ABSTRACT
The in vivo biological activity of IL-2 can be dramatically increased by complexing with anti-IL-2 mAb. Moreover, IL-2/anti-IL-2 mAb immunocomplexes selectively stimulate different subsets of immune cells, depending on the clone of anti-IL-2 mAb that is used. Thus, IL-2/S4B6 mAb complexes strongly stimulate CD122high populations, namely NK and memory CD8+ T cells. They also intermediately stimulate Treg cells. Conversely, IL-2/JES6.1 mAb immunocomplexes have no stimulatory activity for CD122high populations. However, they potently and highly selectively stimulate CD25+ cells (i.e., Treg and activated T cells). IL-2/S4B6 mAb immunocomplexes have also been shown to possess antitumor activity in various mouse tumor models.
Introduction
The history of IL-2 began in 1960, when P. Nowell made an accidental discovery that phytohemagglutinin (PHA), a molecule from kidney beans, could stimulate lymphocytes to divide.Citation1 After a few days in culture, he checked some lymphocytes that had been incubated with PHA and found that they were no longer small, resting cells. Instead, they had transformed into large blastic cells that actually resembled leukemia cells. The mechanism responsible for this remained obscure for more than 20 y after Nowell's discovery. However, in 1965, there were two back-to-back papers in the journal, Nature, which reported activity found in the culture media of stimulated lymphocytes that promoted their proliferation. This was termed the blastogenic factor.Citation2,3 More than 10 y later, the blastogenic factor was found to enable the growth of cytotoxic T lymphocyte lines for several months. These retained their capacity to kill the leukemia cells to which they had been selected.Citation4 In 1978, the same group, which was headed by Kendall A. Smith, published the method to quantify the grow factor activity in culture supernatants of activated lymphocytes in the Journal of Immunology. It was there that they used the term T cell growth factor (TCGF) for the first time.Citation5
Already in 1979, there was evidence that macrophages also produced factor(s) with mitogenic activity for T cells. This was termed the lymphocyte activating factor (LAF).Citation6 However, the group of Kendall A. Smith showed that LAF enhanced the production of TCGF rather than stimulating the proliferation of T cells per se. This explained its mitogenic activity.Citation7,8 Thus, they changed the name of LAF to interleukin1 (IL-1), since it seemed to be first in the sequence. Furthermore, TCGF became IL-2 because it looked like it was second in the sequence. Actually, IL-2 was the first interleukin molecule to be purified (in 1983), identified and characterized in 1981, while IL-1 was purified and identified several years after that.
The biology of IL-2
IL-2 is a secreted glycoprotein with a molecular weight of 15–18 kDa, depending on the species origin and glycosylation. It consists of a single polypeptide chain, which contains four α helices.Citation9 The cytokine IL-2Citation10 is mainly produced by antigen-activated T cells. It promotes the proliferation, differentiation and survival of T lymphocytes, as well as the cytolytic activity of natural killer (NK) cells in the innate immune defense.Citation11-15 Effective T cell activation requires two distinct signals. One occurs when antigenic peptides, which are bound to MHC molecules on APC, are recognized via the T cell antigen receptor (TCR). The second signal occurs following an interaction of costimulatory molecules CD80/86, which are expressed on APCs with counter-receptors CD28, which are expressed on T cells.Citation16-18 These activated T cells initiate the expression and secretion of IL-2, which is subsequently used as an autocrine/paracrine growth factor. This is because these cells possess a complete high-affinity IL-2 receptor. Thus, IL-2 drives the proliferation of activated T cells until it is used up. CD80/86–CD28 interaction plays the most important role in delivering signal 2, which is vital for the sufficient production of IL-2. In the absence of signal 2, the production of IL-2 by TCR-stimulated lymphocytes is about 100-times lower.
Structure and function of IL-2 receptors
IL-2 exerts its pleiotropic activities by binding to either a dimeric receptor, which is composed of IL-2Rβ (CD122) and a common cytokine receptor gamma chain (γc, CD132), or a trimeric receptor, which is composed of IL-2Rα, IL-2Rβ and γc.Citation19 IL-2Rα (CD25) has been termed the “low-affinity” IL-2 receptor (Kd∼10 nM) and it is not involved in signal transduction.Citation20 A dimer of CD122 and CD132 binds IL-2 with an intermediate affinity (Kd∼1 nM) and is present on CD122high populations, namely memory CD8+ T cells (CD3+CD8+CD44highCD122high) and NK cells (CD3−NK1.1+DX5+).Citation21,22 A complex of CD25, CD122 and CD132 binds IL-2 with high affinity (Kd∼10 pM) and it is found mostly on activated T cells and T regulatory cells (Treg, CD3+CD4+CD25+Foxp3+). IL-2 signaling keeps a high level of CD25 expression, thus making positive feedback.Citation10 It is important to note that CD25 is not only found on activated T cells but also, on activated B cells,Citation23 although at significantly lower levels. Furthermore, it has even been expressed on some subpopulations of myeloid-origin cells, namely monocytes, macrophages and dendritic cells.Citation24-26 However, CD25 is absent on resting naive and memory T cells.
Role of IL-2 in the function and homeostasis of the immune system
Steady-state levels of IL-2 in vivo are normally too low to stimulate memory CD8+ and NK cells but are vital for the survival of Treg cells.Citation27,28 These cells are characterized by a high-constitutive expression of CD25, which enables the cells to express a high-affinity trimeric receptor. Thus, they utilize low levels of IL-2. Reflecting their dependency on IL-2, Treg cells disappear after the administration of anti-CD25 mAb.Citation29,30 Treg cells represent an important population of suppressor T cells that are essential in preventing auto-immunity. On the other hand, there is accumulating evidence that suggests Treg cells not only engage in the maintenance of immunologic self-tolerance in the periphery but also, impede immunosurveillance against autologous tumor cells.Citation31,32 Altogether, IL-2 can both potently stimulate T cell growth and NK cell activity, as well as play a role in maintaining peripheral tolerance and support suppressive activity that is mediated via Treg cells.
IL-2 and immunotherapy
For many years, IL-2 has been successfully used as a cancer treatment modality.Citation33 IL-2 was also the first therapy regimen to prove that sole immunological treatment that stimulates T cells can eradicate large, invasive and vascularized tumors in humans. Beginning with the first patient in 1984Citation34 and followed by hundreds of patients in the years after,Citation35,36 IL-2 was approved by the FDA for the treatment of renal carcinoma. Thus, it was the first immunotherapy that was approved for the treatment of cancer patients. The success of IL-2 treatment further escalated in 1998, when it was also approved for the treatment of metastatic melanoma.
Cancer treatment involving IL-2 has also been incorporated in adoptive cell transfer therapy (ACT).Citation37-39 Here, TILs that were isolated from resected tumors or genetically engineered lymphocytes were cultivated with IL-2 to develop specific cytolytic functions against these autologous tumors.Citation40 Once again, this led to an increased percentage of objective responses in patients, more so if the ACT was combined with a systemic treatment like preceding non-myeloablative chemotherapy, total body irradiation or subsequent IL-2 immunotherapy.Citation41
IL-2 immunotherapy was also tested for the treatment of viral infections, namely HIV.Citation42,43 It culminated in phase III clinical trials,Citation44 which stated that, despite increased CD4+ T cells counts, patients that have already been treated by antiretrovirals do not benefit from IL-2 treatment. It is still not known why the benefit of IL-2 mediated increase of CD4+ T cells do not translate into clinically positive effects.
Immunocomplexes of IL-2 and Anti-IL-2 mAb
The idea of IL-2 modification in order to control both its positive and/or negative effects is not brand new. Ranging from DNA vectors that facilitate the expression of IL-2Citation45,46 in situ or direct IL-2 modification via binding IL-2 to various other molecules,Citation47-50 probably one of the most effective ways to harness the potential of IL-2 was published by Boyman et al.Citation51 Here, the authors showed that IL-2 that is complexed with certain anti-IL-2 mAbs exerts extremely high stimulatory activity in vivo.
Anti-IL-2 mAb S4B6 paradoxically stimulates proliferation of memory CD8+ T Cells in vivo
The study of Boyman et al. resolved a long-time puzzle about T cell immunity, where the administration of anti-IL-2 mAb S4B6 leads to the selective transient proliferation and expansion of memory CD8+ T cells. This is similar to the administration of free IL-2. Several other research groups tried to explain this effect by various mechanisms.Citation52-54 However, the study of Boyman et al. showed that all of these explanations were incorrect,Citation51 as the main reason for this paradoxical phenomenon was the formation of IL-2/anti-IL-2 mAb immunocomplexes, i.e., endogenous IL-2 was bound to the injected S4B6 mAb, which were shown to be highly stimulatory in vivo for cells that possessed high levels of CD122.
IL-2/anti-IL-2 mAb immunocomplexes prepared in vitro exert high biological activity in vivo
IL-2/anti-IL-2 mAb immunocomplexes are only superior to IL-2 in vivo. Although IL-2/anti-IL-2 mAb immunocomplexes retain selectivity in vitro, free IL-2 has higher stimulatory activity than IL-2 immunocomplexes in vitro (between two and eight times, depending on the experimental model). This is most probably due to the unhindered access of cells to the IL-2 molecule.Citation51,55 Originally,Citation51 IL-2/anti-IL-2 mAb immunocomplexes were applied via two separate injections, one with free IL-2 and another with S4B6 anti-IL-2 mAb. They can also be prepared by premixing the chosen amount of IL-2 and anti-IL-2 mAb, ideally in a molar ratio 2:1. This is because some reportsCitation56 show that higher amounts of mAb, in the case of IL-2 complexed with anti-IL-2 mAb clone JES6.1, leads to the mild neutralization of IL-2 and, thus, a weaker stimulatory response. Such a premix can then be injected or stored frozen with no or very little loss of activity, respectively (our unpublished data).
Increasing biological activity of cytokine in vivo by respective anti-cytokine mAb is not limited to IL-2
Increasing the biological activity of cytokines in vivo via binding to the corresponding anti-cytokine mAb is not a unique phenomenon that is restricted to IL-2. IL-3,Citation57,58 IL-4,Citation57 IL-6,Citation59,60 IL-7Citation57,61 and GM-CSFCitation62 complexed with a relevant anti-cytokine antibody showed superior activity in comparison to free cytokine. IL-5 and IFN-α most likely fall into this category as well, but very limited data that support this are available.Citation63,64 These mAb-complexed cytokines have been successfully used in various models of antitumor responses, auto-immune diseases, graft tolerance and viral infections.Citation56,61,65,66
IL-2 Immunocomplexes: Selectivity and Cancer Immunotherapy
Two prototypical types of IL-2/anti-IL-2 mAb immunocomplexes, either S4B6-like or JES6.1-like, were described, along with their distinctive features, in terms of structure and effect on target cell subpopulations, based on the different mAb clones that are used for complexing IL-2 (). This is most probably possible due to two distinct and non-overlapping binding epitopes, which allow S4B6 and JES6.1 mAbs to capture a molecule of IL-2 in two different ways, according to its specificity.Citation67 The binding of mAbs to both of the epitopes simultaneously leads to the true neutralization and loss of function of IL-2 both in vitro and in vivo. The IL-2 molecule then becomes totally engulfed and is prevented to create any interactions with the IL-2 receptor.Citation55
Figure 1. Different stimulatory activity of IL-2/JES6.1 and IL-2/S4B6 immunocomplexes. (A) IL-2/JES6.1 immunocomplexes essentially require high-affinity trimeric IL-2 receptor to be expressed on cell surface. Thus, the utilization of IL-2/JES6.1 immunocomplexes is limited to CD25 expressing cells (left part). On the other hand, intermediate affinity dimeric receptor is sufficient to utilize IL-2/S4B6 immunocomplexes being available for much broader spectrum of immune cells (right part). (B) IL-2/JES6.1 immunocomplexes potently and selectively expand Treg cells while not increasing the number of effectors and thus generally promote tolerance. (C) IL-2/S4B6 immunocomplexes stimulate expansion of effectors much strongly than expansion of Treg cells and thus generally promote immunity.
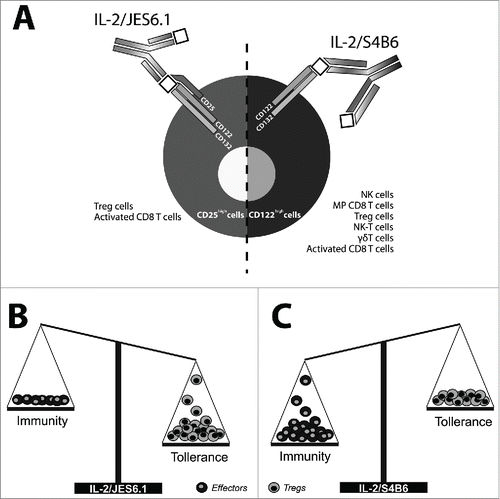
There are several other mAbs binding IL-2 of human and murine origin. Interestingly, they fall into one of these two categories, exerting either IL-2/S4B6-like or IL-2/JES6.1-like effects.
IL-2/S4B6 possess strong stimulatory activity for CD122high cells
IL-2/anti-IL-2 mAb complexes that are based on the clone of anti-IL-2 mAb S4B6 (henceforth IL-2/S4B6) only require a dimeric IL-2 receptor (i.e., βγ dimers) to exert their function (). Thus, IL-2/S4B6 are highly stimulatory for memory CD8+ T cells and NK cells (CD122high populations). However, they also generate moderate (<2-fold) relative increase of Treg cells (CD4+CD25+Foxp3+) and expand γδ T and NKT cells.Citation68 On the other hand, they have minimal effect on CD4+ memory T cells (CD44high) or B cells (B220+).Citation51
IL-2/JES6.1 potently stimulate solely CD25+ cells
Immunocomplexes that are based on the clone of anti-IL-2 mAb JES6.1 (henceforth, IL-2/JES6.1) strictly require CD25 (IL2Rα) to be present on the surface of the target cell, together with CD122 (IL2Rβ) and CD132 (common γc subunit), i.e., high-affinity trimeric IL-2 receptor (). CD25− cell populations are not able to utilize IL-2/JES6.1 even if they express both CD122 and CD132. Thus, IL-2/JES6 is shown to be selectively stimulatory for Treg cells.Citation51 Such expanded Treg cells exhibit similar or higher suppressive activity as normal Treg cells.Citation69 The phenotype of expanded Treg cells is characterized by a considerable, but only transient, increase of molecules that are relevant for suppressive potential (CD25, ICOS, CTLA-4 and GITR) and a mild transient increase in some other markers (CD44, TGF-β, ICAM-1 and PD-1), which are generally present on Treg cells. There is also a considerable build-up of IL-10 mRNA production with a respective increase in the suppressive activity in vitro and with the same transient character as is seen for the surface molecules.Citation56
Activated CD8+ T cells could be dramatically expanded by both IL-2 immunocomplexes
A very similar situation to the expansion of Treg cells arises in the case of activated CD8+ T cells. This is because both IL-2/S4B6 and IL-2/JES6.1 stimulate these cells to expand, although with a slightly different intensity (). Following stimulation through their TCR receptor, an expression of CD25 is induced, thus rendering them sensitive to IL-2/JES6.1. Very high stimulatory activity of both the IL-2/anti-IL-2 mAb immunocomplexes for the CD8+ T cells has been shown both in vitro and in vivo. Furthermore, expansion can reach an increase in relative count of more than three orders of magnitude within one week, leading to the generation of a robust population of memory phenotype CD8+ T cells (CD44highCD122high), which are capable of expressing effector functions upon a TCR signal.Citation55 Furthermore, it has been reported that, in the case of IL-2/S4B6, expanded cells have a central memory phenotype (CD44highCD122high CD62Lhigh) and are able to protect against bacterial infection. However, they exhibit reduced cellular fitness in terms of a lower homeostatic turnover rate and production of cytokines.Citation70
Potential of IL-2 immunocomplexes for cancer immunotherapy
IL-2 immunocomplexes provide a promising opportunity to turn the odds in favor of the patient that is suffering from cancer. However, a clinical level of testing has not been reached and only therapy in experimental animals has been carried out so far.
IL-2/S4B6 were repeatedly shown to possess significant antitumor activity in mice.Citation55,66,68,71 So far, IL-2/JES6.1 have not been reported to be tested for any antitumor activity. The reason for this is most probably their dominant biological activity, i.e., they potently and very selectively expand Treg cells, as has already been mentioned. This fact is likely to be discouraging enough to employ immunocomplexes of this type in cancer immunotherapy ().
However, from our recent experiments, we know that these immunocomplexes surprisingly possess antitumor activity, which is even comparable to IL-2/S4B6 in some tumor models, when given early after tumor inoculation. Furthermore, there are published dataCitation55 that support the idea that IL-2/JES6.1 might also prove as a potent antitumor agent, as they potently expand activated CD8+ T cells and not only Treg cells.
There is an interesting allegory between the prototypical types of IL-2/anti-IL-2 mAb immunocomplexes. On the one hand, both IL-2/S4B6 and IL-2/JES6.1 stimulate regulatory T cell population (Foxp3+ Treg cells). On the other hand, both of them expand effector cell population (activated CD8+ or memory CD8+ T cells). This dual nature of IL-2 immunocomplexes seems mostly critical for IL-2/JES6.1, which can modulate immune reactions based on Treg cells,Citation56,69 as well as functions that require effector cells. From an efficacy and effect's point of view, IL-2/S4B6 driven expansion of memory CD8+ T cells is probably superior to Treg cell expansion ().
The first evidence that IL-2/S4B6 can manifest an antitumor effect in vivo was provided by simultaneously injecting IL-2 expression plasmid and S4B6 mAb in a B16 melanoma model.Citation66 MAb and vector co-application led to a significant reduction of tumor nodules in the lungs of experimental mice. Another display of IL-2/S4B6 mediated antitumor capabilities was shown in combination with a poly(I:C) in RIP-Tag2-HA mice model.Citation71 Two studies have also focused on the direct application of IL-2/S4B6 alone or in combination with anthracycline antibiotic doxorubicin, either unconjugated or covalently bound to a poly(N-(2-hydroxypropyl)metacrylamide) carrier (pHPMA-DOX).Citation55 IL-2/S4B6 applied alone very early after tumor inoculation (several days) showed a significant antitumor effect in two tumor models in vivo, BCL1 leukemia and B16F10 melanoma. This led to the survival of one-third (BCL1) or two-thirds (B16F10) of experimental mice. Unfortunately, the late application of IL-2/S4B6 alone resulted in almost the complete loss of efficacy. Similar to other immunotherapy approaches, IL-2/S4B6 showed that, even though they have a considerable antitumor effect when injected early during tumor progression, they are almost ineffective when used later, i.e., for treatment of advanced tumors.
In such cases, combining different strategies turned out to be more effective. Furthermore, the application of doxorubicinCitation55 or pHPMA-DOXCitation68 prior to IL-2/S4B6 treatment led to significantly prolonged survival or even completely cured experimental mice, which, in the former, suffered from established tumors.
IL-2 complexes: toxicity, mechanisms, structure and beyond
IL-2 is well known for its severe side toxicities and mortality, which are associated with the high-dose applications in tumor therapy regimens.Citation34,72 A high dose of IL-2 associated toxicity can manifest in multiple organ systems, most significantly the heart, kidneys, skin, central nervous system, liver or lungs. Of these, the cardiopulmonary organ systems are the most common in the case of cancer treatmentCitation73 and usually lead to therapy discontinuation. Using a lower dosage to decrease the severity of these toxicities is not an option, as they are either necessary for the effectiveness of IL-2 mediated therapy (bolus applications) or produce the same level of toxicities (infusion application).Citation74
In the majority of IL-2-mediated toxicities, mechanisms of action are rather poorly understood. There are some exceptions, notably the vascular leak syndrome (VLS),Citation73 which is commonly associated with pulmonary toxicity. Originally thought to have been caused by proinflammatory cytokines that are mainly produced by NK cells, Krieg et al.Citation75 have compared the toxicity and effect of free rhIL-2 and rhIL-2 immunocomplexes of S4B6 type (rhIL-2/MAB602) on VLS. IL-2/anti-IL-2 mAb complexes led to a dramatic increase in CD8+ T cells and NK cells, with only mild VLS recorded. This can be compared to free IL-2, where VLS was much more profound and the expansion of the same cell populations was only mild. Moreover, rhIL-2 immunocomplexes that are specific for CD25+ cells also showed severe VLS phenotype in the absence of CD8+ and NK cell expansion. These data, together with experiments that have been conducted in an immunodepleted environment or in absence of CD25, led to the finding that CD25 expressing lung endothelial cells (CD31+) play an important role in IL-2 induced VLS,Citation75 possibly via nitrite oxide (NO) production.Citation76
Most importantly, rhIL-2 complexes that are specific for CD122high cells were about 13-fold less toxic compared to free IL-2. In the latter, both agents were given in five daily doses and the same stimulatory effect was achieved with a 40-fold lower dose of rhIL-2 immunocomplexes, in comparison to free IL-2.Citation75 Our unpublished data also suggest that IL-2/JES6.1, although strongly stimulatory for CD25+ cells, are far less toxic in comparison to free IL-2 than its S4B6-based counterpart. We speculate that either the massive expansion of Treg cells overrides the toxic effect that was reported in the case of IL-2/S4B6 (incl. VLS mediated either by lung endothelial CD31+CD25+ cells or by expanded NK-T cells in the lungs) or that the levels of CD122 and CD132 in lung endothelial CD31+CD25+ cells are too low to allow the strong stimulatory effect of IL-2/JES6.1. There is very little known about the mechanisms of action of the IL-2 immunocomplexes, apart from the finding that their increased biological activity is most probably governed by a considerable prolonged half-life in circulation.Citation55,77 Thus, various parts of the complex have been dissected in order to understand which part accounts more for the superior biological activity of the whole complex.Citation51,55,70,77,78 Such studies have found that the specific interaction of mAb and IL-2 is crucial for increased biological activity, as well as Fc, which is partly due to binding to an neonatal Fc receptor (FcRn), which further increases the half-life of IL-2.
Very recently, the structure of IL-2 binding antibodies S4B6 and JES6.1 came into the rangefinder of several research groups. Based on the functional data that are available, two studies shed more light on the molecular matter of IL-2 binding to anti-IL-2 mAbs, namely S4B6, JES6–5H4Citation79 and JES6.1.Citation67 The first studyCitation79 provided data describing the relevant epitopes for the binding of a human and, most importantly, murine IL-2 to its respective anti-IL-2 mAbs, S4B6 and JES6–5H4. Based on the phage-display method data analysis, S4B6 mAb shows a large overlap in the residues that are critical for binding to JES6–5H4 mAb. This results in a similar biological outcome, as reported earlier.Citation51 This cluster of residues collocates with an interface where CD25 recognizes IL-2, thus giving a molecular gist to the previous functional studies.Citation77,80 All of these corresponding data support the assumption that the binding of S4B6 mAb (and JES6–5H4 mAb alike) blocks or seriously hinders the CD25 recognition of IL-2. Another study of Rojas et al.Citation67 extended the dataset to JES6.1, identifying a completely different cluster of residues that are recognized by this antibody. They described this cluster as non-overlapping, with previously found clusters allowing the binding of S4B6 or JES6–5H4 mAbs. Moreover, their studies also pointed out JES6.1 as an inhibitor of IL-2 binding to CD25. Indeed, most recent published dataCitation81 show that, while S4B6 mAb sterically blocks IL-2 from the interaction with CD25 and stabilizes its interaction with CD122, stimulating all of the IL-2 responding cells and especially CD122high cells (e. g., memory CD8+ and NK cells), JES6.1 mAb blocks interaction of IL-2 with CD122 and CD132. Moreover, in the case of JES6.1 mAb, IL-2 affinity to CD25 is also lowered via a “trigger exchange” mechanism, which leads to the preferred stimulation of CD25high cells, e.g., Treg cells or activated CD8+ T cells.
Another studyCitation80 also added to the understanding of the IL-2 and IL-2 receptor structure and function by presenting a novel protein chimera protein scIL-2/S4B6, where IL-2 was covalently attached to a light chain of anti-IL-2 mAb S4B6 via a flexible oligopeptide spacer. This construct solved some problematic aspects of IL-2 complexes application such as the possible excess of IL-2 or mAb or dissociation-caused loss of IL-2 in low-concentration settings. It also implicated a structural basis and gave some insight into the mechanism of IL-2/anti-IL-2 mAb complexes.
Other approaches have been taken to increase IL-2 potential and untangle the mechanism of action in IL-2 potentiation as creating IL-2-derived muteinCitation82 or IL-2 superkine.Citation83 Such approaches relied on structural adjustments to the IL-2 molecule itself to either disable the CD25 interactions by mutating the CD25 binding interface to boost the effector population influenceCitation82 or remodel the conformation of the whole molecule to the point that it resembles IL-2 bound to CD25.Citation83 Both situations led to increased effector population functions, either by increasing affinity to dimeric IL-2 receptor and thus, stimulating effector T cells and NK cells,Citation83 or by disabling the source of IL-2 for Treg cells by lowering its affinity to CD25.Citation82
Other major applications
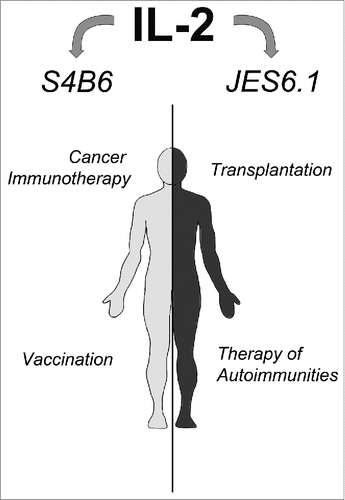
Although IL-2/S4B6-mediated CD8+ T cells expansion is also capable of a significant decrease in viral load during persistent virus infections,Citation84 which could possibly be clinically relevant, it is IL-2/JES6.1 that exert promising potential in other areas than oncological clinical, namely transplantations and auto-immune disease treatment.Citation56,69 Webster et al. covered one of the most pronounced data set on this matter.Citation56 Their works covered the use of IL-2/JES6.1 in mice as a prelude to the allotransplantation of MHC-incompatible pancreatic islets in absence of any other immunosuppression. This led to the indefinite survival of a majority of grafts. It also addressed the induction of an experimental autoimmune encephalomyelitis (EAE) mouse model of sclerosis multiplex and proved that IL-2/JES6.1 pretreatment renders mice resistant to the induction of the disease. IL-2/JES6.1, if combined with rapamycin, could even be used to treat ongoing EAE as well. These and other findings offer a solid platform for further clinical evaluation ().
Figure 2. IL-2 complexed with S4B6 or JES6.1 possesses a potential to be used for therapy of various diseases. IL-2 complexed with S4B6 enormously expands effector immune cells and demonstrates significant potential in cancer immunotherapy and potentiation of vaccination (left part). IL-2 complexed with JES6.1 dramatically expands Treg cells and show promising potential to increase transplantation success rate and to treat auto-immune diseases.
Concluding remarks
IL-2/anti-IL-2 mAb immunocomplexes appear to be promising new tools for the immunotherapy of malignant diseases, as well as the treatment of some other clinically relevant diseases. They are much more potent than IL-2, an FDA approved drug, and their immunostimulatory to toxic effect ratio seems to be superior to free IL-2. The use of CD122high population-stimulating IL-2 complexes (S4B6-like) seems to be a more straightforward approach. However, CD25+ population-stimulating IL-2 complexes (JES6.1-like) could be used, particularly in combination with anticancer vaccines, as they very potently stimulate the expansion of recently activated CD8+ T cells.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Funding
This work was supported by grant 13–12885S from GACR, by RVO: 61388971 and by the project “BIOCEV – Biotechnology and Biomedicine Center of the Academy of Sciences and Charles University” (CZ.1.05/1.1.00/02.0109), from the European Regional Development Fund.
References
- Nowell PC. Phytohemagglutinin: an initiator of mitosis in cultures of normal human leukocytes. Cancer Res 1960; 20:462-6; PMID:14427849
- Kasakura S, Lowenstein L. A factor stimulating DNA synthesis derived from the medium of leukocyte cultures. Nature 1965; 208:794-5; PMID:5868897; http://dx.doi.org/10.1038/208794a0
- Gordon J, MacLean LD. A lymphocyte-stimulating factor produced in vitro. Nature 1965; 208:795-6; PMID:4223737; http://dx.doi.org/10.1038/208795a0
- Gillis S, Smith KA. Long term culture of tumour-specific cytotoxic T cells. Nature 1977; 268:154-6; PMID:145543; http://dx.doi.org/10.1038/268154a0
- Gillis S, Ferm MM, Ou W, Smith KA. T cell growth factor: parameters of production and a quantitative microassay for activity. J Immunol 1978; 120:2027-32; PMID:307029
- Gery I, Gershon RK, Waksman BH. Potentiation of the T-lymphocyte response to mitogens. I. The responding cell. J Exp Med 1972; 136:128-42; PMID:5033417; http://dx.doi.org/10.1084/jem.136.1.128
- Smith KA, Gilbride KJ, Favata MF. Lymphocyte activating factor promotes T-cell growth factor production by cloned murine lymphoma cells. Nature 1980; 287:853-5; PMID:6776414; http://dx.doi.org/10.1038/287853a0
- Smith KA, Lachman LB, Oppenheim JJ, Favata MF. The functional relationship of the interleukins. J Exp Med 1980; 151:1551-6; PMID:6770028; http://dx.doi.org/10.1084/jem.151.6.1551
- Smith KA. Interleukin-2: inception, impact, and implications. Science 1988; 240:1169-76; PMID:3131876; http://dx.doi.org/10.1126/science.3131876
- Boyman O, Sprent J. The role of interleukin-2 during homeostasis and activation of the immune system. Nat Rev Immunol 2012; 12:180-90; PMID:22343569; http://dx.doi.org/10.1126/science.1122927
- Gillis S. Interleukin 2: biology and biochemistry. J Clin Immunol 1983; 3:1-13; PMID:6338024; http://dx.doi.org/10.1007/BF00919133
- Herberman RB. Natural killer cells. Annu Rev Med 1986; 37:347-52; PMID:3518610; http://dx.doi.org/10.1146/annurev.me.37.020186.002023
- Choi YS. Differentiation and apoptosis of human germinal center B-lymphocytes. Immunol Res 1997; 16:161-74; PMID:9212362; http://dx.doi.org/10.1007/BF02786360
- Oppenheim MH, Lotze MT. Interleukin-2: solid-tumor therapy. Oncology 1994; 51:154-69; PMID:8196899; http://dx.doi.org/10.1159/000227330
- Somersalo K. Migratory functions of natural killer cells. Nat Immun 1996; 15:117-33; PMID:9162262
- Johnson JG, Jenkins MK. Co-stimulatory functions of antigen-presenting cells. J Invest Dermatol 1992; 99:62S-5S; PMID:1358981; http://dx.doi.org/10.1111/1523-1747.ep12669010
- Rothenberg EV, Ward SB. A dynamic assembly of diverse transcription factors integrates activation and cell-type information for interleukin 2 gene regulation. Proc Natl Acad Sci U S A 1996; 93:9358-65; PMID:8790334; http://dx.doi.org/10.1073/pnas.93.18.9358
- Schwartz RH. Costimulation of T lymphocytes: the role of CD28, CTLA-4, and B7/BB1 in interleukin-2 production and immunotherapy. Cell 1992; 71:1065-8; PMID:1335362; http://dx.doi.org/10.1016/S0092-8674(05)80055-8
- Stauber DJ, Debler EW, Horton PA, Smith KA, Wilson IA. Crystal structure of the IL-2 signaling complex: paradigm for a heterotrimeric cytokine receptor. Proc Natl Acad Sci U S A 2006; 103:2788-93; PMID:16477002; http://dx.doi.org/10.1073/pnas.0511161103
- Wang HM, Smith KA. The interleukin 2 receptor. Functional consequences of its bimolecular structure. J Exp Med 1987; 166:1055-69; PMID:3116143; http://dx.doi.org/10.1084/jem.166.4.1055
- Nelson BH, Willerford DM. Biology of the interleukin-2 receptor. Adv Immunol 1998; 70:1-81; PMID:9755337; http://dx.doi.org/10.1016/S0065-2776(08)60386-7
- Zhang X, Sun S, Hwang I, Tough DF, Sprent J. Potent and selective stimulation of memory-phenotype CD8+ T cells in vivo by IL-15. Immunity 1998; 8:591-9; PMID:9620680; http://dx.doi.org/10.1016/S1074-7613(00)80564-6
- Brisslert M, Bokarewa M, Larsson P, Wing K, Collins LV, Tarkowski A. Phenotypic and functional characterization of human CD25+ B cells. Immunology 2006; 117:548-57; PMID:16556269; http://dx.doi.org/10.1111/j.1365-2567.2006.02331.x
- Espinoza-Delgado I, Ortaldo JR, Winkler-Pickett R, Sugamura K, Varesio L, Longo DL. Expression and role of p75 interleukin 2 receptor on human monocytes. J Exp Med 1990; 171:1821-6; PMID:2110244; http://dx.doi.org/10.1084/jem.171.5.1821
- Kronin V, Vremec D, Shortman K. Does the IL-2 receptor alpha chain induced on dendritic cells have a biological function? Int Immunol 1998; 10:237-40; PMID:9533452; http://dx.doi.org/10.1093/intimm/10.2.237
- Hodge S, Hodge G, Flower R, Han P. Surface and intracellular interleukin-2 receptor expression on various resting and activated populations involved in cell-mediated immunity in human peripheral blood. Scand J Immunol 2000; 51:67-72; PMID:10632978; http://dx.doi.org/10.1046/j.1365-3083.2000.00644.x
- Fontenot JD, Rasmussen JP, Gavin MA, Rudensky AY. A function for interleukin 2 in Foxp3-expressing regulatory T cells. Nat Immunol 2005; 6:1142-51; PMID:16227984; http://dx.doi.org/10.1038/ni1263
- Malek TR, Bayer AL. Tolerance, not immunity, crucially depends on IL-2. Nat Rev Immunol 2004; 4:665-74; PMID:15343366; http://dx.doi.org/10.1038/nri1435
- Comes A, Rosso O, Orengo AM, Di Carlo E, Sorrentino C, Meazza R, Piazza T, Valzasina B, Nanni P, Colombo MP et al. CD25+ regulatory T cell depletion augments immunotherapy of micrometastases by an IL-21-secreting cellular vaccine. J Immunol 2006; 176:1750-8; PMID:16424205; http://dx.doi.org/10.4049/jimmunol.176.3.1750
- Suvas S, Kumaraguru U, Pack CD, Lee S, Rouse BT. CD4+CD25+ T cells regulate virus-specific primary and memory CD8+ T cell responses. J Exp Med 2003; 198:889-901; PMID:12975455; http://dx.doi.org/10.1084/jem.20030171
- Ko K, Yamazaki S, Nakamura K, Nishioka T, Hirota K, Yamaguchi T, Shimizu J, Nomura T, Chiba T, Sakaguchi S. Treatment of advanced tumors with agonistic anti-GITR mAb and its effects on tumor-infiltrating Foxp3+CD25+CD4+ regulatory T cells. J Exp Med 2005; 202:885-91; PMID:16186187; http://dx.doi.org/10.1084/jem.20050940
- Sakaguchi S. Naturally arising CD4+ regulatory t cells for immunologic self-tolerance and negative control of immune responses. Annu Rev Immunol 2004; 22:531-62; PMID:15032588; http://dx.doi.org/10.1146/annurev.immunol.21.120601.141122
- Rosenberg SA. IL-2: the first effective immunotherapy for human cancer. J Immunol 2014; 192:5451-8; PMID:24907378; http://dx.doi.org/10.4049/jimmunol.1490019
- Rosenberg SA, Lotze MT, Muul LM, Leitman S, Chang AE, Ettinghausen SE, Matory YL, Skibber JM, Shiloni E, Vetto JT et al. Observations on the systemic administration of autologous lymphokine-activated killer cells and recombinant interleukin-2 to patients with metastatic cancer. N Engl J Med 1985; 313:1485-92; PMID:3903508; http://dx.doi.org/10.1056/NEJM198512053132327
- Rosenberg SA, Lotze MT, Muul LM, Chang AE, Avis FP, Leitman S, Linehan WM, Robertson CN, Lee RE, Rubin JT et al. A progress report on the treatment of 157 patients with advanced cancer using lymphokine-activated killer cells and interleukin-2 or high-dose interleukin-2 alone. N Engl J Med 1987; 316:889-97; PMID:3493432; http://dx.doi.org/10.1056/NEJM198704093161501
- Rosenberg SA, Yang JC, Topalian SL, Schwartzentruber DJ, Weber JS, Parkinson DR, Seipp CA, Einhorn JH, White DE. Treatment of 283 consecutive patients with metastatic melanoma or renal cell cancer using high-dose bolus interleukin 2. JAMA 1994; 271:907-13; PMID:8120958; http://dx.doi.org/10.1001/jama.1994.03510360033032
- Griffith KD, Read EJ, Carrasquillo JA, Carter CS, Yang JC, Fisher B, Aebersold P, Packard BS, Yu MY, Rosenberg SA. In vivo distribution of adoptively transferred indium-111-labeled tumor infiltrating lymphocytes and peripheral blood lymphocytes in patients with metastatic melanoma. J Natl Cancer Inst 1989; 81:1709-17; PMID:2810387; http://dx.doi.org/10.1093/jnci/81.22.1709
- Rosenberg SA, Yannelli JR, Yang JC, Topalian SL, Schwartzentruber DJ, Weber JS, Parkinson DR, Seipp CA, Einhorn JH, White DE. Treatment of patients with metastatic melanoma with autologous tumor-infiltrating lymphocytes and interleukin 2. J Natl Cancer Inst 1994; 86:1159-66; PMID:8028037; http://dx.doi.org/10.1093/jnci/86.15.1159
- Rosenberg SA, Packard BS, Aebersold PM, Solomon D, Topalian SL, Toy ST, Simon P, Lotze MT, Yang JC, Seipp CA et al. Use of tumor-infiltrating lymphocytes and interleukin-2 in the immunotherapy of patients with metastatic melanoma. A preliminary report. N Engl J Med 1988; 319:1676-80; PMID:3264384; http://dx.doi.org/10.1056/NEJM198812223192527
- Muul LM, Spiess PJ, Director EP, Rosenberg SA. Identification of specific cytolytic immune responses against autologous tumor in humans bearing malignant melanoma. J Immunol 1987; 138:989-95; PMID:3100623
- Dudley ME, Wunderlich JR, Robbins PF, Yang JC, Hwu P, Schwartzentruber DJ, Topalian SL, Sherry R, Restifo NP, Hubicki AM et al. Cancer regression and autoimmunity in patients after clonal repopulation with antitumor lymphocytes. Science 2002; 298:850-4; PMID:12242449; http://dx.doi.org/10.1126/science.1076514
- Cooper DA, Emery S. Latent reservoirs of HIV infection: flushing with IL-2? Nat Med 1999; 5:611-2; PMID:10371491; http://dx.doi.org/10.1038/9454
- Sereti I, Anthony KB, Martinez-Wilson H, Lempicki R, Adelsberger J, Metcalf JA, Hallahan CW, Follmann D, Davey RT, Kovacs JA et al. IL-2-induced CD4+ T-cell expansion in HIV-infected patients is associated with long-term decreases in T-cell proliferation. Blood 2004; 104:775-80; PMID:15090457; http://dx.doi.org/10.1182/blood-2003-12-4355
- Group I-ES, Committee SS, Abrams D, Levy Y, Losso MH, Babiker A, Collins G, Cooper DA, Darbyshire J, Emery S et al. Interleukin-2 therapy in patients with HIV infection. N Engl J Med 2009; 361:1548-59; PMID:19828532; http://dx.doi.org/10.1056/NEJMoa0903175
- Liu M, Acres B, Balloul JM, Bizouarne N, Paul S, Slos P, Squiban P. Gene-based vaccines and immunotherapeutics. Proc Natl Acad Sci U S A 2004; 101 Suppl 2:14567-71; PMID:15333750; http://dx.doi.org/10.1073/pnas.0404845101
- Parker SE, Khatibi S, Margalith M, Anderson D, Yankauckas M, Gromkowski SH, Latimer T, Lew D, Marquet M, Manthorpe M et al. Plasmid DNA gene therapy: studies with the human interleukin-2 gene in tumor cells in vitro and in the murine B16 melanoma model in vivo. Cancer Gene Ther 1996; 3:175-85; PMID:8725882
- Melder RJ, Osborn BL, Riccobene T, Kanakaraj P, Wei P, Chen G, Stolow D, Halpern WG, Migone TS, Wang Q et al. Pharmacokinetics and in vitro and in vivo anti-tumor response of an interleukin-2-human serum albumin fusion protein in mice. Cancer Immunol Immunother 2005; 54:535-47; PMID:15592670; http://dx.doi.org/10.1007/s00262-004-0624-7
- Klimka A, Yu N, Shami EY. Construction of proteolysis resistant human interleukin-2 by fusion to its protective single chain antibody. Cytokine 2003; 22:134-41; PMID:12842761; http://dx.doi.org/10.1016/S1043-4666(03)00136-4
- Barouch DH, Truitt DM, Letvin NL. Expression kinetics of the interleukin-2/immunoglobulin (IL-2/Ig) plasmid cytokine adjuvant. Vaccine 2004; 22:3092-7; PMID:15297060; http://dx.doi.org/10.1016/j.vaccine.2004.01.065
- Katre NV, Knauf MJ, Laird WJ. Chemical modification of recombinant interleukin 2 by polyethylene glycol increases its potency in the murine Meth A sarcoma model. Proc Natl Acad Sci U S A 1987; 84:1487-91; PMID:3494243; http://dx.doi.org/10.1073/pnas.84.6.1487
- Boyman O, Kovar M, Rubinstein MP, Surh CD, Sprent J. Selective stimulation of T cell subsets with antibody-cytokine immune complexes. Science 2006; 311:1924-7; PMID:16484453; http://dx.doi.org/10.1126/science.1122927
- Ku CC, Murakami M, Sakamoto A, Kappler J, Marrack P. Control of homeostasis of CD8+ memory T cells by opposing cytokines. Science 2000; 288:675-8; PMID:10784451; http://dx.doi.org/10.1126/science.288.5466.675
- Murakami M, Sakamoto A, Bender J, Kappler J, Marrack P. CD25+CD4+ T cells contribute to the control of memory CD8+ T cells. Proc Natl Acad Sci U S A 2002; 99:8832-7; PMID:12084927; http://dx.doi.org/10.1073/pnas.132254399
- Kamimura D, Ueda N, Sawa Y, Hachida S, Atsumi T, Nakagawa T, Sawa S, Jin GH, Suzuki H, Ishihara K et al. Evidence of a novel IL-2/15R β-targeted cytokine involved in homeostatic proliferation of memory CD8+ T cells. J Immunol 2004; 173:6041-9; PMID:15528339; http://dx.doi.org/10.4049/jimmunol.173.10.6041
- Tomala J, Chmelova H, Mrkvan T, Rihova B, Kovar M. In vivo expansion of activated naive CD8+ T cells and NK cells driven by complexes of IL-2 and anti-IL-2 monoclonal antibody as novel approach of cancer immunotherapy. J Immunol 2009; 183:4904-12; PMID:19801515; http://dx.doi.org/10.4049/jimmunol.0900284
- Webster KE, Walters S, Kohler RE, Mrkvan T, Boyman O, Surh CD, Grey ST, Sprent J. In vivo expansion of T reg cells with IL-2-mAb complexes: induction of resistance to EAE and long-term acceptance of islet allografts without immunosuppression. J Exp Med 2009; 206:751-60; PMID:19332874; http://dx.doi.org/10.1084/jem.20082824
- Finkelman FD, Madden KB, Morris SC, Holmes JM, Boiani N, Katona IM, Maliszewski CR. Anti-cytokine antibodies as carrier proteins. Prolongation of in vivo effects of exogenous cytokines by injection of cytokine-anti-cytokine antibody complexes. J Immunol 1993; 151:1235-44; PMID:8393043
- Jones AT, Ziltener HJ. Enhancement of the biologic effects of interleukin-3 in vivo by anti-interleukin-3 antibodies. Blood 1993; 82:1133-41; PMID:8353280
- Heremans H, Dillen C, Put W, Van Damme J, Billiau A. Protective effect of anti-interleukin (IL)-6 antibody against endotoxin, associated with paradoxically increased IL-6 levels. Eur J Immunol 1992; 22:2395-401; PMID:1381315; http://dx.doi.org/10.1002/eji.1830220932
- Martens E, Dillen C, Put W, Heremans H, van Damme J, Billiau A. Increased circulating interleukin-6 (IL-6) activity in endotoxin-challenged mice pretreated with anti-IL-6 antibody is due to IL-6 accumulated in antigen-antibody complexes. Eur J Immunol 1993; 23:2026-9; PMID:8344369; http://dx.doi.org/10.1002/eji.1830230846
- Boyman O, Ramsey C, Kim DM, Sprent J, Surh CD. IL-7/anti-IL-7 mAb complexes restore T cell development and induce homeostatic T Cell expansion without lymphopenia. J Immunol 2008; 180:7265-75; PMID:18490726; http://dx.doi.org/10.4049/jimmunol.180.11.7265
- Rubinstein MP, Salem ML, Doedens AL, Moore CJ, Chiuzan C, Rivell GL, Cole DJ, Goldrath AW. G-CSF/anti-G-CSF antibody complexes drive the potent recovery and expansion of CD11b+Gr-1+ myeloid cells without compromising CD8+ T cell immune responses. J Hematol Oncol 2013; 6:75; PMID:24279871; http://dx.doi.org/10.1186/1756-8722-6-75
- Zabeau L, Van der Heyden J, Broekaert D, Verhee A, Vandekerckhove J, Wu SJ, Chaiken I, Heinrich P, Behrmann I, Tavernier J. Neutralizing monoclonal antibodies can potentiate IL-5 signaling. Eur J Immunol 2001; 31:1087-97; PMID:11298333; http://dx.doi.org/10.1002/1521-4141(200104)31:4%3c1087::AID-IMMU1087%3e3.0.CO;2-Q
- Rosenblum MG, Unger BW, Gutterman JU, Hersh EM, David GS, Frincke JM. Modification of human leukocyte interferon pharmacology with a monoclonal antibody. Cancer Res 1985; 45:2421-4; PMID:3986783
- Hamilton SE, Schenkel JM, Akue AD, Jameson SC. IL-2 complex treatment can protect naive mice from bacterial and viral infection. J Immunol 2010; 185:6584-90; PMID:21037095; http://dx.doi.org/10.4049/jimmunol.1001215
- Kamimura D, Sawa Y, Sato M, Agung E, Hirano T, Murakami M. IL-2 in vivo activities and antitumor efficacy enhanced by an anti-IL-2 mAb. J Immunol 2006; 177:306-14; PMID:16785526; http://dx.doi.org/10.4049/jimmunol.177.1.306
- Rojas G, Cabrera Infante Y, Pupo A, Carmenate T. Fine epitope specificity of antibodies against interleukin-2 explains their paradoxical immunomodulatory effects. MAbs 2014; 6:273-85; PMID:24253188; http://dx.doi.org/10.4161/mabs.27224
- Tomala J, Chmelova H, Strohalm J, Ulbrich K, Sirova M, Rihova B, Kovar M. Antitumor activity of IL-2/anti-IL-2 mAb immunocomplexes exerts synergism with that of N-(2-hydroxypropyl)methacrylamide copolymer-bound doxorubicin conjugate due to its low immunosuppressive activity. Int J Cancer 2011; 129:2002-12; PMID:21165950; http://dx.doi.org/10.1002/ijc.25859
- Liu R, Zhou Q, La Cava A, Campagnolo DI, Van Kaer L, Shi FD. Expansion of regulatory T cells via IL-2/anti-IL-2 mAb complexes suppresses experimental myasthenia. Eur J Immunol 2010; 40:1577-89; PMID:20352624; http://dx.doi.org/10.1002/eji.200939792
- Kamimura D, Bevan MJ. Naive CD8+ T cells differentiate into protective memory-like cells after IL-2 anti IL-2 complex treatment in vivo. J Exp Med 2007; 204:1803-12; PMID:17664293; http://dx.doi.org/10.1084/jem.20070543
- Verdeil G, Marquardt K, Surh CD, Sherman LA. Adjuvants targeting innate and adaptive immunity synergize to enhance tumor immunotherapy. Proc Natl Acad Sci U S A 2008; 105:16683-8; PMID:18936481; http://dx.doi.org/10.1073/pnas.0805054105
- Schwartzentruber DJ. Biologic therapy with interleukin-2: clinical applications. Principles of administration and management of side effects. Philadelphia:Lippincott Williams & Wilkins, 1995.
- Schwartz RN, Stover L, Dutcher J. Managing toxicities of high-dose interleukin-2. Oncology (Williston Park) 2002; 16:11-20; PMID:12469935
- West WH, Tauer KW, Yannelli JR, Marshall GD, Orr DW, Thurman GB, Oldham RK. Constant-infusion recombinant interleukin-2 in adoptive immunotherapy of advanced cancer. N Engl J Med 1987; 316:898-905; PMID:3493433; http://dx.doi.org/10.1056/NEJM198704093161502
- Krieg C, Letourneau S, Pantaleo G, Boyman O. Improved IL-2 immunotherapy by selective stimulation of IL-2 receptors on lymphocytes and endothelial cells. Proc Natl Acad Sci U S A 2010; 107:11906-11; PMID:20547866; http://dx.doi.org/10.1073/pnas.1002569107
- Epstein AL, Mizokami MM, Li J, Hu P, Khawli LA. Identification of a protein fragment of interleukin 2 responsible for vasopermeability. J Natl Cancer Inst 2003; 95:741-9; PMID:12759392; http://dx.doi.org/10.1093/jnci/95.10.741
- Letourneau S, van Leeuwen EM, Krieg C, Martin C, Pantaleo G, Sprent J, Surh CD, Boyman O. IL-2/anti-IL-2 antibody complexes show strong biological activity by avoiding interaction with IL-2 receptor alpha subunit CD25. Proc Natl Acad Sci U S A 2010; 107:2171-6; PMID:20133862; http://dx.doi.org/10.1073/pnas.0909384107
- Phelan JD, Orekov T, Finkelman FD. Cutting edge: mechanism of enhancement of in vivo cytokine effects by anti-cytokine monoclonal antibodies. J Immunol 2008; 180:44-8; PMID:18097002; http://dx.doi.org/10.4049/jimmunol.180.1.44
- Rojas G, Pupo A, Leon K, Avellanet J, Carmenate T, Sidhu S. Deciphering the molecular bases of the biological effects of antibodies against Interleukin-2: a versatile platform for fine epitope mapping. Immunobiology 2013; 218:105-13; PMID:22459271; http://dx.doi.org/10.1016/j.imbio.2012.02.009
- Tomala J, Kovarova J, Kabesova M, Votavova P, Chmelova H, Dvorakova B, Rihova B, Kovar M. Chimera of IL-2 linked to light chain of anti-IL-2 mAb mimics IL-2/anti-IL-2 mAb complexes both structurally and functionally. ACS Chem Biol 2013; 8:871-6; PMID:23419043; http://dx.doi.org/10.1021/cb3007242
- Spangler JB, Tomala J, Luca VC, Jude KM, Dong S, Ring AM, Votavova P, Pepper M, Kovar M, Garcia KC. Antibodies to Interleukin-2 Elicit Selective T Cell Subset Potentiation through Distinct Conformational Mechanisms. Immunity 2015; 42:815-25; PMID:25992858; http://dx.doi.org/10.1016/j.immuni.2015.04.015
- Carmenate T, Pacios A, Enamorado M, Moreno E, Garcia-Martinez K, Fuente D, León K. Human IL-2 mutein with higher antitumor efficacy than wild type IL-2. J Immunol 2013; 190:6230-8; PMID:23677467; http://dx.doi.org/10.4049/jimmunol.1201895
- Levin AM, Bates DL, Ring AM, Krieg C, Lin JT, Su L, Moraga I, Raeber ME, Bowman GR, Novick P et al. Exploiting a natural conformational switch to engineer an interleukin-2 ‘superkine’. Nature 2012; 484:529-33; PMID:22446627; http://dx.doi.org/10.1038/nature10975
- Molloy MJ, Zhang W, Usherwood EJ. Cutting edge: IL-2 immune complexes as a therapy for persistent virus infection. J Immunol 2009; 182:4512-5; PMID:19342623; http://dx.doi.org/10.4049/jimmunol.0804175
