ABSTRACT
Remarkable efficacy of immune checkpoint inhibition has been reported for several types of solid tumors and early studies in gastric adenocarcinoma are promising. A detailed knowledge about the natural biology of immune checkpoints in gastric adenocarcinoma is essential for clinical and translational evaluation of these drugs. This study is a comprehensive analysis of cytotoxic T lymphocyte associated molecule 4 (CTLA-4) and programmed death 1 ligand 1 (PD-L1) expression in gastric adenocarcinoma. PD-L1 and CTLA-4 were stained on tumor sections of 127 Caucasian patients with gastric adenocarcinoma by immunohistochemistry (IHC) and somatic mutation profiling was performed using targeted next-generation sequencing. Expression of PD-L1 and CTLA-4 on lymphocytes in tumor sections, tumor-draining lymph nodes (TDLN) and peripheral blood were studied by flow-cytometry and immune-fluorescence microscopy in an additional cohort. PD-L1 and CTLA-4 were expressed in 44.9% (57/127) and 86.6% (110/127) of the analyzed gastric adenocarcinoma samples, respectively. Positive tumor cell staining for PD-L1 or CTLA-4 was associated with inferior overall survival. Somatic mutational analysis did not reveal a correlation to expression of PD-L1 or CTLA-4 on tumor cells. Expression of PD-1 (52.2%), PD-L1 (42.2%) and CTLA-4 (1.6%) on tumor infiltrating T cells was significantly elevated compared to peripheral blood. Of note, PD-1 and PD-L1 were expressed far higher by tumor-infiltrating lymphocytes than CTLA-4. In conclusion, specific immune checkpoint-inhibitors should be evaluated in this disease and the combination with molecular targeted therapies might be of benefit. An extensive immune monitoring should accompany these studies to better understand their mode of action in the tumor microenvironment.
Abbreviations
| CTLA-4 | = | cytotoxic T lymphocyte associated molecule 4 |
| PBMC | = | peripheral blood mononuclear cells |
| PD-1 | = | programmed death 1 |
| PD-L1 | = | programmed death 1 ligand 1 |
| TDLN | = | tumor-draining lymph nodes |
| UICC | = | Union International Contre le Cancer. |
Introduction
Gastric cancer is the third most common cause of cancer death worldwide.Citation1 Although improved throughout the last decades, the prognosis is still poor. Whereas the majority of patients with early cancers can be cured by surgery alone, the median overall survival for patients diagnosed in a metastatic stage is less than one year.Citation2,3 New treatment options are urgently needed and immunotherapy is a promising option. The presence of mature lymphocytes within the tumor microenvironment has shown to provide a better prognosis in several types of solid tumors, including gastric cancer.Citation4-7 Furthermore, naturally occurring tumor-specific T cells can be detected in various solid tumors, even in metastatic stages.Citation8 Immunosuppressive mechanisms within the tumor microenvironment have been associated with failure of tumor-specific T cells.Citation9 “Immunologic checkpoints” expressed on the surface of tumor cells or cells of the tumor microenvironment promote immune escape by induction of anergy or apoptosis in immune effector cells.Citation10-12 Recently, strategies targeting these immunologic checkpoints with monoclonal antibodies have demonstrated high efficacy representing a major breakthrough in cancer therapy.Citation13-17 One of the best-described immune checkpoints is the programmed cell death protein 1 (PD-1) pathway. Binding of PD-1 to its ligand programmed cell death 1 ligand 1 (PD-L1) leads to a blockade of kinases involved in T cell activation.Citation10,11 Expression of PD-L1 on tumor cells is associated with an inferior prognosis in many malignancies.Citation18-21 Its role in gastric cancer is still controversial as demonstrated in two recent Asian studies.Citation22,23 Cytotoxic T lymphocyte antigen 4 (CTLA-4) is another relevant immune checkpoint molecule. The anti-CTLA-4 antibody ipilimumab is approved for the treatment of malignant melanoma.Citation17,24 Although the prevailing notion is that ipilimumab targets CTLA-4 on T cells, its exact mode of action is still under discussion, as CTLA-4 can be expressed on regulatory lymphocytes as well as on malignant cells.Citation25-29 In an early trial investigating the CLTA-4 antibody tremelimumab in metastatic gastric adenocarcinoma, tumor shrinkage with some long-lasting responses have been described. Of note, this study did not report on CTLA-4 expression on primary tumor cells.Citation30 The aim of our study was to comprehensively analyze expression of CTLA-4, PD-1 and PD-L1 in the tumor microenvironment of gastric adenocarcinoma patients. Furthermore, mutational analysis was performed to give further insights into genomic aberrations associated with expression of these immune checkpoints.
Results
Expression of PD-L1 in gastric adenocarcinoma cells is associated with inferior survival
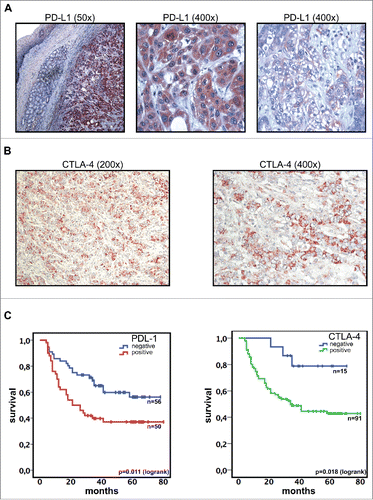
Immunohistochemical analysis revealed expression of PD-L1 in 57 patients (44.9%). The staining pattern in positive samples was homogeneous and mainly cytoplasmatic with some samples showing also membranous staining (). There was no significant correlation to one of the following clinical parameters: Lauren classification, Siewert classification, grading, UICC stage or administration of neoadjuvant chemotherapy (p = 0.17; 0.46; 0.35; 0.34 and 0.99, respectively). Mean overall survival of patients with PD-L1 positive tumors was 39.1 mo compared to 54.2 mo for PD-L1 negative cases (p = 0.011) (). Multivariate analysis identified PD-L1 expression as an independent prognostic factor in primary gastric adenocarcinoma (p = 0.024, Exp(B) = 1.98, ). Additionally, we analyzed 37 metastatic lymph nodes. 15/21 (71.4%) samples with negativity in primary tumors were PD-L1-positive in metastatic lymph nodes, whereas all samples (16/16) with PD-L1 positivity in primary tumors showed also PD-L1 positive staining in metastatic lymph nodes (p < 0.05).
Figure 1. PD-L1 and CTLA-4 expression (analyzed by immunohistochemistry) is associated with inferior survival in gastric adenocarcinoma. (A) Gastric adenocarcinoma cells show a strong expression of PD-L1 (DAB, brown regions). Surrounding normal tissue and intramucosal glands are PD-L1 negative. Exemplary regions with membranous and cytoplasmatic staining are shown in larger magnification. (B) Representative images showing immunohistochemistry staining of CTLA-4 (DAB, brown regions). (C) Expression of PD-L1 or CTLA-4 in primary tumor cells was associated with inferior overall survival.
CTLA-4 is expressed on tumor cells in the majority of gastric adenocarcinoma patients
CTLA-4 was expressed in 86% (110/127) of the analyzed samples (). CTLA-4 negative patients had a significantly superior outcome compared to positive patients (mean overall survival 62.0 mo vs. 44.4 mo, p = 0.018) (). However, CTLA-4 immunohistochemistry was not an independent prognostic factor in multivariate analysis (p = 0.062) (). Correlation of CTLA-4 expression on primary tumor cells to clinical and pathological parameters revealed a correlation of CTLA-4 negativity to higher grading and diffuse type according to the Lauren classification (p = 0.012 and 0.006, respectively) whereas UICC stage (I+II vs. III+IV), age, Siewert classification and use of neoadjuvant chemotherapy were not significantly correlated to CTLA-4 expression (p = 0.37, 0.11, 0.32, and 0.28, respectively). Expression of CTLA-4 on tumor cells in metastatic lymph nodes was not significantly different compared to primary tumor samples (5/5 with negative and 27/32 samples with positive primary tumors showed a positive staining in metastatic cells (p = 0.34)).
PD-L1 and its cognate receptor PD-1 are widely expressed on T and B cells in primary tumors, lymph nodes and peripheral blood of gastric adenocarcinoma patients
In tumor samples, T cells account for 67.0% of CD45+ lymphocytes compared to 72.0% in TDLN (n = 11), 63.9% in peripheral blood of healthy controls (PBMC HC, n = 10) and 69.7% in peripheral blood samples of gastric adenocarcinoma patients (PBMC AC, n = 14 , p = 0.19) (Figs. S1A,B). The percentage of CD8+ cytotoxic T cells was elevated in tumor samples and PBMC AC compared to PBMC HC (39.8% and 28.3% vs. 22.8%, p < 0.05) (Figs. S1A,C). Tumor-infiltrating T cells were mainly of an effector-memory phenotype with 86.8% of tumor-infiltrating T cells showing a CD45RA−CCR7− signature compared to 50.1%, 40.1% and 31.1% in TDLN, PBMC AC and PBMC HC, respectively (p < 0.05). Gastric adenocarcinoma samples (n = 10) contained a significantly increased fraction of T cells expressing PD-L1 (42.2%) compared to 20.3% in TDLN (n = 11). In PBMC HC (20.2%) and PBMC AC (27.3%), this rate was also lower (p < 0.05) (, left plot). The majority of T cells expressing PD-L1 were CD8+ cytotoxic T cells (94.6% in PBMC HC, 92.5% in PBMC AC, 89.4% in TDLN and 85.8% in primary tumor samples, p =0.077) (, central plot). B cells were not increased in primary tumor samples (Fig. S1D). Analysis of tumor-associated B cells revealed a relevant but not statistically significant increase of PD-L1 expressing B cells (9.8% in tumor samples compared to 2.3% in TDLN, 2.9% in PBMC HC and 5.4% in PBMC AC, respectively; p = 0.074) (, right plot). The spatial distribution of T and B cells expressing PD-L1 was further analyzed by four-color immune-fluorescence microscopy. PD-L1+ B cells were predominantly localized in tertiary lymphoid structures, whereas PD-L1+ T cells show a diffuse infiltration of tumor areas and a concentration along the invasive margin of gastric adenocarcinoma ().
Figure 2. PD-1 as well as PD-L1 positive T cells were analyzed by flow cytometry and are increased in the tumor microenvironment of gastric adenocarcinoma. (A) Scatter plots and bar graphs showing results of flow cytometric analyses of PD-L1 expression on T and B cells in gastric adenocarcinoma samples (n = 10), TDLN (n = 11), PBMC HC (n = 10) and PBMC AC (n = 14). (B) Scatter plots and bar graphs showing expression of PD-1 on T cells and T-cell subsets in PBMC HC (n = 10), PBMC AC (n = 14), TDLN (n = 10) and gastric adenocarcinoma samples (n = 10) as assessed by flow cytometry. PD-1+ T cells mainly show an effector-memory phenotype with a CD45RA−/CCR7− signature (right plot). (C) Exemplary density plot showing expression of PD-1 and PD-L1 on T cells (gated on CD45+CD3+ lymphocytes). Scatter plot showing the percentage of T cells coexpressing PD-L1 and PD-1 in tumor samples (n = 11), PBMC HC (n = 10), PBMC AC (n = 10) and TDLN (n = 10). (D) The spatial distribution of tumor-infiltrating T and B cells expressing PD-L1 was analyzed by four-color immune-fluorescence microscopy. In the left image, tumor cells as well as a fraction of tumor infiltrating CD3+ (green) T cells express PD-L1 (red), whereas B cells (CD20+, magenta) in the tumor microenvironment are mainly PD-L1 negative (right image). (E) Confocal microscopy of T cells in the tumor microenvironment. The distribution of PD-1+ (magenta) T cells was similar to PD-L1+ (red) T cells. Two representative images with either expression of PD-1 alone (left image) or coexpression of PD-1 and PD-L1 (right image) are shown.
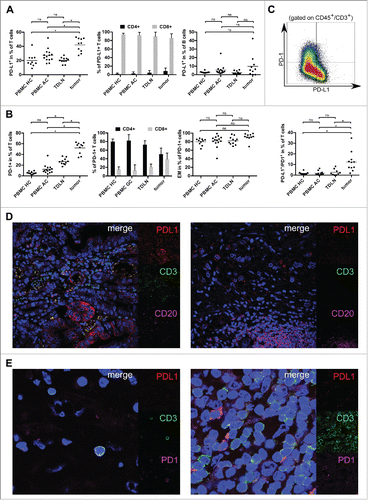
Whereas the majority of T cells in PBMC of healthy controls or cancer patients were PD-1 negative, PD-1+ T cells account for 52.2% of T cells in the tumor microenvironment (26.6% in TDLN, 5.6% in PBMC HC and 12.4% in PBMC AC, p < 0.05 (, left plot). PD-1+ T cells mainly showed an effector-memory phenotype (CD45RA−/CCR7− T cells account for 79.8%, 81.4%, 82.3% and 91.1% of PD-1+ T cells in PBMC HC, PBMC AC, TDLN and gastric adenocarcinoma samples, respectively; p = 0.09) (, right plot). Whereas PD-1+ T cells in PBMC were mainly CD4+ T helper cells, CD8+ T cells account for a relevant fraction of PD1+ tumor-infiltrating T cells (80.0%, 82.1%, 72.8% and 50.8% CD4+ T cells in PBMC HC, PBMC AC, TDLN and tumor samples, respectively; p <0.05) (, central plot). PD-1 and PD-L1 were coexpressed in a subset of T cells (1.7%, 1.9%, 2.6% and 12.4% in PBMC HC, PBMC AC, TDLN and tumor samples, respectively ).
The expression of CTLA-4 is elevated on tumor-infiltrating T cells, especially on regulatory T cells
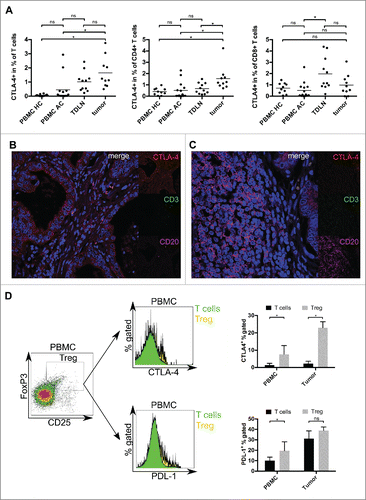
Only a small subset of tumor-infiltrating lymphocytes expressed CTLA-4 (). The percentage of T cells with a positive staining for CTLA-4 was elevated in tumor samples (1.6%) compared to PBMC HC and PBMC AC (0.1% and 0.5%, respectively; p < 0.05) (). The increase compared to TDLN was not significant (1.6% vs. 1.0%, p = 0.27). Further analysis revealed a significant elevation of CTLA-4+ cells in the CD4+ subset of tumor samples (1.6%) compared to PBMC HC, PBMC AC and TDLN (0.4%, 0.5% and 0.7%, respectively; p <0.05) (, central plot), whereas the elevation of CTLA-4 within the CD8+ subset of tumor samples (1.0%) was not significant (0.7%, 0.5% and 2% in PBMC HC, PBMC AC and TDLN, respectively) (, right plot). As these CD4+ T cells could correspond to regulatory T cells in the tumor microenvironment, we analyzed the expression of CTLA-4 and PD-L1 on CD4+FoxP3+CD25+ T cells in an additional analysis. Both immunological checkpoints showed an elevated expression on regulatory T cells: CTLA-4/PD-L1 was expressed on 22.9%/31.1 and 7.6%/10.0% of FoxP3+CD25+ T cells in tumor samples or PBMC of gastric adenocarcinoma patients, respectively ().
Figure 3. Relative proportion and spatial distribution of CTLA-4-expressing T cells and tumor cells in gastric adenocarcinoma were analyzed by flow cytometry and immunofluorescence microscopy. (A) The percentage of CTLA-4 positive T cells was analyzed by flow cytometry and is significantly elevated in gastric adenocarcinoma samples (p <0.05). This difference is mainly based on CD4+ T cells. (B, C) Four color-immunfluorescence microscopy showing CTLA-4 (red) positive tumor cells as well as some CD3+ (green) T cells and CD20+ B cells (magenta). T cells with a coexpression of CTLA-4 (yellow) could be detected in the tumor stroma (B) and close to B cells in peritumoral tertiary lymphoid structures (C). (D) Exemplary density plot and histograms showing expression of CTLA-4 and PD-L1 on CD4+FoxP3+CD25+ regulatory T cells in tumor samples and PBMC of gastric cancer patients (n = 5). Using nuclear staining of FoxP3, we demonstrate a relevant expression of both immune checkpoints on regulatory T cells.
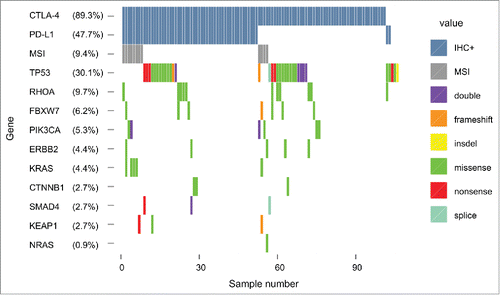
Targeted mutational analyses and correlation with immune checkpoint expression
57 of 113 samples (50.4%) harbored at least one mutation. Frequently detected somatic mutations included TP53 (30.1%), RHOA (9.7%), FBXW7 (6.2%), PI3KCA (5.3%), KRAS (4.4%), ERBB2 (4.4%), CTNNB1 (2.7%), KEAP1 (2.7%), SMAD4 (2.7%) and NRAS (0.9%) (). The amino acid changes in frequently mutated genes are summarized in the supplementary material (Table S1). We did not find a significant correlation between any of the somatic mutations and immune checkpoint expression. To assess whether mutational load is correlated to immune checkpoint expression we analyzed microsatellite-instability (MSI) using quantification of nucleotide loss in non-coding mononucleotide repeats, as recently described.Citation31 Our analysis revealed MSI in 9.4% of the analyzed samples, without correlation to expression of PD-L1 or CTLA-4.
Discussion
Recently, drugs targeting immunological checkpoints have demonstrated remarkable clinical efficacy and therefore reinforce the oncologic armamentarium.Citation13,15,17,32 There are multiple ongoing clinical and translational studies investigating predictive markers for checkpoint inhibitors. Currently, available results suggest a predictive role of PD-L1 expression on primary tumor cells for drugs blocking the PD-1 pathway. However, a relevant number of patients without PD-L1 expression on tumor cells also responded to anti-PD-(L)1 therapies suggesting that other factors like preexisting tumor-specific immune responses and expression of PD-1 or PD-L1 on non-malignant bystander cells might also play an important role.Citation8,13,33 A detailed knowledge about expression and spatial distribution of immune checkpoints is therefore essential. We comprehensively analyzed expression of PD-1, PD-L1 and CTLA-4 in primary tumor samples, PBMC and TDLN of gastric adenocarcinoma patients.
PD-L1 is expressed on malignant cells in a variety of cancers and confers an inferior prognosis.Citation19-21,33 Consistently with these results, we demonstrate a significantly inferior overall survival for gastric adenocarcinoma patients with expression of PD-L1 on tumor cells in primary tumor samples (39.1 mo vs. 54.2 mo, p = 0.011). To the best of our knowledge, this is the first study describing an independent prognostic role of PD-L1-expression on tumor cells in gastric adenocarcinoma for Caucasian patients. Previously, controversial results on the prognostic role of PD-L1 expression in gastric adenocarcinoma have been published in Asian patients. Whereas Wu et al. described an impaired overall survival for patients with PD-L1 positive tumors (5-y overall survival rate 30.2% vs. 64.5%), a recent study by Kim and colleagues showed a trend toward a superior survival for PD-L1 positive patients (83.0% vs. 69.1% 5-y overall survival). Of note, both cohorts are distinct from the patient population investigated in our study as they included a large fraction of early stage cancers treated by subtotal gastrectomy.Citation22,23 Furthermore, gastric adenocarcinoma in Asian patients is genetically and probably immunologically distinct from Causasian patients.Citation34 Although these trials showed conflicting results regarding the prognostic impact, all studies described similar frequencies of PD-L1 expression.Citation22,23 Additionally, we found an increased expression of PD-L1 on metastatic tumor cells further underlining its role in the natural biology of gastric adenocarcinoma. In a previous study, immunohistochemical expression of PD-L1 on tumor-infiltrating lymphocytes was approximately 50% in colorectal cancer, non-small cell lung cancer and melanoma. In that study, expression of PD-L1 on tumor cells as well as on tumor-infiltrating lymphocytes correlated with response to the PD-1 monoclonal antibody nivolumab.Citation33 In our analysis, we detected a significantly higher rate of PD-L1 and PD-1 expression on tumor-infiltrating lymphocytes compared to PBMC and TDLN. PD-1 is upregulated on several lymphocytic subsets after activation.Citation35-37 Interestingly, the expression of PD-1 on T cells has been attributed to T cell exhaustion, which can be overcome by blockade of the PD1-pathway.Citation35,38-40 The presence or absence of a pre-existing immune response to tumor-associated antigens partially represented by tumor-infiltrating memory T cells and/or PD-1+ T cells could be a very important predictive factor for immune checkpoint inhibition.Citation41 Our data clearly demonstrate that several non-malignant cell types in the tumor microenvironment of gastric adenocarcinoma express PD-1 and/or PD-L1, highlighting the importance of a comprehensive assessment of these cellular subsets in future studies investigating immune checkpoint inhibitors.
Although the prevailing notion is a depletion of CTLA-4-expressing T cells in the tumor microenvironment, the exact mode of action for antibodies targeting CTLA-4 is still not fully understood. Several studies suggested a depletion of regulatory T cells. Citation42-45 On the other hand, a negative prognostic impact of CTLA-4-expression on tumor cells has been demonstrated for different types of cancer.Citation25,26,28,29 Expression of CTLA-4 on cancer cells is perhaps underestimated as Contardi et al. described expression of CTLA-4 on 30/34 (88%) tumor cell lines from different origins. We found expression of CTLA-4 on primary tumor cells in the vast majority of analyzed gastric adenocarcinoma samples (86%) and although not statistically significant in multivariate analysis, we show for the first time a potential prognostic role of CTLA-4 expression in gastric adenocarcinoma cells. In contrast to the mainly positive staining on tumor cells, analysis of tumor-infiltrating T cells revealed only low percentages of CTLA-4+ cells (1.6% of T cells). Similar low frequencies have been described for tumor-infiltrating T cells in lung cancer.Citation26 Interestingly, the percentage of CTLA-4+ cells was significantly elevated in tumor samples within the CD4+ subset, potentially reflecting an increase of regulatory T cells.Citation30 In an additional subset-analysis, CTLA-4 as well as PD-L1 expression was elevated on CD4+FoxP3+CD25+ regulatory T cells in PBMC and tumor samples of gastric adenocarcinoma patients (), further highlighting the relevance of this pathway for regulatory T cells. A negative prognostic impact of tumor-infiltrating regulatory T cells has been shown for several types of cancer, including gastric cancer.Citation46-53
The comprehensive mutational analysis in this study is in concordance with the findings of other studies.Citation54 That we could not find a correlation between the genomic profile and expression of immune checkpoint receptors is probably due to the low frequencies of the analyzed mutations. Recently, it has been shown that mutational load correlates to naturally occurring immune responses, probably representing a predictive factor for checkpoint-blockade.Citation55 Using quantification of nucleotide loss in non-coding mononucleotide repeats, we found MSI in 9.4% of the analyzed samples. Although the frequency in our cohort was too low to find a correlation with expression of the analyzed immune checkpoints, this subset could be more susceptible to immune checkpoint blockade, as shown for colorectal cancer.Citation56
In summary, gastric adenocarcinoma shows a remarkable expression of PD-L1 as well as CTLA-4. PD-L1 expression on tumor cells could be confirmed as an independent prognostic factor in gastric cancer. This first indication of a prognostic relevance of CTLA-4 expression in gastric adenocarcinoma should be confirmed in independent cohorts. Furthermore, both proteins show a high expression on tumor-infiltrating lymphocytes, especially regulatory T cells. Of note, CTLA-4 is only expressed by a small subset, whereas PD-1 as well as PD-L1 are expressed by approximately 50% of tumor-infiltrating lymphocytes. These findings are crucial for further investigations of immunotherapeutic approaches in this disease and highlight the importance of comprehensive translational research in future studies of immune checkpoint inhibitors.
Materials and methods
Patient characteristics
We retrospectively included 127 consecutive patients (2007–2011) who underwent total gastrectomy and D2-lymphadenectomy with a diagnosis of gastric adenocarcinoma. Primary tumor material was used for expression analysis of immune checkpoints and comprehensive molecular profiling. 58 (45.7%) patients received perioperative chemotherapy (47 patients epirubicin/platinum/5-FU, 6 patients platinum/5-FU and 5 patients docetaxel/platinum/5-FU).Citation2 The results of histopathological staging (UICC 2009) and clinical parameters are summarized in . Seven patients were lost to follow-up. 67 patients (55.8%) had died at the time of final analysis. Mean follow-up for survivors was 52.9 mo (±14 .5 mo) ranging from 27 to 80 mo. To assess expression of PD-L1 and CTLA-4 on lymphocytes, primary tumor samples, lymph nodes (TDLN) and peripheral blood of 14 additional patients and 10 healthy controls (peripheral blood) were prospectively included. Our local ethics committee approved the study and written informed consent was obtained prior to surgery (No. 11-116 and No. 10-242).
Table 1. Patient characteristics.
Table 2. Correlation of prognosis and expression of PD-L1 and CTLA-4 using Kaplan–Meier analysis and Cox's regression.
Immunohistochemistry
Formalin-fixed and paraffin-embedded tumor samples were retrieved from our institute of pathology. Samples were processed and stained using a Dako EnVision kit (Dako) and PD-L1 (ABIN500467, Acris Antibodies) or CTLA-4 (ABIN738831, BIOSS) primary antibodies (1:100) according to the manufacturer's instructions. Appropriate positive (human testicle/colon) and negative controls for PD-L1 and CTLA-4 were included. An experienced pathologist (U.D.), who was blinded to clinical parameters, assessed expression of PD-L1 and CTLA-4 on tumor cells. Samples with >10% stained tumor cells were considered positive.
IHC doublestaining was performed with mouse anti-human CD20 and rabbit anti-human CD3 (LS Biosciences). Vector Red and Vector SG were used for visualization using Impress HRP/AP anti-mouse/anti-rabbit secondary antibodies according to the manufacturer's instructions (Vectorlabs). For further details, see supplementary methods 2.
Immune-fluorescence microscopy
Mouse anti-human CD20 (1:50, LS Bioscience) or PD-1 (1:50, Abcam), rabbit anti-human PD-L1 or CTLA-4 (1:100, same as IHC), rat anti-human CD3 (1:100, ABD Serotec) and the corresponding secondary antibodies (Alexa Fluor 647, 568 and 488 goat anti-mouse, -rabbit and -rat, respectively (1:400), Life Technologies) were applied sequentially. DAPI (Life Technologies) was used for nuclear staining (see supplementary methods 2). Pictures were achieved on a Leica TCS SP8 gSTED super-resolution microscope. FIJI ImageJ (Version 2.0.0) was used to adjust for brightness and autofluorescence.
Cell isolation from human peripheral blood, tumor tissue and tumor-draining lymph nodes
Peripheral blood was obtained prior to surgery. Mononuclear cells (PBMC) were isolated using Ficoll-Paque PLUS (GE Healthcare Life Sciences). Fresh unfixed tissue from primary tumors and TDLN was provided immediately after surgery. Single cell suspensions were produced using a gentleMACS Dissociator (Miltenyi) with DNase-I (100U/mL, Applichem) and Collagenase IV (320U/mL, Worthington) (see supplementary methods 2).
Flow cytometry
At least 1 × 106 events per sample were acquired on a Gallios 10-color flow cytometer (Beckman Coulter). PD-L1 and CTLA-4 expressing lymphocytes in PBMC, tumor samples and TDLN were identified by multicolor staining using CD3-APC-H7, CD19-APC, CTLA-4-PE (BD), CD4-PerCP-Cy5.5, CD8-PE-Cy7, CD20-Pacific Blue, PD-1-APC, PD-L1-PE-Cy7, CD45RA-AF700, FoxP3-AF647 (Biolegend), CD45-PE-eFlour610 (eBioscience), CCR7-FITC (R+D Systems) and aqua dead cell stain (Life Technologies) (see Fig. S2 for further details).
DNA extraction and targeted sequencing
DNA was extracted from 10 µm sections using the Maxwell™ 16 FFPE Tissue LEV DNA Purification Kit (Promega Corp.) according to the manufacturer's protocol. The resulting DNA concentration was determined with a Qubit™ 2.0 Fluorometer using the Qubit™ dsDNA HS Assay Kit (Life Technologies Corp.). Library preparation was performed using Ion AmpliSeq™ Library Kit 2.0 (Life Technologies) and adapted for sequencing on a MiSeq™ instrument (Illumina) (see supplementary methods for further details). 113 patients had sufficient tumor content and fulfilled quality criteria for sequencing. Finally, variant calling was performed for variants with an allelic fraction > 7% and coverage > 100 reads. Detected variants were annotated using dbSNP build138, ESP5400 and the COSMIC database. MSI was analyzed using quantification of nucleotide loss in non-coding mononucleotide repeats as recently described.Citation31
Data analyses
SPSS version 20 (IBM Corp.) was used for statistical analysis. Chi-square test, Kaplan–Meier survival analysis and Cox-regression analysis were used as appropriate. Patients with R1-Status (9), survival <3 mo (5) and patients lost to follow up (7) were excluded from survival-analyses. p-values <0.05 were considered significant.
Flow cytometry data was analyzed using Kaluza (Version 1.1, Beckman Coulter, Krefeld, Germany) and GraphPad Prism 5 (Graphpad Sofware, Inc.). A one-way ANOVA with multiple comparisons was used to compare means of lymphocytic subsets.
Disclosure of potential conflicts of interest
S.R.—Honoraria for advisory boards from BMS and MSD. Honoraria for invited talks from BMS. Financial support for research projects from AstraZeneca; A.Z.—Honoraria for advisory boards from BMS and MSD; M.H.—Honoraria for advisory boards and research funding from Roche; T.Z.—Honoraria from BMS, Novartis, Merck, Amgen; Research funding from Novartis; M.B.—Honoraria for advisory boards, for invited talks from BMS and financial support for research projects from Astellas, Roche and MSD. All other authors declare no conflicts of interest.
KONI_A_1100789_s02.zip
Download Zip (5.2 MB)Funding
The German “Nolting Stiftung” (to H.S.), the “Sander Stiftung” (Nr. 2014.001.01, to H.S., M.B., A.H.), a “Gerok” local research grant (to H.S.) and “Freie Akademische Gesellschaft Basel” (to S.R.) supported research on immune escape in gastric cancer.
References
- Song M, Kang D, Yang JJ, Choi J-Y, Sung H, Lee Y, Yoon H-S, Choi Y, Kong S-H, Lee H-J et al. Age and sex interactions in gastric cancer incidence and mortality trends in Korea. Gastric Cancer 2015 Jul; 18(3):580-9; PMID:25091081; http://dx.doi.org/10.1007/s10120-014-0411-x.
- Cunningham D, Allum WH, Stenning SP, Thompson JN, Van de Velde CJH, Nicolson M, Scarffe JH, Lofts FJ, Falk SJ, Iveson TJ et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med 2006; 355:11-20; PMID:16822992; http://dx.doi.org/10.1056/NEJMoa055531
- Bang Y-J, Van Cutsem E, Feyereislova A, Chung HC, Shen L, Sawaki A, Lordick F, Ohtsu A, Omuro Y, Satoh T et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet 2010; 376:687-97; PMID:20728210; http://dx.doi.org/10.1016/S0140-6736(10)61121-X
- Nakano O, Sato M, Naito Y, Suzuki K, Orikasa S, Aizawa M, Suzuki Y, Shintaku I, Nagura H, Ohtani H. Proliferative activity of intratumoral CD8(+) T-lymphocytes as a prognostic factor in human renal cell carcinoma: clinicopathologic demonstration of antitumor immunity. Cancer Res 2001; 61:5132-6; PMID:11431351
- Galon J, Costes A, Sanchez-Cabo F, Kirilovsky A, Mlecnik B, Lagorce-Pagès C, Tosolini M, Camus M, Berger A, Wind P et al. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science 2006; 313:1960-4; PMID:17008531; http://dx.doi.org/10.1126/science.1129139
- Lee HE, Chae SW, Lee YJ, Kim MA, Lee HS, Lee BL, Kim WH. Prognostic implications of type and density of tumour-infiltrating lymphocytes in gastric cancer. Br J Cancer 2008; 99:1704-11; PMID:18941457; http://dx.doi.org/10.1038/sj.bjc.6604738
- Wakatsuki K, Sho M, Yamato I, Takayama T, Matsumoto S, Tanaka T, Migita K, Ito M, Hotta K, Nakajima Y. Clinical impact of tumor-infiltrating CD45RO+ memory T cells on human gastric cancer. Oncol Rep 2013; 29:1756-62; PMID:23440298; http://dx.doi.org/10.3892/or.2013.2302
- Turcotte S, Gros A, Tran E, Lee C-CR, Wunderlich JR, Robbins PF, Rosenberg SA. Tumor-reactive CD8+ T cells in metastatic gastrointestinal cancer refractory to chemotherapy. Clin Cancer Res 2014; 20:331-43; PMID:24218514; http://dx.doi.org/10.1158/1078-0432.CCR-13-1736
- Schlößer HA, Theurich S, Shimabukuro-Vornhagen A, Holtick U, Stippel DL, von Bergwelt-Baildon M. Overcoming tumor-mediated immunosuppression. Immunotherapy 2014; 6:973-88; PMID:25341119; http://dx.doi.org/10.2217/imt.14.58
- Pardoll DM. Immunology beats cancer: a blueprint for successful translation. Nat Immunol 2012; 13:1129-32; PMID:23160205; http://dx.doi.org/10.1038/ni.2392
- Sharpe AH, Wherry EJ, Ahmed R, Freeman GJ. The function of programmed cell death 1 and its ligands in regulating autoimmunity and infection. Nat Immunol 2007; 8:239-45; PMID:17304234; http://dx.doi.org/10.1038/ni1443
- Dong H, Strome SE, Salomao DR, Tamura H, Hirano F, Flies DB, Roche PC, Lu J, Zhu G, Tamada K et al. Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat Med 2002; 8:793-800; PMID:12091876; http://dx.doi.org/10.1038/nm0902-1039c
- Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, Powderly JD, Carvajal RD, Sosman JA, Atkins MB et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med 2012; 366:2443-54; PMID:22658127; http://dx.doi.org/10.1056/NEJMoa1200690
- Hamid O, Robert C, Daud A, Hodi FS, Hwu W-J, Kefford R, Wolchok JD, Hersey P, Joseph RW, Weber JS et al. Safety and tumor responses with lambrolizumab (anti-PD-1) in melanoma. N Engl J Med 2013; 369:134-44; PMID:23724846; http://dx.doi.org/10.1056/NEJMoa1305133
- Brahmer JR, Tykodi SS, Chow LQM, Hwu W-J, Topalian SL, Hwu P, Drake CG, Camacho LH, Kauh J, Odunsi K et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med 2012; 366:2455-65; PMID:22658128; http://dx.doi.org/10.1056/NEJMoa1200694
- Brahmer JR, Drake CG, Wollner I, Powderly JD, Picus J, Sharfman WH, Stankevich E, Pons A, Salay TM, McMiller TL et al. Phase I study of single-agent anti-programmed death-1 (MDX-1106) in refractory solid tumors: safety, clinical activity, pharmacodynamics, and immunologic correlates. J Clin Oncol 2010; 28:3167-75; PMID:20516446; http://dx.doi.org/10.1200/JCO.2009.26.7609
- Hodi FS, O'Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, Gonzalez R, Robert C, Schadendorf D, Hassel JC et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med 2010; 363:711-23; PMID:20525992; http://dx.doi.org/10.1056/NEJMoa1003466
- Ohigashi Y, Sho M, Yamada Y, Tsurui Y, Hamada K, Ikeda N, Mizuno T, Kashizuka H, Yane K, Tsushima F et al. Clinical Significance of Programmed Death-1Ligand-1 and Programmed Death-1Ligand-2 Expression in Human Esophageal Cancer. 2005; 11:2947-53; PMID:15837746; http://dx.doi.org/10.1158/1078-0432.CCR-04-1469
- Gao Q, Wang X-Y, Qiu S-J, Yamato I, Sho M, Nakajima Y, Zhou J, Li B-Z, Shi Y-H, Xiao Y-S et al. Overexpression of PD-L1 significantly associates with tumor aggressiveness and postoperative recurrence in human hepatocellular carcinoma. Clin Cancer Res 2009; 15:971-9; PMID:19188168; http://dx.doi.org/10.1158/1078-0432.CCR-08-1608
- Hamanishi J, Mandai M, Iwasaki M, Okazaki T, Tanaka Y, Yamaguchi K, Higuchi T, Yagi H, Takakura K, Minato N et al. Programmed cell death 1 ligand 1 and tumor-infiltrating CD8+ T lymphocytes are prognostic factors of human ovarian cancer. Proc Natl Acad Sci U S A 2007; 104:3360-5; PMID:17360651; http://dx.doi.org/10.1073/pnas.0611533104
- Thompson RH, Kuntz SM, Leibovich BC, Dong H, Lohse CM, Webster WS, Sengupta S, Frank I, Parker AS, Zincke H et al. Tumor B7-H1 is associated with poor prognosis in renal cell carcinoma patients with long-term follow-up. Cancer Res 2006; 66:3381-5; PMID:16585157; http://dx.doi.org/10.1158/0008-5472.CAN-05-4303
- Kim JW, Nam KH, Ahn S-H, Park DJ, Kim H-H, Kim SH, Chang H, Lee J-O, Kim YJ, Lee HS et al. Prognostic implications of immunosuppressive protein expression in tumors as well as immune cell infiltration within the tumor microenvironment in gastric cancer. Gastric Cancer 2016 Jan; 19(1):42-52; PMID:25424150; http://dx.doi.org/10.1007/s10120-014-0440-5
- Wu C, Zhu Y, Jiang J, Zhao J, Zhang X-G, Xu N. Immunohistochemical localization of programmed death-1 ligand-1 (PD-L1) in gastric carcinoma and its clinical significance. Acta Histochem 2006; 108:19-24; PMID:16530813; http://dx.doi.org/10.1016/j.acthis.2006.01.003
- Robert C, Thomas L, Bondarenko I, O'Day S, M D JW, Garbe C, Lebbe C, Baurain J-F, Testori A, Grob J-J et al. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N Engl J Med 2011; 364:2517-26; PMID:21639810; http://dx.doi.org/10.1056/NEJMoa1104621
- Walker LSK, Sansom DM. The emerging role of CTLA4 as a cell-extrinsic regulator of T cell responses. Nat Rev Immunol 2011; 11:852-63; PMID:22116087; http://dx.doi.org/10.1038/nri3108
- Salvi S, Fontana V, Boccardo S, Merlo DF, Margallo E, Laurent S, Morabito A, Rijavec E, Dal Bello MG, Mora M et al. Evaluation of CTLA-4 expression and relevance as a novel prognostic factor in patients with non-small cell lung cancer. Cancer Immunol Immunother 2012; 61:1463-72; PMID:22318401; http://dx.doi.org/10.1007/s00262-012-1211-y
- Mao H, Zhang L, Yang Y, Zuo W, Bi Y, Gao W, Deng B, Sun J, Shao Q, Qu X. New insights of CTLA-4 into its biological function in breast cancer. Curr Cancer Drug Targets 2010; 10:728-36; PMID:20578982; http://dx.doi.org/10.2174/156800910793605811
- Contardi E, Palmisano GL, Tazzari PL, Martelli AM, Falà F, Fabbi M, Kato T, Lucarelli E, Donati D, Polito L et al. CTLA-4 is constitutively expressed on tumor cells and can trigger apoptosis upon ligand interaction. Int J Cancer 2005; 117:538-50; PMID:15912538; http://dx.doi.org/10.1002/ijc.21155
- Laurent S, Queirolo P, Boero S, Salvi S, Piccioli P, Boccardo S, Minghelli S, Morabito A, Fontana V, Pietra G et al. The engagement of CTLA-4 on primary melanoma cell lines induces antibody-dependent cellular cytotoxicity and TNF-α production. J Transl Med 2013; 11:108; PMID:23634660; http://dx.doi.org/10.1186/1479-5876-11-108
- Ralph C, Elkord E, Burt DJ, O'Dwyer JF, Austin EB, Stern PL, Hawkins RE, Thistlethwaite FC. Modulation of lymphocyte regulation for cancer therapy: a phase II trial of tremelimumab in advanced gastric and esophageal adenocarcinoma. Clin Cancer Res 2010; 16:1662-72; PMID:20179239; http://dx.doi.org/10.1158/1078-0432.CCR-09-2870
- Kloth M, Ruesseler V, Engel C, Koenig K, Peifer M, Mariotti E, Kuenstlinger H, Florin A, Rommerscheidt-Fuss U, Koitzsch U et al. Activating ERBB2/HER2 mutations indicate susceptibility to pan-HER inhibitors in Lynch and Lynch-like colorectal cancer. Gut 2015; PMID:26001389; http://dx.doi.org/10.1136/gutjnl-2014-309026
- Robert C, Ribas A, Wolchok JD, Hodi FS, Hamid O, Kefford R, Weber JS, Joshua AM, Hwu W-J, Gangadhar TC et al. Anti-programmed-death-receptor-1 treatment with pembrolizumab in ipilimumab-refractory advanced melanoma: a randomised dose-comparison cohort of a phase 1 trial. Lancet 2014; 384:1109-17; PMID:25034862; http://dx.doi.org/10.1016/S0140-6736(14)60958-2
- Taube JM, Klein A, Brahmer JR, Xu H, Pan X, Kim JH, Chen L, Pardoll DM, Topalian SL, Anders RA. Association of PD-1, PD-1 Ligands, and Other Features of the Tumor Immune Microenvironment with Response to Anti-PD-1 Therapy. Clin Cancer Res 2014; 20:5064-74; PMID:24714771; http://dx.doi.org/10.1158/1078-0432.CCR-13-3271
- Lin SJ, Gagnon-Bartsch JA, Tan IB, Earle S, Ruff L, Pettinger K, Ylstra B, van Grieken N, Rha SY, Chung HC et al. Signatures of tumour immunity distinguish Asian and non-Asian gastric adenocarcinomas. Gut 2015 Nov; 64(11):1721-31; PMID:25385008; http://dx.doi.org/10.1136/gutjnl-2014-308252
- Ahmadzadeh M, Johnson LA, Heemskerk B, Wunderlich JR, Dudley ME, White DE, Rosenberg SA. Tumor antigen-specific CD8 T cells infiltrating the tumor express high levels of PD-1 and are functionally impaired. Blood 2009; 114:1537-44; PMID:19423728; http://dx.doi.org/10.1182/blood-2008-12-195792
- Terme M, Ullrich E, Aymeric L, Meinhardt K, Desbois M, Delahaye N, Viaud S, Ryffel B, Yagita H, Kaplanski G et al. IL-18 induces PD-1-dependent immunosuppression in cancer. Cancer Res 2011; 71:5393-9; PMID:21724589; http://dx.doi.org/10.1158/0008-5472.CAN-11-0993
- Titanji K, Velu V, Chennareddi L, Vijay-Kumar M, Gewirtz AT, Freeman GJ, Amara RR. Acute depletion of activated memory B cells involves the PD-1 pathway in rapidly progressing SIV-infected macaques. J Clin Invest 2010; 120:3878-90; PMID:20972331; http://dx.doi.org/10.1172/JCI43271
- Kaiser AD, Schuster K, Gadiot J, Borkner L, Daebritz H, Schmitt C, Andreesen R, Blank C. Reduced tumor-antigen density leads to PD-1/PD-L1-mediated impairment of partially exhausted CD8+ T cells. Eur J Immunol 2012; 42:662-71; PMID:22144176; http://dx.doi.org/10.1002/eji.201141931
- Krönig H, Julia Falchner K, Odendahl M, Brackertz B, Conrad H, Muck D, Hein R, Blank C, Peschel C, Haller B et al. PD-1 expression on Melan-A-reactive T cells increases during progression to metastatic disease. Int J Cancer 2012; 130:2327-36; PMID:21717461; http://dx.doi.org/10.1002/ijc.26272
- Quigley M, Pereyra F, Nilsson B, Porichis F, Fonseca C, Eichbaum Q, Julg B, Jesneck JL, Brosnahan K, Imam S et al. Transcriptional analysis of HIV-specific CD8+ T cells shows that PD-1 inhibits T cell function by upregulating BATF. Nat Med 2010; 16:1147-51; PMID:20890291; http://dx.doi.org/10.1038/nm.2232
- Badoual C, Hans S, Merillon N, Van Ryswick C, Ravel P, Benhamouda N, Levionnois E, Nizard M, Si-Mohamed A, Besnier N et al. PD-1-expressing tumor-infiltrating T cells are a favorable prognostic biomarker in HPV-associated head and neck cancer. Cancer Res 2013; 73:128-38; PMID:23135914; http://dx.doi.org/10.1158/0008-5472.CAN-12-2606
- Quezada SA, Peggs KS, Curran MA, Allison JP. CTLA4 blockade and GM-CSF combination immunotherapy alters the intratumor balance of effector and regulatory T cells. J Clin Invest 2006; 116:1935-45; PMID:16778987; http://dx.doi.org/10.1172/JCI27745
- Duraiswamy J, Kaluza KM, Freeman GJ, Coukos G. Dual blockade of PD-1 and CTLA-4 combined with tumor vaccine effectively restores T-cell rejection function in tumors. Cancer Res 2013; 73:3591-603; PMID:23633484; http://dx.doi.org/10.1158/0008-5472.CAN-12-4100
- Chen H, Liakou CI, Kamat A, Pettaway C, Ward JF, Tang DN, Sun J, Jungbluth AA, Troncoso P, Logothetis C et al. Anti-CTLA-4 therapy results in higher CD4+ICOShi T cell frequency and IFN-gamma levels in both nonmalignant and malignant prostate tissues. Proc Natl Acad Sci U S A 2009; 106:2729-34; PMID:19202079; http://dx.doi.org/10.1073/pnas.0813175106
- Liakou CI, Kamat A, Tang DN, Chen H, Sun J, Troncoso P, Logothetis C, Sharma P. CTLA-4 blockade increases IFNgamma-producing CD4+ICOShi cells to shift the ratio of effector to regulatory T cells in cancer patients. Proc Natl Acad Sci U S A 2008; 105:14987-92; PMID:18818309; http://dx.doi.org/10.1073/pnas.0806075105
- Perrone G, Ruffini PA, Catalano V, Spino C, Santini D, Muretto P, Spoto C, Zingaretti C, Sisti V, Alessandroni P et al. Intratumoural FOXP3-positive regulatory T cells are associated with adverse prognosis in radically resected gastric cancer. Eur J Cancer 2008; 44:1875-82; PMID:18617393; http://dx.doi.org/10.1016/j.ejca.2008.05.017
- Shen Z, Zhou S, Wang Y, Li R, Zhong C, Liang C, Sun Y. Higher intratumoral infiltrated Foxp3+ Treg numbers and Foxp3+/CD8+ ratio are associated with adverse prognosis in resectable gastric cancer. J Cancer Res Clin Oncol 2010; 136:1585-95; PMID:20221835; http://dx.doi.org/10.1007/s00432-010-0816-9
- Kim H-I, Kim H, Cho HW, Kim SY, Song KJ, Hyung WJ, Park C-G, Kim C-B. The ratio of intra-tumoral regulatory T cells (Foxp3+)/helper T cells (CD4+) is a prognostic factor and associated with recurrence pattern in gastric cardia cancer. J Surg Oncol 2011; 104:728-33; PMID:21792941; http://dx.doi.org/10.1002/jso.22038
- Kashimura S, Saze Z, Terashima M, Soeta N, Ohtani S, Osuka F, Kogure M, Gotoh M. CD83(+) dendritic cells and Foxp3(+) regulatory T cells in primary lesions and regional lymph nodes are inversely correlated with prognosis of gastric cancer. Gastric Cancer 2012; 15:144-53; PMID:22083420; http://dx.doi.org/10.1007/s10120-011-0090-9
- Zhou S, Shen Z, Wang Y, Ma H, Xu S, Qin J, Chen L, Tao H, Zhen Z, Chen G et al. CCR7 expression and intratumoral FOXP3+ regulatory T cells are correlated with overall survival and lymph node metastasis in gastric cancer. PLoS One 2013; 8:e74430; PMID:24040244; http://dx.doi.org/10.1371/journal.pone.0074430
- Sinicrope FA, Rego RL, Ansell SM, Knutson KL, Foster NR, Sargent DJ. Intraepithelial effector (CD3+)/regulatory (FoxP3+) T-cell ratio predicts a clinical outcome of human colon carcinoma. Gastroenterology 2009; 137:1270-9; PMID:19577568; http://dx.doi.org/10.1053/j.gastro.2009.06.053
- Krystufkova E, Sekerkova A, Striz I, Brabcova I, Girmanova E, Viklicky O. Regulatory T cells in kidney transplant recipients: the effect of induction immunosuppression therapy. Nephrol Dial Transplant 2012; 27:2576-82; PMID:22167587; http://dx.doi.org/10.1093/ndt/gfr693
- Ino Y, Yamazaki-Itoh R, Shimada K, Iwasaki M, Kosuge T, Kanai Y, Hiraoka N. Immune cell infiltration as an indicator of the immune microenvironment of pancreatic cancer. Br J Cancer 2013; 108:914-23; PMID:23385730; http://dx.doi.org/10.1038/bjc.2013.32
- Kim S, Lee J, Hong ME, Do I-G, Kang SY, Ha SY, Kim ST, Park SH, Kang WK, Choi M-G et al. High-throughput sequencing and copy number variation detection using formalin fixed embedded tissue in metastatic gastric cancer. PLoS One 2014; 9:e111693; PMID:25372287; http://dx.doi.org/10.1371/journal.pone.0111693
- Rizvi NA, Hellmann MD, Snyder A, Kvistborg P, Makarov V, Havel JJ, Lee W, Yuan J, Wong P, Ho TS et al. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science (80-) 2015; 348:124-8; PMID:25765070; http://dx.doi.org/10.1126/science.aaa1348
- Lipson EJ, Sharfman WH, Drake CG, Wollner I, Taube JM, Anders RA, Xu H, Yao S, Pons A, Chen L et al. Durable cancer regression off-treatment and effective reinduction therapy with an anti-PD-1 antibody. Clin Cancer Res 2013; 19:462-8; PMID:23169436; http://dx.doi.org/10.1158/1078-0432.CCR-12-2625