ABSTRACT
T cell infiltration at the tumor site has been identified as a major predictor for the efficacy of adoptive T cell therapy. The chemokine C-C motif ligand 22 (CCL22) is highly expressed by immune cells in murine and human pancreatic cancer. Expression of its corresponding receptor, C-C chemokine receptor type 4 (CCR4), is restricted to regulatory T cells (Treg). We show that transduction of cytotoxic T cells (CTL) with CCR4 enhances their immigration into a pancreatic cancer model. Further, we show that binding of CCR4 with CCL22 strengthens the binding of T cell LFA-1 to dendritic cell (DC) ICAM-1 and increases CTL activation. In vivo, in a model of subcutaneous pancreatic cancer, treatment of tumor-bearing mice with CCR4-transduced CTL led to the eradication of established tumors in 40% of the mice. In conclusion, CCR4 overexpression in CTL is a promising therapeutic strategy to enhance the efficacy of adoptive T cell transfer (ACT).
Abbreviations
| ACT | = | adoptive T cell transfer |
| APC | = | antigen-presenting cell |
| CCL2 | = | chemokine C-C motif ligand |
| CCR | = | C-C chemokine receptor |
| CTL | = | cytotoxic T lymphocytes |
| DC | = | dendritic cell |
| ELISA | = | Enzyme-Linked Immunosorbent Assay |
| GFP | = | green fluorescent protein |
| ICAM-1 | = | intercellular adhesion molecule-1 |
| IL-2 | = | interleukin-2 |
| IFNγ | = | interferon gamma |
| LFA-1 | = | lymphocyte function-associated antigen-1 |
| OVA | = | Ovalbumine |
| PBMC | = | peripheral blood mononuclear cells |
| Treg | = | regulatory T cell |
| Teff | = | effector T cell. |
Introduction
ACT is a powerful approach for the treatment of different cancer types.Citation1 ACT uses tumor-specific T cells either isolated from the patient´s own tumor or rendered tumor specific through transduction with a given T cell or chimeric antigen receptor.Citation2-4 The transfer of tumor antigen-specific cytotoxic T lymphocytes (CTL) can induce complete disease remission in some patients with metastatic melanoma,Citation5 Epstein-Barr virus-positive non-Hodgkin lymphoma,Citation6 acute lymphatic leukemia,Citation7 B cell lymphomaCitation8 or nasopharyngeal carcinoma.Citation9 However, the capacity of adoptively transferred T cells to invade the tumor and to induce an efficient antitumor immune response is limited. Thus, only a small subgroup of patients benefit from ACT in the long term.Citation10
Infiltration of CTL or other immune cells into the tumor is mainly regulated by the local chemokine milieu. Several chemokines are known to attract immunosuppressive cell populations that shield tumor cells from the host's immune response.Citation11,12 Chemotherapy and irradiation of tumors can enhance the migration of T cells into tumors, among other by altering the chemokine profile of the tumor environment. The induction of apoptosis and necrosis in tumor cells by chemotherapy and irradiation generates an inflammatory reaction, which promotes the recruitment of T cells into the tumor.Citation13-15 However, a limitation is the lack of specificity and the high toxicity of these therapies. Citation16 To circumvent these obstacles and to enhance specificity, we aimed to genetically modify CTL ex vivo prior to ACT to improve their entry into the tumor.
Determinants of T cell infiltration into tumors include adhesion molecules that enable lymphocytes to attach to and pass the endothelial barrier of blood vesselsCitation2,17,18 and chemokine gradients sensed by receptors expressed on CTLs to attract T cells chemotactically toward tumors.Citation19 The endothelial integrin intercellular adhesion molecule 1 (ICAM-1) and its receptor lymphocyte function-associated antigen 1 (LFA-1) are mandatory for the process of extravasation.Citation20 Moreover, the interaction of LFA-1 on T cells with ICAM-1 on antigen-presenting cells (APC), is a prerequisite for APC-mediated T cell activation.Citation21 The affinity of integrin receptors can be regulated by activation of chemokine receptors. CCR7, for example activates LFA-1 through a process known as inside-out-signaling: Binding of CCR7 by its ligand CCL21 changes the conformation of LFA-1 and its affinity for ICAM-1 is strongly increased.Citation22
The chemokine CCL22 is expressed in many tumors and mediates the recruitment of Treg into the tumor tissue.Citation11,23 The corresponding chemokine receptor CCR4 is highly expressed by Treg, whereas CTL lack CCR4 expression. Citation24 We hypothesized that a strategy increasing the migration of CTL into the tumor could improve the therapeutic efficacy of ACT. In this context, CCR4 may be a promising candidate to increase CTL tumor infiltration and potentially to enhance antitumor effects of CTL by increasing the LFA-1 affinity for ICAM-1.
In this study, we show that the transduction of CCR4 into CTL enhances the LFA-1-mediated binding to DCs and increases the activation of CTL. We demonstrate that adoptively transferred CTL overexpressing CCR4 accumulate in pancreatic cancer and induce increased antitumor immune responses. We also show CCL22 expression in patient pancreatic cancer specimens as evidence that T-cell transduction with CCR4 may warrant further investigations for the treatment of human pancreatic cancer.
Results
CCL22 is over-expressed in experimental tumors of pancreatic cancer cells
We aimed to identify chemokines with strong intratumoral expression and with no expression of their corresponding chemokine receptors on CTL to explore unique chemoattractant stimuli for these cells. We hypothesized that the de novo expression of such chemokine receptors in CTL prior to adoptive transfer could increase the capability of these chemokines to attract CTL into the tumor and to improve the therapeutic efficacy of ACT. In order to identify appropriate chemokines, we screened established subcutaneously induced murine Panc02-OVA tumors for C-C chemokine expression by real-time PCR (). The strongest expression was found for the chemokines CCL2, CCL6, CCL7 and CCL22 (). The CCL22-specific receptor CCR4 is not expressed on CTL. In contrast, CCR4 is highly expressed on Tregs and guides these cells into the tumor tissue. Citation11 Thus, the de novo expression of CCR4 in CTL could be a promising approach to increase tumor-directed migration of CTL in ACT. To validate the potential of CCL22 to selectively attract CCR4-expressing cells into the tumor tissue, we quantified the expression of CCL22 on protein level in tumor and in other organs of Panc02-OVA tumor-bearing mice by ELISA. Expression of CCL22 was strongest in the tumor and peripheral lymph nodes (), suggesting that CCR4-mediated migration of T cells would be preferentially directed to these sites. In these tumors, we could identify CD11c-positive immune cells as the main source of CCL22-production (Fig. S1). For the second ligand of CCR4, CCL17, only low concentrations were detected in the same tissues (Fig. S2). Normal murine pancreas did not express detectable levels of either chemokine. We next investigated the expression of CCR4 on T cells in tumor-bearing mice. Cell populations from tumor, peripheral lymph nodes, spleen, lung and blood of Panc02-OVA tumor-bearing mice were analyzed for CCR4 expression on non-T cells (CD3neg.), CTL (CD3+CD8+), Teff (CD3+CD4+CD25neg.) and Treg (CD3+CD4+CD25+) (). In all analyzed compartments, CCR4 was preferentially expressed on Treg (). These experiments identify the CCL22–CCR4 axis as a potential target to improve CTL migration into Panc02-OVA tumors.
Figure 1. CCL22 is expressed in murine pancreatic tumors. (A) Panc02-OVA tumors were dissected and quantitative real-time PCR was used to assess mRNA levels of all known C-C chemokines. (B) Murine CCL22 protein concentrations were quantified in different organs of tumor-bearing mice using ELISA. (C) Using anti-CCR4 antibodies, non-T cells (CD3neg.), CTL (CD3+CD8+), Teff (CD3+CD4+CD25neg.) and Treg (CD3+CD4+CD25+) harvested from tumors and different organs of tumor bearing mice were stained and MFIs for CCR4 were determined using flow cytometry. Data are presented as mean of biological triplicates ± SEM and are representative of two independent experiments.
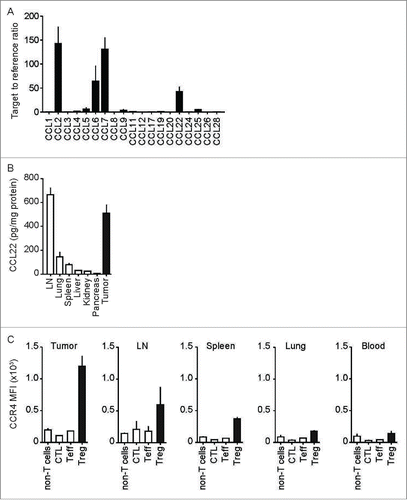
Figure 2. CCL22 attracts CCR4- but not CCR4del-transduced T cells. (A, B) Migration of CCR4-GFP- and CCR4del-GFP-transduced T cells toward increasing concentrations of CCL22 (0, 10, 50 and 100 ng/mL) was analyzed in a transwell migration assay. (C) Panc02-OVA-CCL22dox tumor cells with doxycycline-inducible CCL22 expression were plated in the bottom well of a transwell plate with (+) and without (−) doxycycline and anti-CCL22 antibody. Numbers of migrated CCR4-GFP- and CCR4del-GFP-transduced OT-1 T cells were determined by flow cytometry and tumor cell lysis induced by migrated cells was evaluated by LDH release. Data are presented as mean of biological triplicates ± SEM and are representative of two independent similar experiments. p values were determined by the unpaired Student's t-test. ***p < 0.001.
CCR4-transduced CTL specifically migrate toward CCL22 and effectively kill tumor cells in vitro
To test the ability of CCR4 to promote CTL migration, we transduced OVA-specific T cells from OT-1 transgenic mice with CCR4-GFP or with a non-functional mutant of CCR4 (CCR4del-GFP) (Fig. S3). In the trans-well assay, CCR4-GFP but not CCR4del-GFP expression mediated specific and dose-dependent migration of the transduced T-cells toward CCL22 (). Specificity of migration was confirmed by enrichment of GFP-expressing cells in CCR4-GFP but not in CCR4del-GFP-transduced OT-1 T cells (). Dose-dependent migration and GFP enrichment of CCR4-GFP cells was also observed toward CCL17 (Fig. S4). CCL22 neutralization by antibody completely abrogated migration (). To exclude that the overexpression of CCR4 alters the effector function of T cells, migration toward and cytotoxicity against Panc02-OVA-CCL22dox cells, a tumor cell line with doxycycline (Dox)-inducible expression of CCL22 were analyzed. We could show that tumor cell-derived CCL22 strongly promoted CCR4-GFP but not CCR4del-GFP-transduced OT-1 T cell migration (). By analyzing cytotoxicity of the migrated cells, we could further show that CCR4-GFP-transduced CTL efficiently lysed Panc02-OVA-CCL22dox tumor cells. CCL22-blockade abrogated both migration and subsequent tumor cell lysis (). Thus, transduction of CTL with CCR4 strongly enhances migration toward CCL22-expressing cells and promotes tumor cell lysis.
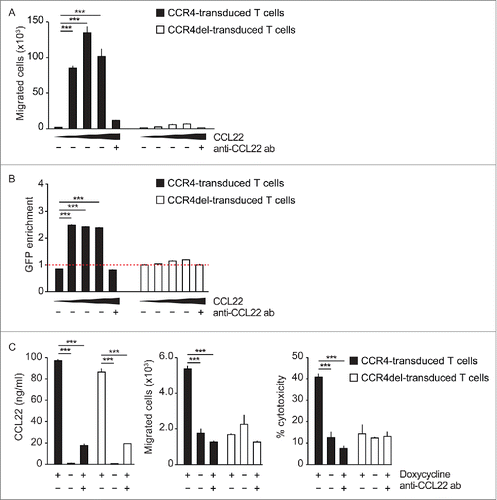
Figure 3. CCL22 enhances LFA-1 - ICAM-1 binding and results in stronger CTL activation. (A) CCR4- and CCR4del-transduced OT-1 T cells stained with PKH67 (green) and PKA26 (red), respectively, were co-cultured with OVA257–264-loaded DC of wild type (WT) or CCL22-deficient (KO) mice in the presence (+) or absence (−) of anti-ICAM-1 antibody for 6 h and were then imaged by confocal microscopy. The ratio of CCR4- to CCR4del-transduced T cells within DC—T cell clusters was quantified. (B and C) CCR4-GFP- and CCR4del-GFP-transduced T cells were incubated with (+) and without (−) CCL22 and ICAM-1 binding affinity was evaluated either (B) by visualizing T cell-bound Fc-tagged ICAM-1 using an APC-linked anti-Fc antibody for flow cytometry or (C) with a calcein-based adhesion assay. (D) CCR4del- or CCR4-transduced OT-1 CTL were cultured with OVA257–264-loaded WT DC and recombinant CCL22 in the presence (+) or absence (−) of anti-ICAM-1. IL-2 and IFNγ release was analyzed by ELISA. All data are presented as mean of biological replicates ± SEM and are representative of two to three independent experiments. The displayed clusters are representative of 40 randomly selected and blindly evaluated clusters of two independent experiments. p values were determined by the unpaired Student's t-test. *p < 0.05; ** p < 0.01; ***p < 0.001; ns, not significant.
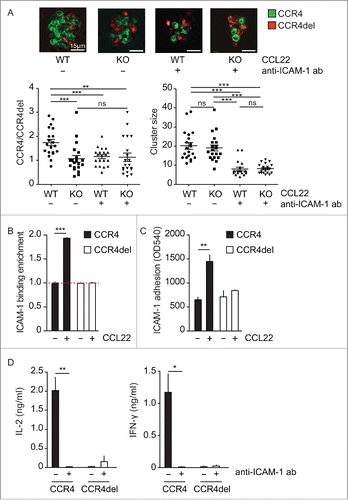
Figure 4. Transfer of CCR4-transduced OT-1 (T)cells induces regression of established tumors. (A) Panc02-OVA tumor-bearing CD45.2+ CD90.2+ mice (WT) were i.v. injected with CCR4-GFP-transduced CD45.1+ and CCR4del-GFP-transduced CD90.1+ OT-1 CTL. One week after injection the GFP distribution among all transferred marker cells was analyzed by flow cytometry. The amount of CCR4-transduced T cells was normalized to the amount of CCR4del-transduced T cells. (B, C) Mice bearing established subcutaneous Panc02-OVA tumors were i.v. injected 6 and 12 d after tumor induction with 2 × 106 OT-1 T cells transduced with GFP, CCR4 or CCR4del and tumor growth and survival was monitored every second day. (D) Tumor-free mice were re-challenged by subcutaneous injection of a lethal number (0.5 × 106) of Panc02-OVA tumor cells. Data are presented as mean ± SEM of eight mice and sensored at the time, the first mice had to be sacrificed due to the predefined endpoints of the study and are representative for two independent experiments. For re-challenge experiments, all cured mice were used. Significance of tumor growth was calculated by two-way ANOVA with Bonferroni post-test correction, differences in survival were analyzed by log-rank test and p value of cell enrichment by unpaired Student's t-test. *p < 0.05; **p < 0.01; ***p < 0.001.
CCR4 enhances ICAM-1-dependent T cell activation
Substantial amounts of CCL22 are produced by mature DC in vivo.Citation25 The process of CTL priming against tumor antigen requires the interaction of DC with the corresponding T cells. To investigate whether CCR4 expression in CTL influences DC-CTL interactions and in consequence CTL activation, co-cultures of both cell types were imaged in vitro and analyzed for T cell activation. CCR4- and CCR4del-transduced OT-1 T cells were mixed at a 1:1 ratio and were co-cultured with OVA-primed DC derived from either wild-type or CCL22-deficient mice. After 6 h DC-CTL clusters were analyzed by confocal microscopy for the ratio of CCR4 to CCR4del within the clusters () and CCL22 concentration in the co-culture supernatant was measured by ELISA (Fig. S5). Interestingly, clusters with CCL22-expressing DC derived from wild-type mice contained almost twice as many CCR4-expressing CTL as CCR4del-expressing CTL (). In contrast, equal amounts of CCR4- and CCR4del-transduced CTL clustered around DC derived from CCL22-deficient mice. These findings indicate that DC-derived CCL22 induces CCR4-mediated cell contacts between DC and CTL. An important factor of DC-T cell aggregation is the interaction of LFA-1 on T cells with ICAM-1 on DC.Citation21 As chemokines can affect LFA-1-ICAM-1 interaction,Citation22 we aimed to test whether this ligand-receptor pair mediates the enhanced clustering of CCR4-expressing CTL. Indeed, blocking of ICAM-1 completely abrogated the preferential accumulation of CCR4-expressing CTL (). In addition, ICAM-1 blockade resulted in a significant reduction of cluster size in all conditions, irrespective of CCL22 expression (). To elucidate whether CCL22 binding to CCR4 on T cells indeed enhances T cell LFA-1 affinity for ICAM-1, we analyzed the binding of recombinant ICAM-1 to CCL22-stimulated T cells. Indeed, in the presence of CCL22, 2fold more CCR4-transduced T cells bound recombinant ICAM-1 than in the absence of CCL22, whereas no ICAM-1 binding increase was observed on CCR4del-transduced () or on untransduced GFP-negative T cells (Fig. S6A). Binding of ICAM-1 to CCL22-stimulated CCR4-transduced T cells was LFA-1 specific, as preincubation with an LFA-1 blocking antibody, completely abrogated ICAM-1 binding (Fig. S6B). We next tested the adhesion of T cells to immobilized (plate-bound) ICAM-1. CCL22 pretreatment significantly increased the adhesion of CCR4-transduced CTL to ICAM-1, while the binding of CCR4del-transduced cells was not affected by CCL22 (). These results suggest that CCL22-CCR4 interactions indeed increase ICAM-1 to LFA-1 binding and thus enhances DC-T cell interaction. To test the functional consequence of the strengthened interaction of CCR4-transduced T cells with DC, we analyzed the activation of CCR4- and CCR4del-transduced CTL by DC. In the presence of CCL22, the recognition of OVA presented on DC by CCR4-transduced OT-1 CTL was markedly increased compared to CCR4-del-transduced OT-1 CTL, as measured by IL-2 and IFNγ release (). Again, the addition of ICAM-1 blocking antibody abrogated the CCL22-induced increase of T cell activation (). These results suggest that the binding of ICAM-1 to LFA-1 contributes to the CCL22-induced enhancement of CCR4- over CCR4del-transduced CTL-DC interaction.
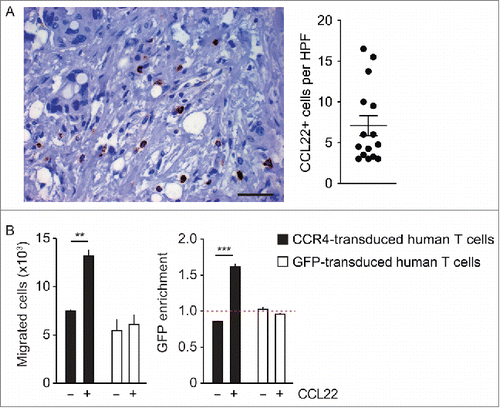
Figure 5. CCL22 is expressed in human pancreatic cancer tissue and induces the migration of CCR4-transduced human T cells. (A) Paraffin-embedded samples of 15 patients suffering from human pancreatic ductal adenocarcinoma were stained with anti-human CCL22 antibody (400-fold magnification, scale bar 50 µm). The average number of CCL22-expressing cells per high power field was calculated separately for every case. (B) Primary T cells obtained from human peripheral blood mononuclear cells (PBMC) were transduced with CCR4-GFP or GFP and were used for a transwell migration assay toward CCL22 (50 ng/mL). Numbers and GFP expression of migrated cells were analyzed by flow cytometry. Data are shown as mean of triplicates ± SEM and are representative for two independent similar experiments with human T cells. p values were determined by the unpaired Student's t-test. **p < 0.01; ***p < 0.001.
CCR4-transduced CTL enhance the efficacy of adoptive T cell transfer in a subcutaneous Panc02 murine tumor model
To examine whether CCR4 expression can increase the therapeutic efficacy of ACT, we made use of the Panc02-OVA syngeneic tumor, which expresses CCL22 and is known to be widely resistant to ACT. We mixed CD45.1+ CCR4-GFP-transduced OT-1 T cells and CD90.1+ CCR4del-GFP-transduced OT-1 T cells at a 1:1 ratio and adoptively transferred these cells into Panc02-OVA tumor-bearing CD45.2+CD90.2+ mice. One week after transfer, the GFP distribution among all transferred marker cells was analyzed in the spleen, the peripheral lymph nodes and the tumors by flow cytometry. Remarkably, CCR4-transduced T cells specifically enriched over CCR4del-transduced T cells in the tumor tissue but not in the spleen or total peripheral lymph nodes (). When we analyzed lymph node sites individually, we found a slight enrichment in the axillary ipsilateral but not in the ipsilateral or contralateral inguinal lymph nodes (Fig. S7). Next, we treated mice bearing established Panc02-OVA tumors twice, at days 6 and 12, with either GFP-, CCR4del- or CCR4-transduced OT-1 T cells (by i.v. injection). Treatment with CCR4-transduced T cells resulted in inhibition of tumor growth and cured four out of eight mice compared to one out of eight mice (p < 0.05) in the control groups treated with GFP- or CCR4del-transduced OT-1 T cells (). Tumor-free mice remained cured for the duration of the observation period (up to 70 d) and were protected from re-challenge with a lethal dose of Panc02-OVA tumor cells (), suggesting established immunity against the Panc02-OVA cells and long-term persistence of the transferred T cells. These results suggest that CCR4 expression increases the migration of adoptively transferred T cells preferentially into the tumor and thereby enhances their therapeutic efficacy.
CCL22 is expressed by human pancreatic cancer and human CCR4-transduced CTL migrate toward CCL22
To examine whether CCL22 may be a promising target for improving ACT against human malignancies, we analyzed CCL22 expression in human pancreatic adenocarcinoma, the entity recapitulated in the murine model we used so far. In immunohistochemistry, CCL22 was expressed in all of 15 analyzed pancreatic cancer samples. It was expressed in cells that corresponded in size, shape and localization to infiltrating leukocytes, but not to cancer cells (). To test if the impact of CCR4-transduction in murine T cells would also translate into human T cells, we retrovirally transduced primary T cells obtained from human peripheral blood mononuclear cells (PBMC) with human CCR4-GFP or GFP alone (Fig. S8). CCR4-GFP but not GFP transduction increased migration of transduced primary T cells toward CCL22 (). Among the migrated cells, GFP-positive cells preferentially migrated upon CCR4-GFP transduction, but not in the control condition, indicating a specific chemotactic effect mediated through CCR4 (). In summary, we show here that CCR4 transduction induces specific migration of primary human T cells toward CCL22, a chemokine that is expressed in human pancreatic cancers. Thus, in analogy to our findings in the murine tumor model of pancreatic cancer cells, CCR4 transduction of human T cells may be capable to improve adoptive T cell therapy in patients with pancreatic cancer.
Discussion
In patients suffering from hematological malignancies, ACT is a powerful treatment modality to treat even refractory disease.Citation26,27 However, a major limitation for the use of ACT in the treatment of solid tumors is the impaired access of immune cells to the tumor tissue, resulting in limited efficacy.Citation28 Strategies to improve tissue infiltration by adoptively transferred T cells, especially in tumor entities such as pancreatic ductal adenocarcinoma which feature dense and extended stroma, are critical for ACT success.Citation2 We could recently show that transduction of CTL with a marker antigen may enable bispecific antibodies to specifically engage these T cells to the tumor cell and enhance ACT efficacy.Citation29 Similarly, we could demonstrate using a novel PD1-CD28-fusion receptor, that these T cells can be rendered resistance against PD-L1 driven immune suppression. Citation30 In these studies, while we initially hypothesized an enhancement of T cell infiltration, we found few infiltrating T cells in the tumor. Furthermore, after initial response, the tumors relapsed, suggesting that a more extensive T cell infiltration is required for effective and persistent ACT effects.
Treg, in contrast to CTL, can be found in large numbers in experimental and human tumors.Citation11 The main mechanism for the attraction of immunosuppressive cells and the relative repulsion of CTL from the tumor is the chemokine profile present in the tumor micromilieu.Citation19 In the preclinical tumor model studied here, we could identify the CCL22 - CCR4 axis as central for Treg tumor infiltration, as has been suggested previously for other diseases and models.Citation11,31 We reasoned that we could target this axis therapeutically to enhance ACT efficacy. We demonstrate a strong therapeutic impact of T cell transduction with CCR4 in a syngeneic tumor model, accompanied by the accumulation of CCR4-transduced OT-1-T cells in the tumor. Neither CCL22 nor CCL17 are expressed by pancreatic cancer cells, as shown in the present study, but by the surrounding immune cells both in mice and humans. Our results extend previous findings where CCR4 has been used to redirect T cells to the tumor cells in xenograft models.Citation32 The present approach is novel in the sense that previous strategies have used chemokine receptors to redirect T cells to the tumor cells directlyCitation32-34 while none have tried to attract T cells by employing a chemokine secreted by non-tumor cells in or around the tumor, such as CCL22 in our model. Attracting T cells to the tumor tissue, instead of directly to the tumor cell, may be beneficial, since we could show that CCL22 also strengthens the interaction with APC such as DC and boosts antigen recognition in an integrin ICAM-1-dependent manner.
The previous studies on chemokine receptor-enhanced recruitment of T cells to tumors have been performed in xenograft models in immunodeficient mice.Citation32,33 In these models, counteracting effects of immunosuppressive cells, such as Treg, are excluded and the transplanted tumors are the only tissue that expresses human chemokines for attracting human CTL. In contract, the results of the present study demonstrate the efficiency of CCR4-transduced CTL for tumor treatment in immunocompetent mice.
It is known that the function of integrin receptors can be regulated by certain chemokines and their receptors. Engagement of CCL21 to CCR7 changes the conformation of LFA-1 and thereby increases the affinity of LFA-1 for ICAM-1.Citation22,35 Since DC express large amounts of ICAM-1, the interactions of LFA-1 and ICAM-1 are part of the immunological synapse formed by T cells and DC.Citation36 Our results show that CCR4 transduction not only increases the infiltration of adoptively transferred CTL into the tumor but also enhances the ICAM-1 - LFA-1-dependent interaction with antigen-presenting DC. This in turn is a crucial step for the activation of CTL. At this interface, CCR4-transduced T cells seem to outcompete CCR4del-transduced, potentially due to a limited amount of interaction sites for T cells per DC.Citation37 Thus, in the absence of CCL22 from DC or CCR4 on T cell the cluster composition but not the cluster size is altered.
While our data confirmed previously reported expression of CCL22 in murine pancreatic cancer,Citation38 little is known about the expression of CCL22 in human pancreatic cancer. To test if the CCL22 - CCR4 axis could, in principle, be targeted in human cancer, we analyzed tissue specimens from 15 pancreatic ductal adenocarcinoma patients by immunohistochemistry. In all tumor samples, CCL22 expressing cells were found. Interestingly, infiltrating immune cells, but not tumor cells, appeared to be responsible for intratumoral CCL22 expression, as has been suggested before in other tumor entities.Citation39,40 We could recently identify CD14+ and CD68+ myeloid cells as the origin of CCL22 secretion at the tumor site in breast-cancer patients Citation41. However, if our expression data from the Panc02-OVA-model holds for pancreatic cancer, there the secreting cells may be rather CD11c+ myeloid cells. CCL22 is homeostatically expressed by DCs in lymph nodes and other lymphatic tissues Citation42,43. Thus, attracting antigen-specific T cells to tumor-distant sites may be important in the safety assessment of the strategy, especially if the antigen chosen is not tumor selective and may become activated outside of the tumor tissue. However, under homeostatic conditions, CCL22 is not relevant for entry of T cells in the lymph node under homeostatic conditions but is controlled through CCL19 and CCL21 Citation44. As a consequence, we could not find an overall enrichment in lymph nodes but only in distinct anatomical location at the edge of the tumor site. Our data provide evidence that CCR4-transduced T cells potentially rather drain into tumor associated nodes which may reduce the risk of offsite T cell activation through unspecific redirection.
In summary, our results indicate that CCR4 transduction of CTL may be a promising new approach for the therapy of patients with a CCL22-expressing tumor microenvironment. Given that arming T cells with CCR4 can only affect T cell activation in an antigen-specific manner, we suggest that equipping T cells with such a navigation system may enhance T cell efficacy without impacting safety.
We suggest that an analysis of the chemokine expression profile of human cancers may help to identify entities amenable for a disease specific chemokine-based targeting strategy to enhance the efficacy of ACT.
Methods
Cell lines
The murine pancreatic cancer cell line Panc02 and its ovalbumin-transfected counterpart Panc02-OVA have been previously described.Citation45 The CCL22-expressing Panc02-OVA-CCL22dox and MC38-OVA-CCL22dox tumor cell lines were generated by lentiviral transduction with a construct containing a Dox-inducible CCL22 expression cassette. The transduction protocol has been described in detail.Citation46 The packaging cell line Plat-E was a kind gift of W. Uckert (Berlin, Germany) and HEK 293T cells were obtained from ATCC (Manassas, USA). T cell line Jurkat was purchased from Life technologies (USA). All cells were cultured in DMEM with 10% fetal bovine serum (FBS, Life Technologies), 1% penicillin and streptomycin (PS) and 1% L-glutamine (all from PAA, Germany). 1 µg/mL puromycin and 10 µg/mL blasticidin (both Sigma, Germany) were added to the Plat-E medium. Primary murine and human T cells were cultured in RPMI 1640 with 10% FBS, 1% PS, 1% Lglutamine, 1% sodium pyruvate, 1 mM HEPES and 50 µM β-mercaptoethanol (PAA, Germany and Sigma, Germany).
Animal experiments
C57BL/6 mice transgenic for a T cell receptor specific for ovalbumin (OT-1) were purchased from The Jackson Laboratory, USA (stock number 003831). OT-1 mice were crossed with CD45.1 congenic marker mice (obtained from The Jackson Laboratory, stock number 002014) or with CD90.1 congenic marker mice (a kind gift from R. Obst, Munich, Germany) to generate CD45.1-OT-1 and CD90.1-OT-1 mice, respectively. CCL22 knockout mice were obtained from KOMP, USA. For animal experiments, C57BL/6 mice were purchased from Janvier, France. Tumors were induced by subcutaneous injection of 2 × 106 tumor cells and mice were treated by i.v. injection of T cells as indicated. For re-challenge experiments, mice were injected subcutaneously with 0.5 × 106 tumor cells in the flank opposite to the initial tumor. All experiments were randomized and blinded. Tumor growth and condition of mice was monitored every other day. All animal experiments were approved by the local regulatory agency (Regierung von Oberbayern).
Generation of new fusion constructs
All constructs were generated by overlap extension PCR and recombinant expression cloning into the retroviral pMP71 vector, as follows: CCR4-GFP consists of murine CCR4 (Uniprot Entry P51680 amino acids 1–360) linked to GFP; the CCR4del-GFP consists of murine CCR4 amino acids 1–313 linked to GFP and the human CCR4-GFP consists of human CCR4 (Uniprot Entry P51679 amino acids 1–360) linked to GFP.
Murine T cell transduction
The retroviral vector pMP71 (kindly provided by C. Baum, Hannover) was used for transfection of the ecotrophic packaging cell line Plat-E. Transduction protocols have been described in detail.Citation29 In brief, for primary murine T cell transduction Plat-E cells were transfected and the produced retrovirus was used to transduce T cells. T cells were stimulated first by addition of anti-CD3 antibody, anti-CD28 antibody (eBioscience, clones 145-2C11 and 37.51, respectively) and IL-2, and subsequently by addition of anti-CD3 beads, anti-CD28 beads (Life technologies) and human IL-15 (Peprotech, Germany).
Human T cell transduction
CCR4-GFP was cloned into the retroviral vector pMP71. pMP71-CCR4-GFP or pMP71-GFP was used for transduction of human T cells. Transduction protocol has been described in detail.Citation29 In brief, for human T cell transduction, HEK-293T cells were triple-transfected with the respective retroviral vector together with the plasmids pcDNA3.1-MLVg/p and pALF10A1 (kindly provided by W. Uckert, Berlin, Germany). The produced retrovirus was used to transduce T cells. T cells were stimulated using anti-CD3 and anti-CD28 antibodies (clones HIT3a and CD28.2, eBioscience) and IL-2 (Peprotech).
Flow cytometry
Multi-color flow cytometry was performed using a BD FACS Canto II (BD bioscience, Germany). Single cell suspensions were obtained from spleen, lymph nodes, lung, blood and tumor and were stained with following antibodies: anti-CD3e-APC (clone 145–2C11, Biolegend), anti-CD4-APC/Cy7 (clone GK1.5, Biolegend), anti-CD8-PerCP (clone 53–6.7, Biolegend), anti-CD25 (clone 3C7, Biolegend), anti-IFNγ-FITC (clone XMG1.2, Biolegend), anti-mouse CD45.1 (APC, Clone A20, eBioscience), anti-mouse CD90.1-PeCy7 (clone OX7, Biolegend), anti-FOXP3-Pacific BlueTM (clone MF-14, Biolegend) and anti-CCR4-PE/Cy7 (clone 2G12, Biolegend). For isolating tumor-infiltrating lymphocytes, tumors were mechanically disrupted, incubated with 1 mg/mL collagenase and 0.05 mg/mL DNAse (both from Sigma Aldrich) and passed through a cell strainer. Single cell suspensions were layered on a gradient of 44% Percoll (Biochrome, Berlin, Germany) and 67% Percoll prior to centrifugation at 800 g for 30 min. Lymphocytes were obtained from the interphase, were washed with PBS and used for flow cytometry analysis.
Migration and killing assays
Cell migration was evaluated using transwell plates (Corning) as previously described.Citation47 In brief, 1 × 106 CCR4-GFP- or CCR4del-GFP-transduced CTL or Jurkat cells were placed onto a 5 µm pore filter in the upper chamber of a transwell plate with the lower chamber containing different concentrations of CCL22 or CCL17 (both Peprotech). For antagonizing migration through neutralization of CCL22 10 ng/mL, anti-CCL22 antibody (clone 158132, R&D) was added to the lower chamber. After 3 h incubation at 37°C the migrated cells in the lower chamber were analyzed by flow cytometry. For migration assays in combination with killing assays, 5 × 105 CCR4-GFP- or CCR4del-GFP-transduced OT-1 CTL were placed in the upper chamber of a transwell plate containing 1 × 105 Panc02-OVA-CCL22dox or MC38-OVA-CCL22dox tumor cells with Dox-inducible CCL22 expression in the lower chamber in the presence or absence of 2 µg/mL Dox (Sigma-Aldrich) and 10 ng/mL anti-CCL22 antibody. After 3 h incubation at 37°C the upper chamber was removed. The CTL-mediated lysis of tumor cells in the lower was measured by LDH release (Promega) after another 6 h incubation at 37°C. The percentage of cytotoxicity was normalized as follows: % lysis = (release of the target condition–spontaneous release of tumor and T cells) / (maximal release of tumor cells–spontaneous release of tumor and T cells) . The concentration of CCL22 in the supernatant of the tumor cells was measured by ELISA (R&D Systems, Minneapolis, MN, USA).
Cytokine assays of tissue lysates
Tissue homogenates were resuspended in lysis buffer (BioRad Laboratories, Hercules, CA, USA) and were centrifuged. Total protein concentration was measured by Bradford assay (BioRad Laboratories). All samples were diluted to a protein concentration of 10 mg/mL and CCL17 and CCL22 concentrations were measured by ELISA (R&D Systems). The final cytokine concentration was calculated as pg cytokine per mg protein in the respective lysate.
RNA isolation and quantitative real-time PCR analysis
Total RNA was extracted from subcutaneous tumors using High Pure RNA Isolation Kit (Qiagen, Valencia, CA) according to the manufacturer's instructions. 1 µg of RNA was converted to cDNA using the Revert Aid First strand cDNA Synthesis Kit (Fermentas, St. Leon-Rot, Germany). Quantitative real-time PCR amplification was performed with the Light Cycler TaqMan Master (Roche Diagnostics, Mannheim, Germany) on a LightCycler 2.0 instrument (Roche Diagnostics) together with the Universal Probe Library System (Roche Diagnostics). Relative gene expression is shown as a ratio of the expression level of the gene of interest to that of hypoxanthine phosphoribosyltransferase (HPRT) RNA. Quantitative real-time PCR primers were obtained from Metabion (Planegg, Germany; for primer sequences see Table S1).
ICAM-1 adhesion and flow cytometry assay
For ICAM-1 adhesion assays, 107 T cells transduced with CCR4 or CCR4del were labeled with 10 µg/mL Calcein (Life Technologies). Flat bottom 96-well plates were coated with100 µg/mL ICAM-1 (R&D) for 1 h and blocked using 2% BSA for 30 min. After washing with PBS, T cells were plated in 200 µL PBS at a concentration of 2 × 106 cells per mL and incubated for 1 h at 37°C. After washing 3 times with 200 µL PBS, remaining cells were lysed using 200 µL of 10% Triton X-100 and centrifugation at 800 g for 5 min. 100 µL of the supernatants were transferred into new plates and fluorescence was measured using an ELISA reader. For ICAM-1 binding assays, 0.1 × 106 CCR4-GFP- or CCR4del-GFP-transduced T cells were incubated with 200 ng/mL CCL22 (Peprotech) in the presence or absence of 10 µg/mL anti-LFA-1 antibody (clone H155–78, Biolegend) in a total volume of 50 µL cell adhesion buffer (PBS containing 10% FCS, 1 mM MgCl2 and 1 mM CaCl2). After adding 50 µL of recombinant mouse ICAM1/human Fc chimera (10 µg/mL, R&D) and incubating for 15 min at room temperature, cells were washed using cell adhesion buffer and fixated with 1% PFA at 4°C for 30 min. Subsequently, cells were stained for 30 min at 4°C using an APC linked anti-human Fc antibody (clone HP6017, Biolegend) and analyzed using flow cytometry.
Confocal microscopy and cytokine secretion assay
CCR4del- and CCR4-transduced OT-1 CTL were labeled with PKH-26 and PKH-67 (Sigma, Germany), respectively, according to the manufacturer's instruction. PKH-labeled CCR4- and CCR4del-transduced CTL (5 × 104) were cultured in the presence or absence of 5 µg/mL anti-ICAM-1 antibody (clone YNI.7.4, BioXCell) with non-labeled splenic DC (5 × 103) in 96-well non-tissue round bottom plates as described before.Citation48 DC were isolated with CD11c-microbeads (Miltenyi Biotec, Germany) from splenic single cell suspensions of C57BL/6 or CCL22-deficient mice. After 6 h culture in the presence of 5 µg/mL CpG 1826 (Coley Pharmaceutical Group) and 1 µg/mL OVA257–264 peptide (InvivoGen) cells were gently transferred to a glass-bottomed dish and used for confocal microscopy. The CCL22 concentration in the supernatants was analyzed by ELISA. For cytokine secretion assays 2 × 105 CCR4del- or CCR4-transduced OT-1 CTL were cultured in the presence or absence of 5 µg/mL anti-ICAM-1 antibody with splenic DC (5 × 103). After 2, 4, 6, 8 and 10 h culture in the presence of 5 µg/mL CpG 1826, 1 µg/mL OVA257–264 peptide and 50 ng/mL CCL22 (Peprotech) IL-2 and IFNγ levels were analyzed by ELISA (R&D Systems) in the culture supernatants.
Patient samples and tissue microarray (TMA) construction
Formalin-fixed, paraffin-embedded tumor tissue of 15 patients with confirmed pancreatic ductal adenocarcinoma was retrieved from the archives of the Institute of Pathology of the Ludwig-Maximilians Universität München. TMA consisting of two cores of histologically confirmed PDAC tumor tissue, each 1.5 mm diameter, was constructed using a semiautomatic tissue arrayer (Beecher Instruments). Clinicopathological patient data was retrieved from the original pathology reports. This retrospective analysis was carried out according to the recommendations of the local ethics committee of the Medical Faculty of the Ludwig-Maximilians-Universität München.
CCL22 immunohistochemistry and microscopy
Immunohistochemical staining was performed on 2 to 3 µm thick TMA sections after deparaffinization and rehydration using the rabbit anti-human CCL22 antibody (1:350; Peprotech) and an alkaline phosphatase-conjugated secondary antibody for detection, including appropriate positive and negative control tissues. In each tissue core, two peritumoral regions containing high amounts of CCL22+ cells were examined and the number of CCL22+ cells of two high power fields (HPF) was determined. The average number of CCL22+ cells in each case was finally calculated by division by four. Exemplary microscopical images were acquired at 400-fold magnification using a camera-equipped Zeiss Axioskop microscope (Zeiss) and Zeiss Axiovision imaging software.
Statistical analysis
All data are presented as mean +/− SEM and the statistical significance of differences were determined by the two-tailed Student's t-test. Differences in tumor size were analyzed using two-way ANOVA with Bonferroni posttest corrections. Differences in survival were analyzed by log-rank (Mantel–Cox) test. Statistical analyses were performed using GraphPad Prism 6 (GraphPad Software). p values < 0.05 were considered significant.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
KONI_S_1105428.zip
Download Zip (4.1 MB)Acknowledgments
SE and SK are member of the German Center for Lung Research. SG received a stipend from the German Cancer Aid. Parts of this work have been performed for the doctoral theses of SG and MC at the Ludwig-Maximilians-Universität München.
Funding
This work was supported by the international doctoral program “i-Target: Immunotargeting of cancer” funded by the Elite Network of Bavaria (to SK and SE), the Melanoma Research Alliance (grant number N269626 to SK and SE), the Wilhelm Sander Stiftung (grant number 2014.018.1 to SE and SK), the Graduiertenkolleg 1202 “Oligonucleotides in cell biology and therapy” funded by the Deutsche Forschungsgemeinschaft (to SE, DA and SK), the German Cancer Aid (to MR, DA and SK), the Else-Kröner Fresenius Stiftung (grant number 2014_A204 to SK), the Marie-Slodowska Curie innovative training network “IMMUTRAIN: training network for the immunotherapy of cancer,” funded under the H2020 program of the European Union (to SE and SK) and by LMU Munich's Institutional Strategy LMUexcellent within the framework of the German Excellence Initiative (to SE and SK).
References
- Rosenberg SA. Raising the bar: the curative potential of human cancer immunotherapy. Sci Transl Med 2012; 4:127ps8; PMID:22461638; http://dx.doi.org/10.1126/scitranslmed.3003634
- Kalos M, June CH. Adoptive T cell transfer for cancer immunotherapy in the era of synthetic biology. Immunity 2013; 39:49-60; PMID:23890063; http://dx.doi.org/10.1016/j.immuni.2013.07.002
- Morgan RA, Chinnasamy N, Abate-Daga D, Gros A, Robbins PF, Zheng Z, Dudley ME, Feldman SA, Yang JC, Sherry RM et al. Cancer regression and neurological toxicity following anti-MAGE-A3 TCR gene therapy. J Immunother 2013; 36:133-51; PMID:23377668; http://dx.doi.org/10.1097/CJI.0b013e3182829903
- Dudley ME, Gross CA, Somerville RP, Hong Y, Schaub NP, Rosati SF, White DE, Nathan D, Restifo NP, Steinberg SM et al. Randomized selection design trial evaluating CD8+-enriched versus unselected tumor-infiltrating lymphocytes for adoptive cell therapy for patients with melanoma. J Clin Oncol: Off J Am Soc Clin Oncol 2013; 31:2152-9; PMID:23650429; http://dx.doi.org/10.1200/JCO.2012.46.6441
- Dudley ME, Wunderlich J, Nishimura MI, Yu D, Yang JC, Topalian SL, Schwartzentruber DJ, Hwu P, Marincola FM, Sherry R et al. Adoptive transfer of cloned melanoma-reactive T lymphocytes for the treatment of patients with metastatic melanoma. J Immunother 2001; 24:363-73; PMID:11565838; http://dx.doi.org/10.1097/00002371-200107000-00012
- Bollard CM, Aguilar L, Straathof KC, Gahn B, Huls MH, Rousseau A, Sixbey J, Gresik MV, Carrum G, Hudson M et al. Cytotoxic T lymphocyte therapy for Epstein-Barr virus+ Hodgkin's disease. J Exp Med 2004; 200:1623-33; PMID:15611290; http://dx.doi.org/10.1084/jem.20040890
- Maude SL, Frey N, Shaw PA, Aplenc R, Barrett DM, Bunin NJ, Chew A, Gonzalez VE, Zheng Z, Lacey SF et al. Chimeric antigen receptor T cells for sustained remissions in leukemia. N Engl J Med 2014; 371:1507-17; PMID:25317870; http://dx.doi.org/10.1056/NEJMoa1407222
- Kochenderfer JN, Dudley ME, Kassim SH, Somerville RP, Carpenter RO, Stetler-Stevenson M, Yang JC, Phan GQ, Hughes MS, Sherry RM et al. Chemotherapy-refractory diffuse large B-cell lymphoma and indolent B-cell malignancies can be effectively treated with autologous T cells expressing an anti-CD19 chimeric antigen receptor. J Clin Oncol. 2015 Feb 20; 33(6):540-9; PMID:25154820; http://dx.doi.org/10.1200/JCO.2014.56.2025.
- Straathof KC, Bollard CM, Popat U, Huls MH, Lopez T, Morriss MC, Gresik MV, Gee AP, Russell HV, Brenner MK et al. Treatment of nasopharyngeal carcinoma with Epstein-Barr virus—specific T lymphocytes. Blood 2005; 105:1898-904; PMID:15542583; http://dx.doi.org/10.1182/blood-2004-07-2975
- Slaney CY, Kershaw MH, Darcy PK. Trafficking of T cells into tumors. Cancer Res. 2014 Dec 15; 74(24):7168-74; PMID:25477332; http://dx.doi.org/10.1158/0008-5472.
- Curiel TJ, Coukos G, Zou L, Alvarez X, Cheng P, Mottram P, Evdemon-Hogan M, Conejo-Garcia JR, Zhang L, Burow M et al. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med 2004; 10:942-9; PMID:15322536; http://dx.doi.org/10.1038/nm1093
- Schlecker E, Stojanovic A, Eisen C, Quack C, Falk CS, Umansky V, Cerwenka A. Tumor-infiltrating monocytic myeloid-derived suppressor cells mediate CCR5-dependent recruitment of regulatory T cells favoring tumor growth. J Immunol 2012; 189:5602-11; PMID:23152559; http://dx.doi.org/10.4049/jimmunol.1201018
- Ganss R, Ryschich E, Klar E, Arnold B, Hammerling GJ. Combination of T-cell therapy and trigger of inflammation induces remodeling of the vasculature and tumor eradication. Cancer Res 2002; 62:1462-70; PMID:11888921
- Hong M, Puaux AL, Huang C, Loumagne L, Tow C, Mackay C, Kato M, Prévost-Blondel A, Avril MF, Nardin A et al. Chemotherapy induces intratumoral expression of chemokines in cutaneous melanoma, favoring T-cell infiltration and tumor control. Cancer Res 2011; 71:6997-7009; PMID:21948969; http://dx.doi.org/10.1158/0008-5472.CAN-11-1466
- Matsumura S, Wang B, Kawashima N, Braunstein S, Badura M, Cameron TO, Babb JS, Schneider RJ, Formenti SC, Dustin ML et al. Radiation-induced CXCL16 release by breast cancer cells attracts effector T cells. J Immunol 2008; 181:3099-107; PMID:18713980; http://dx.doi.org/10.4049/jimmunol.181.5.3099
- June CH. Adoptive T cell therapy for cancer in the clinic. J Clin Invest 2007; 117:1466-76; PMID:17549249; http://dx.doi.org/10.1172/JCI32446
- Kunkel EJ, Campbell DJ, Butcher EC. Chemokines in lymphocyte trafficking and intestinal immunity. Microcirculation 2003; 10:313-23; PMID:12851648; http://dx.doi.org/10.1080/mic.10.3-4.313.323
- Madri JA, Graesser D. Cell migration in the immune system: the evolving inter-related roles of adhesion molecules and proteinases. Dev Immun 2000; 7:103-16; PMID:11097205; http://dx.doi.org/10.1155/2000/79045
- Franciszkiewicz K, Boissonnas A, Boutet M, Combadiere C, Mami-Chouaib F. Role of chemokines and chemokine receptors in shaping the effector phase of the antitumor immune response. Cancer Res 2012; 72:6325-32; PMID:23222302; http://dx.doi.org/10.1158/0008-5472.CAN-12-2027
- Takeichi T, Mocevicius P, Deduchovas O, Salnikova O, Castro-Santa E, Buchler MW, Schmidt J, Ryschich E. alphaL beta2 integrin is indispensable for CD8+ T-cell recruitment in experimental pancreatic and hepatocellular cancer. Int J Cancer 2012; 130:2067-76; PMID:21647874; http://dx.doi.org/10.1002/ijc.26223
- Evans R, Patzak I, Svensson L, De Filippo K, Jones K, McDowall A, Hogg N. Integrins in immunity. J Cell Sci 2009; 122:215-25; PMID:19118214; http://dx.doi.org/10.1242/jcs.019117
- Kliche S, Worbs T, Wang X, Degen J, Patzak I, Meineke B, Togni M, Moser M, Reinhold A, Kiefer F et al. CCR7-mediated LFA-1 functions in T cells are regulated by 2 independent ADAP/SKAP55 modules. Blood 2012; 119:777-85; PMID:22117043; http://dx.doi.org/10.1182/blood-2011-06-362269
- Gobert M, Treilleux I, Bendriss-Vermare N, Bachelot T, Goddard-Leon S, Arfi V, Biota C, Doffin AC, Durand I, Olive D et al. Regulatory T cells recruited through CCL22/CCR4 are selectively activated in lymphoid infiltrates surrounding primary breast tumors and lead to an adverse clinical outcome. Cancer Research 2009; 69:2000-9; PMID:19244125; http://dx.doi.org/10.1158/0008-5472.CAN-08-2360
- Iellem A, Mariani M, Lang R, Recalde H, Panina-Bordignon P, Sinigaglia F, D'Ambrosio D. Unique chemotactic response profile and specific expression of chemokine receptors CCR4 and CCR8 by CD4(+)CD25(+) regulatory T cells. J Exp Med 2001; 194:847-53; PMID:11560999; http://dx.doi.org/10.1084/jem.194.6.847
- Tang HL, Cyster JG. Chemokine Up-regulation and activated T cell attraction by maturing dendritic cells. Science 1999; 284:819-22; PMID:10221917; http://dx.doi.org/10.1126/science.284.5415.819
- Kochenderfer JN, Rosenberg SA. Treating B-cell cancer with T cells expressing anti-CD19 chimeric antigen receptors. Nat Rev Clin Oncol 2013; 10:267-76; PMID:23546520; http://dx.doi.org/10.1038/nrclinonc.2013.46
- Porter DL, Kalos M, Zheng Z, Levine B, June C. Chimeric antigen receptor therapy for B-cell malignancies. J Cancer 2011; 2:331-2; PMID:21716851; http://dx.doi.org/10.7150/jca.2.331
- Kershaw MH, Westwood JA, Parker LL, Wang G, Eshhar Z, Mavroukakis SA, White DE, Wunderlich JR, Canevari S, Rogers-Freezer L et al. A phase I study on adoptive immunotherapy using gene-modified T cells for ovarian cancer. Clin Cancer Res 2006; 12:6106-15; PMID:17062687; http://dx.doi.org/10.1158/1078-0432.CCR-06-1183
- Kobold S, Steffen J, Chaloupka M, Grassmann S, Henkel J, Castoldi R, Zeng Y, Chmielewski M, Schmollinger JC, Schnurr M et al. Selective bispecific T cell recruiting antibody and antitumor activity of adoptive T cell transfer. J Natl Cancer Inst 2015; 107:1-8; PMID:25424197; http://dx.doi.org/10.1093/jnci/djv146
- Kobold S, Grassmann S, Chaloupka M, Lampert C, Wenk S, Kraus F, Rapp M, Düwell P, Zeng Y, Schmollinger JC et al. Impact of a new fusion receptor on PD-1-mediated immunosuppression in adoptive T cell therapy. J Natl Cancer Inst 2015; 107:1-10; PMID: 26105028; http://dx.doi.org/10.1093/jnci/djv146
- Pere H, Montier Y, Bayry J, Quintin-Colonna F, Merillon N, Dransart E, Badoual C, Gey A, Ravel P, Marcheteau E et al. A CCR4 antagonist combined with vaccines induces antigen-specific CD8+ T cells and tumor immunity against self antigens. Blood 2011; 118:4853-62; PMID:21908423; http://dx.doi.org/10.1182/blood-2011-01-329656
- Di Stasi A, De Angelis B, Rooney CM, Zhang L, Mahendravada A, Foster AE, Heslop HE, Brenner MK, Dotti G, Savoldo B. T lymphocytes coexpressing CCR4 and a chimeric antigen receptor targeting CD30 have improved homing and antitumor activity in a Hodgkin tumor model. Blood 2009; 113:6392-402; PMID:19377047; http://dx.doi.org/10.1182/blood-2009-03-209650
- Moon EK, Carpenito C, Sun J, Wang LC, Kapoor V, Predina J, Riley JL, June CH, Albelda SM. Expression of a functional CCR2 receptor enhances tumor localization and tumor eradication by retargeted human T cells expressing a mesothelin-specific chimeric antibody receptor. Clin Cancer Res 2011; 17:4719-30; PMID:21610146; http://dx.doi.org/10.1158/1078-0432.CCR-11-0351
- Peng W, Ye Y, Rabinovich BA, Liu C, Lou Y, Zhang M, Whittington M, Yang Y, Overwijk WW, Lizée G et al. Transduction of tumor-specific T cells with CXCR2 chemokine receptor improves migration to tumor and antitumor immune responses. Clin Cancer Res 2010; 16:5458-68; PMID:20889916; http://dx.doi.org/10.1158/1078-0432.CCR-10-0712
- Bolomini-Vittori M, Montresor A, Giagulli C, Staunton D, Rossi B, Martinello M, Constantin G, Laudanna C. Regulation of conformer-specific activation of the integrin LFA-1 by a chemokine-triggered Rho signaling module. Nat Immunol 2009; 10:185-94; PMID:19136961; http://dx.doi.org/10.1038/ni.1691
- Grakoui A, Bromley SK, Sumen C, Davis MM, Shaw AS, Allen PM, Dustin ML. The immunological synapse: a molecular machine controlling T cell activation. Science 1999; 285:221-7; PMID:10398592; http://dx.doi.org/10.1126/science.285.5425.221
- Kedl RM, Rees WA, Hildeman DA, Schaefer B, Mitchell T, Kappler J, Marrack P. T cells compete for access to antigen-bearing antigen-presenting cells. J Exp Med 2000; 192:1105-13; PMID:11034600; http://dx.doi.org/10.1084/jem.192.8.1105
- Vernon PJ, Loux TJ, Schapiro NE, Kang R, Muthuswamy R, Kalinski P, Tang D, Lotze MT, Zeh HJ 3rd. The receptor for advanced glycation end products promotes pancreatic carcinogenesis and accumulation of myeloid-derived suppressor cells. J Immunol 2013; 190:1372-9; PMID: 23269246; http://dx.doi.org/10.4049/jimmunol.1201151
- Hefetz-Sela S, Stein I, Klieger Y, Porat R, Sade-Feldman M, Zreik F, Nagler A, Pappo O, Quagliata L, Dazert E et al. Acquisition of an immunosuppressive protumorigenic macrophage phenotype depending on c-Jun phosphorylation. Proc Natl Acad Sci U S A. 2014 Dec 9; 111(49):17582-7; PMID:25422452; http://dx.doi.org/10.1073/pnas.1409700111.
- Tsujikawa T, Yaguchi T, Ohmura G, Ohta S, Kobayashi A, Kawamura N, Fujita T, Nakano H, Shimada T, Takahashi T et al. Autocrine and paracrine loops between cancer cells and macrophages promote lymph node metastasis via CCR4/CCL22 in head and neck squamous cell carcinoma. Int J Cancer 2013; 132:2755-66; PMID:23180648; http://dx.doi.org/10.1002/ijc.27966
- Anz D, Rapp M, Eiber S, Koelzer VH, Thaler R, Haubner S, Knott M, Nagel S, Golic M, Wiedemann GM et al. Suppression of intratumoral CCL22 by type I interferon inhibits migration of regulatory T cells and blocks cancer progression. Cancer Res. 2015 Nov 1; 75(21):4483-93; PMID:26432403; http://dx.doi.org/10.1158/0008-5472.
- Godiska R, Chantry D, Raport CJ, Sozzani S, Allavena P, Leviten D, Mantovani A, Gray PW. Human macrophage-derived chemokine (MDC), a novel chemoattractant for monocytes, monocyte-derived dendritic cells, and natural killer cells. J Exp Med 1997; 185:1595-604; PMID:9151897; http://dx.doi.org/10.1084/jem.185.9.1595
- Arias MA, Pantoja AE, Jaramillo G, Paris SC, Shattock RJ, Garcia LF, Griffin GE. Chemokine receptor expression and modulation by Mycobacterium tuberculosis antigens on mononuclear cells from human lymphoid tissues. Immunology 2006; 118:171-84; PMID:16771852; http://dx.doi.org/10.1111/j.1365-2567.2006.02352.x
- Luther SA, Bidgol A, Hargreaves DC, Schmidt A, Xu Y, Paniyadi J, Matloubian M, Cyster JG. Differing activities of homeostatic chemokines CCL19, CCL21, and CXCL12 in lymphocyte and dendritic cell recruitment and lymphoid neogenesis. J Immunol 2002; 169:424-33; PMID:12077273; http://dx.doi.org/10.4049/jimmunol.169.1.424
- Jacobs C, Duewell P, Heckelsmiller K, Wei J, Bauernfeind F, Ellermeier J, Kisser U, Bauer CA, Dauer M, Eigler A et al. An ISCOM vaccine combined with a TLR9 agonist breaks immune evasion mediated by regulatory T cells in an orthotopic model of pancreatic carcinoma. Int J Cancer 2011; 128:897-907; PMID:20473889; http://dx.doi.org/10.1002/ijc.25399
- Bauernfeind F, Rieger A, Schildberg FA, Knolle PA, Schmid-Burgk JL, Hornung V. NLRP3 inflammasome activity is negatively controlled by miR-223. J Immunol 2012; 189:4175-81; PMID:22984082; http://dx.doi.org/10.4049/jimmunol.1201516
- Schaniel C, Pardali E, Sallusto F, Speletas M, Ruedl C, Shimizu T, Seidl T, Andersson J, Melchers F, Rolink AG et al. Activated murine B lymphocytes and dendritic cells produce a novel CC chemokine which acts selectively on activated T cells. J Exp Med 1998; 188:451-63; PMID:9687523; http://dx.doi.org/10.1084/jem.188.3.451
- Onishi Y, Fehervari Z, Yamaguchi T, Sakaguchi S. Foxp3+ natural regulatory T cells preferentially form aggregates on dendritic cells in vitro and actively inhibit their maturation. Proc Natl Acad Sci U S A 2008; 105:10113-8; PMID:18635688; http://dx.doi.org/10.1073/pnas.0711106105
