 is also reportedly cleaved to a soluble form (sCD25)Citation28 with Kd = 30 nM following T cell activation.Citation29 We next monitored sCD25 in the culture supernatant following treatment with mAb2-3 or control mAb. The data revealed that the level of sCD25 in the supernatant was increased when Tregs were treated with mAb2-3 in a dose-dependent manner () and this effect was positively correlated with the time of incubation (Fig. S10D–F). To further determine the function of mAb2-3-mediated sCD25 shedding on Treg survival, Tregs were cultured with IL-2 and/or mAb2-3/control IgG1. As expected, IL-2 showed the capacity to maintain Treg survival, but interestingly, the positive effect of IL-2 on Treg survival was inhibited by mAb2-3 (). These results demonstrate that mAb2-3 engagement of CCR4 on Tregs leads to modulation of the IL-2/IL-2R complex that can result in increased Treg death.
sCD25 shedding by mAb2-3 is shared by CCR4 ligands CCL17/22 and mediated through metalloproteinase 9 (MMP-9)
Since mAb2-3 can block CCL17/22 interaction with CCR4, we sought to understand whether CCL17/22 engagement of CCR4 might induce sCD25 shedding similarly to mAb2-3. In Fig. S11, both CCL17 and CCL22 ligands showed the activity to induced sCD25 shedding which has not been previously reported. In addition, several studies have shown that matrix metalloproteinase 9 (MMP-9) possesses the capacity to cleave CD25.Citation32-34 To investigate the mechanisms of action of mAb2-3, CCL17, and CCL22 in inducing sCD25 shedding, the cell cultures were treated with MMP-9 inhibitor or control MMP inhibitor. Interestingly, the MMP-9 inhibitor reduced sCD25 in all treatment groups with no effect observed from the control inhibitor (Fig. S11). These results indicate that mAb2-3 treatment leads to the cleavage of CD25 in a similar manner as CCL17/22,Citation30,31 suggesting that mAb2-3 possesses agonist activities and shares the capacity with the CCR4 ligands to activate MMP-9 function and sCD25 cleavage, with the end result being loss of IL-2 binding.
Discussion
The tumor microenvironment contains a plethora of mixed immune cell types that play a paradoxical role in tumor immunosurveillance by either activating antitumor responses or promoting tumor progression.Citation35,36 It is now recognized that Treg infiltration is widespread in many cancers where they are presumed to have a role in mediating local immunosuppression.Citation19,37 One important role of human mAbs in cancer immunotherapy lies in their capacity to reverse the immune dysregulation and in our current study human anti-CCR4 mAb, mAb2-3, has been used to deplete or block Treg functional activity. We demonstrate that circa 85% of peripheral blood CD4+CD25+FoxP3+ Tregs overexpress CCR4 compared to Teffs and they are responsible for the majority of suppressor activity. In vitro studies demonstrated that CCR4+ Treg depletion by mAb2-3 inhibits Treg chemotactic activity to CCL22-expressing OvCA cells and restores Teff proliferation and anti-OvCA immunity. In a humanized mouse model bearing an OvCA xenograft, both mAb2-3 IgG1 and IgG4 isotypes showed therapeutic capability to modulate human Treg function and enhance antitumor activity.
MAb2-3 has both shared and distinct properties from Mogamulizumab, a defucosylated humanized anti-CCR4 IgG1 mAb that is approved by the Japanese FDA for the treatment of chemotherapy naive and relapsed/refractory adult T cell leukemia and for relapsed/refractory peripheral T cell lymphoma (PTCL) and cutaneous T cell leukemia (CTCL). Both mAbs have modified Fc functions to enhance ADCC activityCitation21,38 and are effective in depleting CCR4+ tumor cells in humanized mouse models.Citation39,40 In a subset of ATL patients, the CD4+CD25+Foxp3+ cells appear to function as autologous Tregs.Citation41,42 Two reports show a reduction of peripheral blood Tregs in ATLCitation22 and CTCLCitation43 patients treated with this mAb drug. Additional clinical studies with Mogamulizumab are underway in US and Europe for treatment of advanced and/or metastatic CCR4− solid tumors with the goal of depleting infiltrating Tregs. In contrast to Mogamulizumab, mAb2-3 additionally mediates potent complement-dependent cytotoxicity (CDC) that may be the result of an optimal orientation of the Fc region, allowing the angle of attachment to be permissive for complement pore formation.Citation21 Another distinguishing feature is that mAb2-3 recognizes a conformational epitope that encompasses the N-terminal domain and the extracellular loops that mediates biological signaling.Citation21,44-47 In this regard, mAb2-3 treatment of Mac1 cells and Tregs led to enhanced sCD25 shedding, a property that was shared by the CCR4 ligands CCL22 and CCL17. This shared activity raises the possibility that mAb2-3 has agonist activity and triggers cell activation which results in CD25 cleavage. Studies of CCR4 signaling through CCL22/CCL17 binding have shown evidence of PI(3) kinase/AKT activation.Citation30,31 In addition, distinct conformations of CCR4 have been reported to respond differently to the two ligands, a property that is supported by our evidence that CCL22 and mAb2-3 are more potent activators of sCD25 shedding than is CCL17.Citation48,49
High level CD25 expression on Tregs leads to formation of the trimeric high affinity IL-2 receptor that supports greater IL-2 binding which has been shown to be required for survival.Citation50 The increased cleavage of CD25 will result in decreased affinity of IL-2 for the IL-2βγR complex on Tregs. Our data demonstrate that mAb2-3 treatment also leads to blockade of IL-2 uptake by Tregs and inhibition of IL-2-mediated survival which may play a role in the in vivo antitumor effects seen with non-immunodepleting mAb2-3 IgG4. Elevations in sCD25 have been reported following anti-CTLA-4 mAb treatment of multiple myeloma patients although the mechanisms of sCD25 release were not investigated.Citation51 sCD25 functions as both a surrogate marker of T cell activation as well as an indicator of subsequent cellular death and therefore it could be expected to play varying roles depending on the local environment in regards to IL-2 concentration, cellular activation state, and the amount of sCD25 present. In addition, the role of CD25 in immunomodulation is likely dependent on the local inflammatory milieu, with molecules capable of modulating surface CD25 expression playing a key role in defining immune responsiveness.Citation32 Indeed, while sCD25 has been reported to act as a decoy to inhibit antitumor T cell responses,Citation52 other studies have shown that mDCs can lend their CD25 to primed T cell in trans to facilitate early high affinity IL-2 signaling.Citation53,54 Whether sCD25 can function in trans to activate Teff cells should be further investigated.
The new therapeutic approach of modulating T cell immune responses to restore antitumor immunity has shown encouraging results in the clinic. Strategies of targeting CD25 with anti-CD25 mAbs or IL-2-toxin fusion proteins in both murine models and humans have demonstrated that depletion of Tregs leads to tumor rejection by enhancing antitumor immune responses.Citation55,56 However, these strategies also affect activated Teffs and DCs, resulting in toxicity due to lack of specificity for Tregs.Citation57 The primary action of anti-CTLA-4 blocking antibodies is to restore CD28 binding to CD80/CD86 and remove an inhibitory checkpoint on proliferation and function to enhance Teff activity.Citation58 However, anti-CTLA4 also inhibits Treg function mainly though antibody-dependent cellular phagocytosis (ADCP) that is dependent on the presence of FcγR-expressing macrophages within the tumor microenvironment.Citation59 The clinical data for anti-CTLA-4 mAb blockade has shown objective responses in patients with melanoma, but significant antitumor effects were only observed in a minority of the OvCA patients.Citation60 The PD-1/PD-L1 is an inhibitory immune checkpoint pathway of lymphocyte activation. Recent studies using PD-1 blocking antibody showed clinical benefit in patients with malignancies.Citation61,62 However, a trial on the use of anti-PD-1 IgG4 in OvCA patients revealed a response rate of only 23% (3/13 patients).Citation63 These results document an urgent need to develop new human antibody drugs that are effective in restoring host anti-OvCA immunity. The development of novel therapeutic strategies that specifically target Tregs and abolish their suppressive function will provide an important new translational pathway to pursue.
In this study, the biological functions of both IgG1 and IgG4 isotypes of mAb2-3 were tested and showed similar capacity to block CCR4+Treg migration in vitro but revealed their different mechanisms of action in vivo. In particular, mAb2-3 IgG1 induced a profound immunodepletion of Tregs as evidenced by in vivo clearance studies and decreased tumor cell infiltration. In OvCA xenograft studies, mAb2-3 IgG1 treatment led to marked inhibition of tumor cell growth and in two animal studies the mice showed significant weight loss. In contrast, the IgG4 isotype appeared to work primarily through ligand-receptor blockade. In vivo trafficking studies showed that this isotype caused blockade of Treg chemotaxis to CCL22 secreting OvCA tumors and a decrease in tumor cell infiltration. The IgG4 isotype also caused a slower and less complete depletion of Tregs. The slower in vivo clearance observed for IgG4-mediated depletion of Tregs may be through a different mechanism of action as a recent report showed that IgG4 isotype has similar ADCP capacity to IgG1.Citation64 In addition, mAb2-3 IgG4 treatment showed lesser antitumor effect, however, the mice had no weight loss. These results suggest that the two mAb2-3 isotypes may have unique roles at different stages of OvCA disease with IgG4 treatment having a possibly preferred role at earlier stages when tumor burden is smaller and immune dysfunction is more easily reversed.
Development of an effective immune-based therapy for OvCA is urgently needed due to the high recurrence and mortality rates with current treatments. Recent studies using WBCs from ascites and peripheral blood of OvCA patients have demonstrated upregulation of surface markers that are associated with T cell exhaustion (e.g. PD-1, TIM-3) as well as an increase in Tregs.Citation65 This suggests that immune dysregulation is not just localized to tumor-associated lymphocytes (TALs) and TILs but is a systemic manifestation of the host. Patient derived tumor models (PDX) in immunocompromised mice are being used increasingly to test personalized cancer therapies but have been primarily focused on assessing the effects of small molecule drugs because the mouse models lack patient immune cells.Citation66 An alternative strategy toward advancing antibody-based cancer immunotherapies is to establish a PDX mouse model in which the critical interactions between the patient's tumor and immune cells can be interrogated. Here, we show in a prototypic model using allogeneic immune cells that TP-T cell immunity can be established and that the biological activity of Tregs can be modulated by the administration of mAb2-3, including assessing the role of the IgG1 and IgG4 isotypes in modulating tumor growth by Treg depletion and blockage activities, respectively. Moreover, this model may also allow combination immunotherapy to be assessed as there is growing laboratory and clinical evidence that concurrently targeting PD-1 and CTLA-4 leads to more profound effects on modulation of antitumor immunity in vivo.Citation67 The development of an HGSOC patient PDX model that could pre-clinically assess the antitumor efficacy of mAb2-3 alone and in combination with other immunotherapies would be particularly important to rapidly advance this promising new treatment for OvCA.
Materials and methods
Determining molecular densities on the surfaces of T cells
T cells were incubated with PacBlue-anti-CD3, BV570-anti-CD4, APC-anti-CD25, PE-Cy7-anti-CD127, PE-Cy5-anti-CD45RA, PerCP-Cy5.5-CCR7, and PE-anti-CCR4 mAbs at the concentration recommended in the datasheet. Cells were stained in 100 μL of FACS buffer (PBS supplemented with 5 mM EDTA and 1% BSA) at 4°C for 30 min. T cells were gated into different T cell subsets according to the CD markers and analyzed for PE fluorescent intensity. The fluorescent intensities were compared to standard calibration BD QuantiBRITE PE Beads (BD Biosciences, San Jose, CA) to determine the total number of molecules per cell/bead, which were divided by the cell/bead surface area to obtain site densities.
Treg suppression and survival assay
CD4+CD25− T cells were labeled with CFSE (BioLegend, San Diego, CA) at the concentration of 5 μM and cultured in 96-well plates at 5 × 104 cells/well in the presence and absence of 20 µg/mL phytohemagglutinin (PHA, Sigma, St. Louis, MO) as positive and negative control for T cell proliferation, respectively. CD4+ and CD4+CCR4− Tregs were isolated using Treg Cell Enrichment Kit (StemCell, Vancouver, Canada) and mAb2-3-conjugated Dynabeads M-280 (Life Technologies, Carlsbad, CA). 5 × 103 CD4+ and CD4+CCR4− Tregs were individually incubated with CFSE-labeled CD4+CD25− T cells at 37°C for 7 d. To measure the proliferation of CFSE-labeled T cells, co-cultured cells were stained with Viability Dye eFluor 506 (eBioscience, San Diego, CA) and then the live CFSE+ cells were gated and analyzed using flow cytometry.
For survival assay, CD4+CD127dim/−CD49d− Tregs were isolated from PBMCs using Treg Cell Enrichment Kit. 1 × 105 Tregs were cultured with 0.5 IU/mL IL-2, 20 µg/mL mAb2-3 IgG1, and 20 µg/mL control IgG1 separately or in combinations, and incubated at 37oC for 5 d Cells were then stained with Viability Dye (eBioscience) and analyzed using flow cytometry.
Cells
OvCA cell lines, IGROV-1, OVCAR-5, and OVCAR-8, were incubated at 37°C in a 5% CO2-containing atmosphere and were provided courtesy of Dr Ronny I. Drapkin at 2013. OVCAR-5 and OVCAR-8 were cultured in RPMI-1640 (Life Technologies) supplemented with 10% fetal bovine serum (FBS) and 1% penicillin/streptomycin (Life Technologies). IGROV-1 was cultured in 10% FBS and 1% penicillin/streptomycin Dulbecco's modification of Eagle medium (DMEM, Life Technologies). Luciferase-expressed IGROV-1 and T cells were stably transduced with a luciferase reporter retrovirus and authenticated by detecting luminescence. IGROV-1 and OVCAR-8 were genotyped at the Broad Institute at 2011. No additional authentication of these cell lines was conducted by the authors.
Animals
Six–eight weeks-old female NOD.Cg-Prkdcscid Il2rgtm1Wjl/SzJ (NSG) mice (The Jackson Laboratory, Bar Harbor, ME) were used in this study. 2 × 106 or 5 × 106 luciferase-expressing IGROV-1 cells were injected into the dorsolateral flank subcutaneously (s.c.) in NSG mice and incubated for one or three days, respectively. Then, mice were randomly assigned into different groups and treated with IGROV-1-primed T cells (4 × 106 or 1 × 107) and 3 mg/kg mAb2-3 IgG1, mAb2-3 IgG4 and control mAb (twice a week for five weeks) by i.v. injection. Body weight and tumor size were measured using digital calipers and Xenogen in vivo imaging system (Xenogen IVIS-200, Alameda, CA). Tumor volumes were calculated as length×(width)2×0.52. Animal care was carried out in accordance with the guidelines of Animal Care and Use Committee of Dana-Farber Cancer Institute (Boston, MA).
Chemotaxis
T cells (1 × 106 cells/well) were placed in transwell migration wells (Corning, Tewksbury, MA) with or without mAb2-3 for 5 h at 37°C. Migrated cells were harvested from the bottom chamber containing OvCA cell-cultured medium or 100 ng/mL human CCL22 (R&D Systems) and enumerated by FACS analysis. The OvCA cell-cultured medium was harvested from the supernatant of IGROV-1-, OVCAR-5-, and OVCAR-8-cultured medium (1 × 106 cells/mL). T cell migration was calculated as a percentage relative to culture or CCL22-supplemented medium.
Establishment of tumor-primed T (TP-T) cells
PBMCs (2 × 106/mL) were incubated with autologous IGROV-1-pulsed DCs (2 × 105/mL) in complete medium containing recombinant IL-2 (30 IU/mL) and IL-7 (5 ng/mL). Cells were incubated in 50-mL tissue culture flasks at 37°C in 5% CO2 incubator. PBMCs were re-stimulated with lysate-pulsed autologous DCs every two weeks, and the cultures were fed every 5 d with fresh medium containing recombinant IL-2 and IL-7. After three to four cycles of antigen stimulation and selection, TP-T cells were established, and cells were expanded in complete medium containing recombinant IL-2 and IL-7 for two weeks and were subjected to functional tests.
Analysis of cytokine production
TP-T cells (1 × 105) were incubated with autologous IGROV-1-pulsed DCs (2 × 104), unpulsed DCs (2 × 104), or Dynabeads Human T-Activator CD3/CD28 (Life Technologies) in the complete medium at 37°C. After 48-h incubation, the supernatant was harvested and IFNγ was detected using Human IFNγ Reagent Kit (Pierce Biotechnology, Rockford, IL) and Meso Scale Discovery Sector Imager 2400 (MSD, Rockville, MD). In addition, TP-T cells were incubated with mAb2-3-conjugated beads to deplete CCR4+ TP-T cells. TP-T or CCR4− TP-T cells (1 × 105) were incubated with IGROV-1 (1 × 104) in the presence or absence of 20 µg/mL of mAb2-3 IgG1 or IgG4 in complete medium. After 24 and 48 h incubation, IFNγ production by TP-T cells was assessed by MSD and intracellular FACS analysis.
Supernatant cytokines, IL-2, and sCD25 detection
Cytokines and soluble CD25 were detected in cell culture supernatants using ELISA Ready-SET-Go! Kits for IL-2, IL-10, and TGF-β (eBioscience) and Human IL-2 sRα ELISA Set (BD Biosciences) according to manufacturer instructions. Samples were diluted (when necessary) in RPMI-1640 medium. For IL-10 and TGF-β, autologous CD4+CD25 and CD4+CD25+ T cells (1:1) were cultured with 10% FBS RPMI-1640 medium in anti-CD3/28 (1/0.5 µg/mL) coated plates in the presence or absence of 20 µg/mL antibodies. For IL-2, Teffs and Tregs alone or co-culture were incubated with 10% FBS RPMI-1640 medium in the presence or absence of exogenous IL-2 or antibodies. In the presence of exogenous IL-2 (20 IU/mL), 2 × 105 Mac-1 cells were cultured with or without antibodies (mAb2-3 or anti-CD25, including anti-TAC and control mAbs). For competition assay, Mac-1 cells were stained with biotinylated IL-2 in the presence or absence of different concentration of antibodies and then detected by FACS. For sCD25 study, Mac-1 cells or Tregs were incubated with mAb2-3, CCL17, or CCL22 in the presence and absence of MMP-9 inhibitor (CAS 1177749-58-4) or GM6001, negative control from Calbiochem (EMD Biosciences, San Diego, CA). Cells were incubated for 12, 24, and 48 h and then cultured supernatant were harvested for ELISA.
Statistical analyses
Data were analyzed using two-sided unpaired Student t-test and two-way ANOVA for in vitro and in vivo experiments, respectively. *, **, and *** indicate p value < 0.05, 0.01, and 0.005, respectively.
Disclosure of potential conflicts of interest
MAb2-3 has been licensed for clinical development from Dana-Farber Cancer Institute. Dr Marasco has a financial interest in the company but receives no research support and no materials from the company were used in this research.
KONI_A_1090075_supplementary_material.zip
Download Zip (40.7 MB)Funding
This study was supported by grants from DFCI Women's Cancer Program grant (WAM), Honorable Tina Brozman Foundation (RD), the Dr Miriam and Sheldon G. Adelson Medical Research Foundation (RD), NIH U01 CA-152990 (RD) and the Robert and Debra First Fund (RD).
References
- Stewart BW, Wild CP. World Cancer Report 2014. France: WHO Press, World Health Organization
- Agarwal R, Kaye SB. Ovarian cancer: strategies for overcoming resistance to chemotherapy. Nat Rev Cancer 2003; 3:502-16; PMID:12835670; http://dx.doi.org/10.1038/nrc1123
- DiSaia PJ, Bloss JD. Treatment of ovarian cancer: new strategies. Gynecol Oncol 2003; 90:S24-32; PMID:12928003; http://dx.doi.org/10.1016/S0090-8258(03)00341-X
- Disis ML, Rivkin S. Future directions in the management of ovarian cancer. Hematol Oncol Clin North Am 2003; 17:1075-85; PMID:12959192; http://dx.doi.org/10.1016/S0889-8588(03)00054-6
- Chatterjee M, Tainsky MA. Autoantibodies as biomarkers for ovarian cancer. Cancer Biomark 2010; 8:187-201; PMID:22045353; http://dx.doi.org/10.3233/CBM-2011-0213.
- Duraiswamy J, Kaluza KM, Freeman GJ, Coukos G. Dual blockade of PD-1 and CTLA-4 combined with tumor vaccine effectively restores T-cell rejection function in tumors. Cancer Res 2013; 73:3591-603; PMID:23633484; http://dx.doi.org/10.1158/0008-5472.CAN-12-4100
- Phan GQ, Yang JC, Sherry RM, Hwu P, Topalian SL, Schwartzentruber DJ, Restifo NP, Haworth LR, Seipp CA, Freezer LJ et al. Cancer regression and autoimmunity induced by cytotoxic T lymphocyte-associated antigen 4 blockade in patients with metastatic melanoma. Proc Natl Acad Sci U S A 2003; 100:8372-7; PMID:12826605; http://dx.doi.org/10.1073/pnas.1533209100
- Hodi FS, Mihm MC, Soiffer RJ, Haluska FG, Butler M, Seiden MV, Davis T, Henry-Spires R, MacRae S, Willman A et al. Biologic activity of cytotoxic T lymphocyte-associated antigen 4 antibody blockade in previously vaccinated metastatic melanoma and ovarian carcinoma patients. Proc Natl Acad Sci U S A 2003; 100:4712-7; PMID:12682289; http://dx.doi.org/10.1073/pnas.0830997100
- Soares KC, Rucki AA, Wu AA, Olino K, Xiao Q, Chai Y, Wamwea A, Bigelow E, Lutz E, Liu L et al. PD-1/PD-L1 blockade together with vaccine therapy facilitates effector T-cell infiltration into pancreatic tumors. J Immunother 2015; 38:1-11; PMID:25415283; http://dx.doi.org/10.1097/CJI.0000000000000062
- Anagnostou VK, Brahmer JR. Cancer Immunotherapy: A Future Paradigm Shift in the Treatment of Non-Small Cell Lung Cancer. Clin Cancer Res 2015; 21:976-84; PMID:25733707; http://dx.doi.org/10.1158/1078-0432.CCR-14-1187
- Boon T, Cerottini JC, Van den Eynde B, van der Bruggen P, Van Pel A. Tumor antigens recognized by T lymphocytes. Annu Rev Immunol 1994; 12:337-65; PMID:8011285; http://dx.doi.org/10.1146/annurev.iy.12.040194.002005
- Sakaguchi S, Yamaguchi T, Nomura T, Ono M. Regulatory T cells and immune tolerance. Cell 2008; 133:775-87; PMID:18510923; http://dx.doi.org/10.1016/j.cell.2008.05.009
- Wood KJ, Sakaguchi S. Regulatory T cells in transplantation tolerance. Nat Rev Immunol 2003; 3:199-210; PMID:12658268; http://dx.doi.org/10.1038/nri1027
- Valzasina B, Piconese S, Guiducci C, Colombo MP. Tumor-induced expansion of regulatory T cells by conversion of CD4+CD25- lymphocytes is thymus and proliferation independent. Cancer Res 2006; 66:4488-95; PMID:16618776; http://dx.doi.org/10.1158/0008-5472.CAN-05-4217
- Woo EY, Chu CS, Goletz TJ, Schlienger K, Yeh H, Coukos G, Rubin SC, Kaiser LR, June CH. Regulatory CD4(+)CD25(+) T cells in tumors from patients with early-stage non-small cell lung cancer and late-stage ovarian cancer. Cancer Res 2001; 61:4766-72; PMID:11406550
- Curiel TJ, Coukos G, Zou L, Alvarez X, Cheng P, Mottram P, Evdemon-Hogan M, Conejo-Garcia JR, Zhang L, Burow M et al. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med 2004; 10:942-9; PMID:15322536; http://dx.doi.org/10.1038/nm1093
- Baecher-Allan C, Anderson DE. Regulatory cells and human cancer. Semin Cancer Biol 2006; 16:98-105; PMID:16378733; http://dx.doi.org/10.1016/j.semcancer.2005.11.003
- Zhang L, Conejo-Garcia JR, Katsaros D, Gimotty PA, Massobrio M, Regnani G, Makrigiannakis A, Gray H, Schlienger K, Liebman MN et al. Intratumoral T cells, recurrence, and survival in epithelial ovarian cancer. N Engl J Med 2003; 348:203-13; PMID:12529460; http://dx.doi.org/10.1056/NEJMoa020177
- Kurose K, Ohue Y, Sato E, Yamauchi A, Eikawa S, Isobe M, Nishio Y, Uenaka A, Oka M, Nakayama E. Increase in activated Treg in TIL in lung cancer and in vitro depletion of Treg by ADCC using an antihuman CCR4 mAb (KM2760). J Thorac Oncol 2015; 10:74-83; PMID:25325779; http://dx.doi.org/10.1097/JTO.0000000000000364
- Preston CC, Maurer MJ, Oberg AL, Visscher DW, Kalli KR, Hartmann LC, Goode EL, Knutson KL. The ratios of CD8+ T cells to CD4+CD25+ FOXP3+ and FOXP3- T cells correlate with poor clinical outcome in human serous ovarian cancer. PloS one 2013; 8:e80063; PMID:24244610; http://dx.doi.org/10.1371/journal.pone.0080063
- Chang DK, Sui J, Geng S, Muvaffak A, Bai M, Fuhlbrigge RC, Lo A, Yammanuru A, Hubbard L, Sheehan J et al. Humanization of an anti-CCR4 antibody that kills cutaneous T-cell lymphoma cells and abrogates suppression by T-regulatory cells. Mol Cancer Ther 2012; 11:2451-61; PMID:22869555; http://dx.doi.org/10.1158/1535-7163.MCT-12-0278
- Sugiyama D, Nishikawa H, Maeda Y, Nishioka M, Tanemura A, Katayama I, Ezoe S, Kanakura Y, Sato E, Fukumori Y et al. Anti-CCR4 mAb selectively depletes effector-type FoxP3+CD4+ regulatory T cells, evoking antitumor immune responses in humans. Proc Natl Acad Sci U S A 2013; 110:17945-50; PMID:24127572; http://dx.doi.org/10.1073/pnas.1316796110
- Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol 2003; 4:330-6; PMID:12612578; http://dx.doi.org/10.1038/ni904
- Bruhns P, Iannascoli B, England P, Mancardi DA, Fernandez N, Jorieux S, Daëron M. Specificity and affinity of human Fcgamma receptors and their polymorphic variants for human IgG subclasses. Blood 2009; 113:3716-25; PMID:19018092; http://dx.doi.org/10.1182/blood-2008-09-179754
- Domcke S, Sinha R, Levine DA, Sander C, Schultz N. Evaluating cell lines as tumour models by comparison of genomic profiles. Nat Commun 2013; 4:2126; PMID:23839242; http://dx.doi.org/10.1038/ncomms3126
- Vignali DA, Collison LW, Workman CJ. How regulatory T cells work. Nat Rev Immunol 2008; 8:523-32; PMID:18566595; http://dx.doi.org/10.1038/nri2343
- Richardson JH, Sodroski JG, Waldmann TA, Marasco WA. Phenotypic knockout of the high-affinity human interleukin 2 receptor by intracellular single-chain antibodies against the α subunit of the receptor. Proc Natl Acad Sci U S A 1995; 92:3137-41; PMID:7724529; http://dx.doi.org/10.1073/pnas.92.8.3137
- Sheu BC, Hsu SM, Ho HN, Lien HC, Huang SC, Lin RH. A novel role of metalloproteinase in cancer-mediated immunosuppression. Cancer Res 2001; 61:237-42; PMID:11196168
- Jacques Y, Le Mauff B, Boeffard F, Godard A, Soulillou JP. A soluble interleukin 2 receptor produced by a normal alloreactive human T cell clone binds interleukin 2 with low affinity. J Immunol 1987; 139:2308-16; PMID:2821110
- Cronshaw DG, Owen C, Brown Z, Ward SG. Activation of phosphoinositide 3-kinases by the CCR4 ligand macrophage-derived chemokine is a dispensable signal for T lymphocyte chemotaxis. J Immunol 2004; 172:7761-70; http://dx.doi.org/10.4049/jimmunol.172.12.7761
- Nakagawa M, Schmitz R, Xiao W, Goldman CK, Xu W, Yang Y, Yu X, Waldmann TA, Staudt LM. Gain-of-function CCR4 mutations in adult T cell leukemia/lymphoma. J Exp Med 2014; 211:2497-505; PMID:25488980; http://dx.doi.org/10.1084/jem.20140987
- Brusko TM, Wasserfall CH, Hulme MA, Cabrera R, Schatz D, Atkinson MA. Influence of membrane CD25 stability on T lymphocyte activity: implications for immunoregulation. PloS One 2009; 4:e7980; PMID:19956753; http://dx.doi.org/10.1371/journal.pone.0007980
- De Paiva CS, Yoon KC, Pangelinan SB, Pham S, Puthenparambil LM, Chuang EY, Farley WJ, Stern ME, Li DQ, Pflugfelder SC. Cleavage of functional IL-2 receptor α chain (CD25) from murine corneal and conjunctival epithelia by MMP-9. J Inflamm 2009; 6:31; PMID:19878594; http://dx.doi.org/10.1186/1476-9255-6-31
- Mortier E, Bernard J, Plet A, Jacques Y. Natural, proteolytic release of a soluble form of human IL-15 receptor α-chain that behaves as a specific, high affinity IL-15 antagonist. J Immunol 2004; 173:1681-8; PMID:15265897; http://dx.doi.org/10.4049/jimmunol.173.3.1681
- Lanca T, Silva-Santos B. The split nature of tumor-infiltrating leukocytes: Implications for cancer surveillance and immunotherapy. Oncoimmunology 2012; 1:717-25; PMID:22934263; http://dx.doi.org/10.4161/onci.20068
- Zou W. Immunosuppressive networks in the tumour environment and their therapeutic relevance. Nat Rev Cancer 2005; 5:263-74; PMID:15776005; http://dx.doi.org/10.1038/nrc1586
- Ishida T, Ueda R. Immunopathogenesis of lymphoma: focus on CCR4. Cancer Sci 2011; 102:44-50; PMID:21044233; http://dx.doi.org/10.1111/j.1349-7006.2010.01767.x
- Niwa R, Shoji-Hosaka E, Sakurada M, Shinkawa T, Uchida K, Nakamura K, Matsushima K, Ueda R, Hanai N, Shitara K. Defucosylated chimeric anti-CC chemokine receptor 4 IgG1 with enhanced antibody-dependent cellular cytotoxicity shows potent therapeutic activity to T-cell leukemia and lymphoma. Cancer Res 2004; 64:2127-33; PMID:15026353; http://dx.doi.org/10.1158/0008-5472.CAN-03-2068
- Han T, Abdel-Motal UM, Chang DK, Sui J, Muvaffak A, Campbell J, Zhu Q, Kupper TS, Marasco WA. Human anti-CCR4 minibody gene transfer for the treatment of cutaneous T-cell lymphoma. PloS one 2012; 7:e44455; PMID:22973452; http://dx.doi.org/10.1371/journal.pone.0044455
- Ito A, Ishida T, Yano H, Inagaki A, Suzuki S, Sato F, Takino H, Mori F, Ri M, Kusumoto S et al. Defucosylated anti-CCR4 monoclonal antibody exercises potent ADCC-mediated antitumor effect in the novel tumor-bearing humanized NOD/Shi-scid, IL-2Rgamma(null) mouse model. Cancer Immunol Immunother 2009; 58:1195-206; PMID:19048251; http://dx.doi.org/10.1007/s00262-008-0632-0
- Yano H, Ishida T, Inagaki A, Ishii T, Kusumoto S, Komatsu H, Iida S, Utsunomiya A, Ueda R. Regulatory T-cell function of adult T-cell leukemia/lymphoma cells. Int J Cancer 2007; 120:2052-7; PMID:17278106; http://dx.doi.org/10.1002/ijc.22536
- Karube K, Ohshima K, Tsuchiya T, Yamaguchi T, Kawano R, Suzumiya J, Utsunomiya A, Harada M, Kikuchi M. Expression of FoxP3, a key molecule in CD4CD25 regulatory T cells, in adult T-cell leukaemia/lymphoma cells. Br J Haematol 2004; 126:81-4; PMID:15198736; http://dx.doi.org/10.1111/j.1365-2141.2004.04999.x
- Ni X, Jorgensen JL, Goswami M, Challagundla P, Decker WK, Kim YH, Duvic MA. Reduction of regulatory T cells by Mogamulizumab, a defucosylated anti-CC chemokine receptor 4 antibody, in patients with aggressive/refractory mycosis fungoides and Sezary syndrome. Clin Cancer Res 2015; 21:274-85; PMID:25376389; http://dx.doi.org/10.1158/1078-0432.CCR-14-0830
- Katritch V, Cherezov V, Stevens RC. Diversity and modularity of G protein-coupled receptor structures. Trends Pharmacol Sci 2012; 33:17-27; PMID:22032986; http://dx.doi.org/10.1016/j.tips.2011.09.003
- Lagerstrom MC, Schioth HB. Structural diversity of G protein-coupled receptors and significance for drug discovery. Nat Rev Drug Discov 2008; 7:339-57; PMID:18382464; http://dx.doi.org/10.1038/nrd2518
- Rosenbaum DM, Rasmussen SG, Kobilka BK. The structure and function of G-protein-coupled receptors. Nature 2009; 459:356-63; PMID:19458711; http://dx.doi.org/10.1038/nature08144
- Vaidehi N, Kenakin T. The role of conformational ensembles of seven transmembrane receptors in functional selectivity. Curr Opin Pharmacol 2010; 10:775-81; PMID:20933468; http://dx.doi.org/10.1016/j.coph.2010.09.004
- Viney JM, Andrew DP, Phillips RM, Meiser A, Patel P, Lennartz-Walker M, Cousins DJ, Barton NP, Hall DA, Pease JE. Distinct conformations of the chemokine receptor CCR4 with implications for its targeting in allergy. J Immunol 2014; 192:3419-27; http://dx.doi.org/10.4049/jimmunol.1300232; PMID:24563252
- Santulli-Marotto S, Boakye K, Lacy E, Wu SJ, Luongo J, Kavalkovich K, Coelho A, Hogaboam CM, Ryan M. Engagement of two distinct binding domains on CCL17 is required for signaling through CCR4 and establishment of localized inflammatory conditions in the lung. PloS One 2013; 8:e81465; PMID:24339934; http://dx.doi.org/10.1371/journal.pone.0081465
- Boyman O, Kovar M, Rubinstein MP, Surh CD, Sprent J. Selective stimulation of T cell subsets with antibody-cytokine immune complexes. Science 2006; 311:1924-7; PMID:16484453; http://dx.doi.org/10.1126/science.1122927
- Hannani D, Vetizou M, Enot D, Rusakiewicz S, Chaput N, Klatzmann D, Desbois M, Jacquelot N, Vimond N, Chouaib S et al. Anticancer immunotherapy by CTLA-4 blockade: obligatory contribution of IL-2 receptors and negative prognostic impact of soluble CD25. Cell Res 2015; 25:208-24; PMID:25582080; http://dx.doi.org/10.1038/cr.2015.3
- Lindqvist CA, Christiansson LH, Simonsson B, Enblad G, Olsson-Stromberg U, Loskog AS. T regulatory cells control T-cell proliferation partly by the release of soluble CD25 in patients with B-cell malignancies. Immunology 2010; 131:371-6; PMID:20518821; http://dx.doi.org/10.1111/j.1365-2567.2010.03308.x
- Wuest SC, Edwan JH, Martin JF, Han S, Perry JS, Cartagena CM, Matsuura E, Maric D, Waldmann TA, Bielekova B. A role for interleukin-2 trans-presentation in dendritic cell-mediated T cell activation in humans, as revealed by daclizumab therapy. Nat Med 2011; 17:604-9; PMID:21532597; http://dx.doi.org/10.1038/nm.2365
- Wiendl H, Gross CC. Modulation of IL-2Ralpha with daclizumab for treatment of multiple sclerosis. Nat Rev Neurol 2013; 9:394-404; PMID:23732529; http://dx.doi.org/10.1038/nrneurol.2013.95
- Kudo-Saito C, Schlom J, Camphausen K, Coleman CN, Hodge JW. The requirement of multimodal therapy (vaccine, local tumor radiation, and reduction of suppressor cells) to eliminate established tumors. Clin Cancer Res 2005; 11:4533-44; PMID:15958639; http://dx.doi.org/10.1158/1078-0432.CCR-04-2237
- Morse MA, Hobeika AC, Osada T, Serra D, Niedzwiecki D, Lyerly HK, Clay TM. Depletion of human regulatory T cells specifically enhances antigen-specific immune responses to cancer vaccines. Blood 2008; 112:610-8; PMID:18519811; http://dx.doi.org/10.1182/blood-2008-01-135319
- Curtin JF, Candolfi M, Fakhouri TM, Liu C, Alden A, Edwards M, Lowenstein PR, Castro MG. Treg depletion inhibits efficacy of cancer immunotherapy: implications for clinical trials. PloS one 2008; 3:e1983; PMID:18431473; http://dx.doi.org/10.1371/journal.pone.0001983
- Dilek N, Poirier N, Hulin P, Coulon F, Mary C, Ville S, Vie H, Clémenceau B, Blancho G, Vanhove B. Targeting CD28, CTLA-4 and PD-L1 costimulation differentially controls immune synapses and function of human regulatory and conventional T-cells. PloS one 2013; 8:e83139; PMID:24376655; http://dx.doi.org/10.1371/journal.pone.0083139
- Simpson TR, Li F, Montalvo-Ortiz W, Sepulveda MA, Bergerhoff K, Arce F, Roddie C, Henry JY, Yagita H, Wolchok JD et al. Fc-dependent depletion of tumor-infiltrating regulatory T cells co-defines the efficacy of anti-CTLA-4 therapy against melanoma. J Exp Med 2013; 210:1695-710; PMID:23897981; http://dx.doi.org/10.1084/jem.20130579
- Hodi FS, Butler M, Oble DA, Seiden MV, Haluska FG, Kruse A, Macrae S, Nelson M, Canning C, Lowy I et al. Immunologic and clinical effects of antibody blockade of cytotoxic T lymphocyte-associated antigen 4 in previously vaccinated cancer patients. Proc Natl Acad Sci U S A 2008; 105:3005-10; PMID:18287062; http://dx.doi.org/10.1073/pnas.0712237105
- Ansell SM, Lesokhin AM, Borrello I, Halwani A, Scott EC, Gutierrez M, Schuster SJ, Millenson MM, Cattry D, Freeman GJ et al. PD-1 blockade with nivolumab in relapsed or refractory Hodgkin's lymphoma. N Engl J Med 2015; 372:311-9; PMID:25482239; http://dx.doi.org/10.1056/NEJMoa1411087
- Wolchok JD, Kluger H, Callahan MK, Postow MA, Rizvi NA, Lesokhin AM, Segal NH, Ariyan CE, Gordon RA, Reed K et al. Nivolumab plus ipilimumab in advanced melanoma. N Engl J Med 2013; 369:122-33; PMID:23724867; http://dx.doi.org/10.1056/NEJMoa1302369
- Swaika A, Hammond WA, Joseph RW. Current state of anti-PD-L1 and anti-PD-1 agents in cancer therapy. Mol Immunol 2015 Oct; 67(2 Pt A):4-17; PMID:25749122; http://dx.doi.org/10.1016/j.molimm.2015.02.009
- Nesspor TC, Raju TS, Chin CN, Vafa O, Brezski RJ. Avidity confers FcgammaR binding and immune effector function to aglycosylated immunoglobulin G1. J Mol Recognit 2012; 25:147-54; PMID:22407978; http://dx.doi.org/10.1002/jmr.2155
- Severson JJ, Serracino HS, Mateescu V, Raeburn CD, McIntyre RC, Sams SB, Haugen BR, French JD. PD-1+Tim-3+ CD8+ T Lymphocytes Display Varied Degrees of Functional Exhaustion in Patients with Regionally Metastatic Differentiated Thyroid Cancer. Cancer Immunol Res 2015; 3(6):620-30; PMID:25701326; http://dx.doi.org/10.1158/2326-6066.CIR-14-0201
- Choi SY, Lin D, Gout PW, Collins CC, Xu Y, Wang Y. Lessons from patient-derived xenografts for better in vitro modeling of human cancer. Adv Drug Deliv Rev 2014; 79-80:222-37; PMID:25305336; http://dx.doi.org/10.1016/j.addr.2014.09.009.
- Das R, Verma R, Sznol M, Boddupalli CS, Gettinger SN, Kluger H, Callahan M, Wolchok JD, Halaban R, Dhodapkar MV et al. Combination therapy with anti-CTLA-4 and anti-PD-1 leads to distinct immunologic changes in vivo. J Immunol 2015; 194:950-9; http://dx.doi.org/10.4049/jimmunol.1401686
