ABSTRACT
Abcc3, a member of the ATP-binding cassette transporter superfamily, plays a role in multidrug resistance. Here, we found that Abcc3 is highly expressed in blood-derived NK cells but not in CD8+ T cells. In GL261 glioma-bearing mice treated with the alkylating agent temozolomide (TMZ) for 5 d, an early increased frequency of NK cells was observed. We also found that Abcc3 is strongly upregulated and functionally active in NK cells from mice treated with TMZ compared to controls. We demonstrate that Abcc3 is critical for NK cell survival during TMZ administration; more importantly, Akt, involved in lymphocyte survival, is phosphorylated only in NK cells expressing Abcc3. The resistance of NK cells to chemotherapy was accompanied by increased migration and homing in the brain at early time points. Cytotoxicity, evaluated by IFNγ production and specific lytic activity against GL261 cells, increased peripherally in the later phases, after conclusion of TMZ treatment. Intra-tumor increase of the NK effector subset as well as in IFNγ, granzymes and perforin-1 expression, were found early and persisted over time, correlating with a profound modulation on glioma microenvironment induced by TMZ. Our findings reveal an important involvement of Abcc3 in NK cell resistance to chemotherapy and have important clinical implications for patients treated with chemo-immunotherapy.
Introduction
Current therapeutic options for glioblastoma (GBM) patients include chemotherapy with the alkylating agent TMZ.Citation1,2 Recent data have suggested that some chemotherapeutic agents, previously viewed as immunosuppressive, possess immune-modulatory effectsCitation3,4 and influence the vaccine-induced immune response affecting the quality and efficacy of the T cell responseCitation5 or enhancing the immunogenicity of dying tumor cells.Citation3,4 TMZ leads to transient lymphodepletionCitation6 and may interfere with regulatory T cell (Treg) trafficking to the tumor,Citation7 thereby creating a “time-window” for improved efficacy of vaccinations; moreover, dendritic cell (DC) immunotherapy may increase TMZ sensitivity.Citation8 Preclinical evidence has implicated the inhibition of glioma growth by NK cellsCitation9,10 and recently, we reported a significant, positive correlation of NK cell response and survival of patients affected by recurrent GBM treated with DCs loaded with autologous tumor lysates.Citation11 Treg depletion by TMZ could relieve the suppression of NK cells restoring the innate antitumor response.Citation12
Previous attempts have been made to decipher the mechanisms through which NK cells are more radio- and chemotherapy resistant than other lymphoid cells. It has been observed that NK cells express high levels of P-glycoprotein 1 (P-gp1), a transmembrane transporter encoded by the multidrug-resistance 1 (MDR1) gene, as well as MRP1 (Abcc1) and MRP2 (Abcc2).Citation13 Discrepancies were found in terms of the expression and function in T cells of multidrug-resistance proteins, specifically P-gp1 and Abcc1.Citation14,15
In a clinical trial currently active at our institution (DENDR1 - EUDRACT No. 2008-005035-15), 24 patients with first diagnosis of GBM have been treated with DCs loaded with autologous tumor lysate together with standard radiotherapy and chemotherapy with TMZ. Peripheral blood lymphocytes (PBLs) from patients were analyzed by flow cytometry for immunotherapy follow-up. Their ratio of vaccine/baseline frequencies (V/B ratio) was correlated with the progression-free survival (PFS) of each patient. The increased V/B ratio of NK cells but not CD8+ T cells was significantly associated with prolonged PFS (Pellegatta et al., manuscript in preparation).
To investigate the specific contribution of TMZ-based chemotherapy to differential responses of NK and T cells, we used the GL261 pre-clinical model of glioma.
We found that blood-derived NK cells (but not CD8+ T cells) are resistant to and activated by TMZ. Multidrug resistance is primarily associated with Abcc3 expression (a member of the MRP family), which was upregulated and functionally active in NK cells during TMZ treatment. Furthermore, NK cells displayed migratory and cytotoxic activities that were positively influenced by TMZ.
Results
Local and systemic NK cell frequency is positively influenced by TMZ
Nine days after intracranial implantation of GL261 gliomas, immune competent glioma-bearing mice were treated with intraperitoneal injections (i.p.) of 5 mg/kg TMZ or DMSO for 5 d (). To characterize the effect of TMZ on the immune system, PBLs and tumor-infiltrating lymphocytes (TILs) were harvested at different time points, and immune cell populations quantified using flow cytometry. TMZ induced rapid and reversible lymphopenia: CD8+ T cells decreased significantly at 48 h, after two administrations of chemotherapy (p < 0.0001 vs. controls) and quickly increased at 72 h (p < 0.01 vs. 48h; ). On the contrary, peripheral blood NK cells increased significantly at early time point, doubled 72 h after the first TMZ administration and remained higher than controls throughout the entire treatment (). To assess a possible delayed effect of TMZ on immune cells, we performed similar evaluations at day 19, 5 d after ending chemotherapy. We did not observe a significant difference between CD8+ T cells in the blood of TMZ-treated mice compared to controls () while NK cells were still increased in blood of TMZ- compared to vehicle-treated mice (). In non-glioma-bearing mice, TMZ induced a modulation of CD8+ T lymphocytes and NK cells similar to TMZ-treated tumor bearing mice (Fig. S1).
Figure 1. TMZ treatment influences local and peripheral immune cell frequency. (A) Experimental schema of in vivo treatment. C57BL6 were i.c. injected with GL261 cells and treated for 5 d with i.p. injection of 5 mg/kg TMZ or vehicle (DMSO) 9 d after tumor implantation. On days 9–13 and 19 after tumor implantation (24–96 and 240 h after TMZ treatment), n = 5 mice per group/each time point were sacrificed for immune monitoring. (B) Peripheral percentages of CD8+T cells (CD8+CD3+): 22.2 ± 1.2% vs. 15.5 ± 0.2% at 24 h; 21.1 ± 2.0% vs. 5.5 ± 1.0% at 48 h; 19.8 ± 1.4% vs. 10.8 ± 1.3% at 72 h; 22.4 ± 2.1 vs. 16.6 ± 2.1% at 96 h; 22.6 ± 0.4% vs. 17.3± 1.4% at 240 h, controls vs. TMZ-treated mice, respectively; *p < 0.01; ***p <0.0001. (C) Percentages of blood NK cells (NK1.1+CD3−): 4.8 ± 1.2% vs. 9.8 ± 3.2% at 24 h; 7.2 ± 1.2% vs. 13.3 ± 1.2% at 48 h; 6.3 ± 1.6% vs. 20.2 ± 1.9% at 72 h; 10.2 ± 2.1% vs. 20.6 ± 2.3% at 96 h, 7.2 ± 2.1% vs. 21.8 ± 2.3% at 240 h, controls vs. TMZ-treated mice respectively; *p < 0.01; ***p < 0.0001. (D) Tumor-infiltrating CD8+ T cells: 10.3 ± 1.2% vs. 10.5 ± 0.2% at 24 h; 15.2 ± 2.1% vs. 6.5 ±1.0% at 48 h; 16.3 ± 2.7% vs. 11.2 ± 2.6% at 72 h; 15.7 ± 2.2% vs. 19.3 ± 1.1% at 96 h, 18.2 ± 2.2% vs. 20.4 ± 2.3% at 240 h, controls vs. TMZ-treated mice respectively; **p < 0.001. (E) Tumor-infiltrating NK cells during and after TMZ administration: 7.2 ± 12.5% vs. 12.5 ± 7.4% at 24 h; 9.1 ± 4.5% vs. 21.1 ± 5.3% at 48 h; 11.3 ± 5.6% vs. 36.2 ± 8.1% at 72 h; 14.5 ± 7.6% vs. 39.2 ± 9.2% at 96 h; 21.4 ± 6.5% vs. 48.9 ± 7.7% at 240 h, controls vs. treated mice respectively; *p < 0.01; ***p < 0.0001. (F) Characterization of immune infiltration in gliomas by IF at 72 h after beginning of TMZ treatment. (G) Quantitative determination of TILs by flow cytometry obtained from the same groups of gliomas used for IF studies.
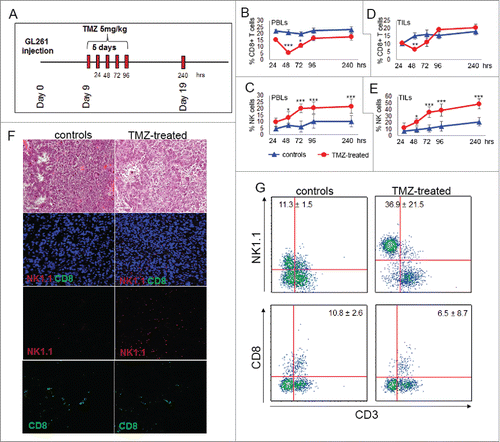
Tumor-infiltrating immune cells were isolated from fresh gliomas by Percoll gradient and quantified by flow cytometry as NK1.1+CD3− and CD8+ CD3+: at 48 h CD8+ T cells decreased in TMZ-treated mice compared with controls (); at 240 h no differences were found between TMZ-treated mice and controls (p = 0.3; ). NK cells in the tumor showed a similar pattern of systemic NK cells, increasing significantly at 72 h after the first TMZ administration and continued to be significantly elevated at 240 h (p < 0.005 at both time points, ).
Evidence of a vigorous immune cell infiltration in response to TMZ compared with the vehicle at 72 h was confirmed by hematoxilin and eosin staining. In situ immunofluorescence confirmed the predominant infiltration of NK cells located into the tumor mass (). Quantitative determination of TILs from the same groups of treatment was obtained by flow cytometry ().
In another set of experiments, immune cells from spleen, cervical lymph nodes and bone marrow were analyzed: the results did not indicate a significant influence of TMZ on lymphocytes in these organs (not shown).
These results show that trafficking and NK cell homing to the tumor are positively influenced by TMZ administration.
Expression of genes involved in drug resistance and chemotaxis is upregulated in NK cells from TMZ-treated mice
To further characterize the molecular effects of TMZ, we compared gene expression profiles of NK cells obtained by magnetic sorting of PBLs from TMZ- and vehicle-treated glioma-bearing mice (n = 50/ group) 72 h after treatment onset. We used the GeneChip Mouse Gene 2.0 ST Array and identified differentially expressed genes (DEGs) using a ≥2-fold-change (FC) threshold for transcript comparisons. A robust difference was observed between the transcriptome levels of the two NK cell groups (Table S1) and 211 DEGs passed the FC cut-off.
Based on Gene Ontology annotations, the transcripts were grouped because of their involvement in multidrug resistance, anti-apoptosis and migration. We focused the validation experiments on genes indicating the relationship of NK cells with drug-resistance. In particular, three upregulated genes were related to ABC drug transporters: Abcc3, Abca9 and Abca6.Citation16 Other genes were related to the inhibition of apoptosis (CD5L and Naip1) and cell survival (Ednrb, Gata6 and Fgfr1), an indication of the predisposition of NK cells to resist the cytotoxic effect of TMZ.Citation17-20
In addition, data from genes regulating cytoskeleton organization, microtubule-based movement, actin polymerization and chemotaxis (Ccr1, Efnb2, Alox15, Lbp and Lrp1) supported the idea that NK cells from TMZ-treated mice migrated more than NK cells from controls.Citation21,22 Notably, at this time point, downregulated genes were related to NK cell-mediated cytotoxicity (GzmD, GzmE, GzmG and GmzC) and to secretory pathway or inflammatory response (Scgb1a1 and Elane).Citation23
Gene expression profiling was also performed on CD8+T cells purified from the same mice. No significant differences could be observed between CD8+ T cells sorted from TMZ-treated mice and those of controls (Table S1).
Overall, these findings suggest that TMZ influences the activity of NK cells by activating pathways relevant in the acquisition of chemo-resistance.
NK cells respond to chemotherapy by over-expressing Abcc3
The validation of ABC transporter over-expression was performed by real time PCR. The analysis revealed that two of the three ABC transporters, Abcc3 and Abca6, were significantly upregulated in peripheral NK cells from TMZ-treated mice compared to controls (3.46 ± 0.01-fold; p < 0.0001, and 2.75 ± 0.045-fold; p < 0.001, respectively; ).
Figure 2. Abcc3 is responsible for NK cell chemo-resistance. (A and B) Relative expression of Abca6 and Abcc3 transporters in blood NK cells at 72 h, **p < 0.001. (C and D) Time course of Abca6 and Abcc3 expression in PBLs from naïve mice (n = 20) treated in vitro with 1 μM TMZ or DMSO; *p = 0.01, **p < 0.005 and ***p < 0.0001. (E and F) Abcc3 levels in NK and CD8+ T cells from glioma-bearing mice (n = 5/group) after three treatments of TMZ (red line) or DMSO (green line). (G and H) multidrug-resistance activity assay in NK and CD8+ T cells from PBLs of naive mice (n = 25) treated in vitro with 1 μM TMZ or DMSO for 4 h. MAF values > 25 are indicative of multidrug-resistant phenotype.
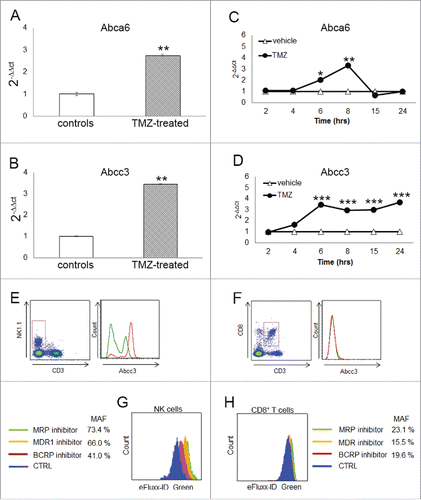
To investigate in vitro the expression of Abca6 and Abcc3 during TMZ administration, we treated PBLs from naive mice with 1 µM TMZ or DMSO at different time points. The dosage was determined according to TMZ concentrations measured in the plasma of patients treated with “standard” schedule.Citation24 The upregulation of Abca6 expression was observed only after 6 and 8 h of TMZ treatment (2.0 ± 0.1-fold and 3.3 ± 0.1-fold, respectively vs. DMSO-treated PBLs; p = 0.01, p < 0.005; ). On the contrary, the upregulation of Abcc3 expression was detectable after 4 h (p < 0.05) and increased over the time during TMZ treatment, suggesting a direct effect of chemotherapy on its expression ().
The remarkable increase of Abcc3 expression on the surface of NK cells was confirmed in vivo. At flow cytometry, NK cells (but not CD8+ T cells) displayed a high basal expression of Abcc3 (31.2 ± 0.8% Abcc3+NK cells vs. 2.0 ± 0.6% Abcc3+CD8+ T cells; p < 0.00001). Moreover, NK cells from TMZ-treated mice exhibited a significant upregulation of Abcc3 compared to controls during chemotherapy (31.2 ± 0.8% vehicle NK cells vs. 59.8 ± 1.1% TMZ-treated NK cells, p < 0.0001; , left). No significant difference in Abcc3 expression was found in CD8+ T cells from TMZ-treated mice compared to controls.(2.0 ± 0.6% vehicles vs. 1.5 ± 0.5% TMZ-treated mice, , right).
These results show that Abcc3 is differentially expressed in NK cells compared to CD8+ T cells and is increased in NK cells from TMZ-treated glioma-bearing mice compared with controls.
Abcc3 expressed in NK cells is functionally active
To investigate whether Abcc3 expression is related to a greater ABC transporter activity and a drug-resistant phenotype, we used a flow cytometry assay to measure the efflux activity of the three clinically most important ABC transporter families involved in cancer multidrug resistance. The assay is based on determining the fluorescence intensities of cells after a short incubation with a fluorescent substrate in the presence or absence (control) of specific ABC transporter inhibitors. Inhibition of active ABC transporters results in increased fluorescence intensity due to the accumulation of the substrate. PBLs from naïve mice were treated in vitro with 1 µM TMZ or DMSO for 4 h.
NK and CD8+ T cells were gated on PBLs and a multidrug-resistance activity factor (MAF) was calculated. Cells exhibiting drug resistance have increased fluorescence and a MAF greater than 25%. TMZ-NK cells showed greater fluorescence in the presence of a multidrug-resistance protein inhibitor for MRP, of which Abcc3 is a key member resulting in MAF = 73.4%.().
TMZ-treated NK cells were also tested for the MDR and BCRP inhibitors included in the assay, confirming a high efflux activity. MRP exhibited the strongest efflux activity (p < 0.001; ). Results obtained with a lower dose of TMZ suggested that the efflux activity was dose-dependent in NK cells (Fig. S2). No evidence of a resistant phenotype was found in TMZ-treated CD8+ T cells, showing a MAF < 25% for all ABC transporter families ().
These findings highlight the rapid activation of ABC multidrug-resistance transporters in NK cells but not in CD8+T lymphocytes during TMZ treatment, supporting the NK cell ability to react to the cytotoxic effects of chemotherapy.
Abcc3 is critical for survival and expansion of NK cells during TMZ administration
To determine whether Abcc3 is required for NK cell survival, we treated PBLs from naïve mice with 1 μM TMZ or DMSO for 2, 6 and 15 h. Apoptotic cells were measured by flow cytometry on gated NK and CD8+ T cell populations using Annexin V and Propidium Iodide (PI) staining ().
Figure 3. NK cells exhibit resistance to TMZ-induced apoptosis. (A and C) Apoptosis induced by TMZ treatment in NK and CD8+ T cells from PBLs of naive mice (n = 20). EA and LA represent early and late apoptosis, respectively. (B) Early and late apoptosis of Abcc3+ and Abcc3− NK cells treated in vitro with 1 μM TMZ. (D) Western blot analysis and densitometric quantification of pAkt in blood immune-separated NK and CD8+ T cells of naïve mice (n = 20) treated with 1 μM TMZ or DMSO. (E) Intracellular staining of pAkt in blood-derived Abcc3+NK and Abcc3+CD8+ T cells from glioma-bearing mice (n = 4/group) at 72 h. (F) Representative dot plots showing apoptosis in NK cells treated for 4 h in vitro with 1 μM TMZ and 25 μM Abcc3 inhibitor added to the medium 30 min before (pre-MK571) or after (post MK571) the pharmacological treatment. *p < 0.01, **p < 0.001 and ***p < 0.0001.
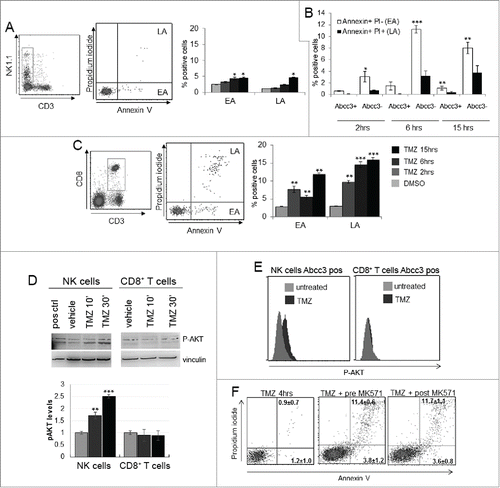
Cells in early apoptosis (EA) were Annexin V positive and PI negative, cells in late apoptosis (LA) or dead were Annexin V and PI positive. NK cells showed a low percentage of early apoptotic cells that slightly increased after 6 and 15 h (p < 0.01; ). Abcc3 negative NK cells showed a higher percentage of apoptotic cells compared to Abcc3 positive NK cells in response to TMZ (). CD8+ T cells showed a remarkable increase of early and LA after 2 h and later (p < 0.005, p < 0.001; ).
Because the pathways involving Akt activation could promote lymphocyte survival,Citation25,26 we investigated Akt activation by analyzing phosphorylation in PBLs in response to chemotherapy. NK cells and CD8+ T cells were purified from naïve mice and treated with 1 μM TMZ or DMSO in vitro at two different time points. Western blots showed a basal phosphorylation of Akt in NK cells with a time-dependent increase of pAkt with TMZ treatment (). On the contrary, in CD8+ T cells, no significant difference in Akt activation was detected in TMZ-treated cells (). We confirmed these results by analyzing Akt activation in NK and CD8+ T cells from glioma-bearing mice treated with TMZ or vehicle. We sacrificed mice after the third TMZ (or DMSO) administration every 5 min for 30 min, and Akt activation in PBLs was measured by a flow cytometry phospho-specific staining (Miltenyi Biotec). Akt phosphorylation was only detected in NK cells from TMZ-treated mice expressing Abcc3 (p = 0.01 vs. vehicle-treated mice; ). Abcc3+CD8+ T cells from TMZ-treated and control mice did not exhibit Akt phosphorylation (2.9 ± 1.2% TMZ-treated vs. 3.3 ± 0.9% control mice; ).
Inactivation of Abcc3 function by its specific inhibitor MK571 induced a significant increase of NK cell apoptosis in PBLs treated with 1 μM TMZ for 4h ().
Together, these results confirm the important role of Abcc3 in survival and response to cytotoxic effects of chemotherapy.
NK cell migration and maturation are positively influenced by TMZ
To validate the signature related to migration and chemotaxis in NK cells from TMZ-treated glioma-bearing mice, we measured NK cell migration using the transwell system. NK cells purified from PBLs of TMZ-treated glioma-bearing mice and controls were evaluated for their ability to migrate toward conditioned medium derived from GL261 cells treated with 150 µM TMZ or DMSO for 24 h. We observed a 1.8-fold increase in the migration of NK cells from TMZ-treated mice toward the supernatant from TMZ-stimulated GL261 compared to the vehicle supernatant (p < 0.005). Migration of NK cells from treated mice increased by 2.3- and 30-fold compared with NK cells from control mice (p < 0.0001, ).
Figure 4. The developmental stage of NK cells from TMZ-treated mice was influenced by TMZ. (A) Migration of NK cells from PBLs of TMZ-treated and control mice (n = 30/group) at 72 h toward medium from DMSO-treated (white bars) and TMZ-treated (striped bars) GL261 cells. **p < 0.005; ***p < 0.0001. (B) CD49b and CD49d integrin surface expression in blood-derived NK cells from TMZ-treated and control mice (n = 4/group) isolated at 72 h; p < 0.001. (C and D) Representative dot plots of the NK cell four-developmental stages basing on surface expression of CD27 and CD11b in blood of TMZ- and vehicle-treated mice on days 12 (C) and 19 (D); p < 0.01. (E and F) Representative dot plots showing CD27 and CD11b expression in tumor-infiltrating NK cells isolated on day 12 (E) and 19 (F) from gliomas, p < 0.005.
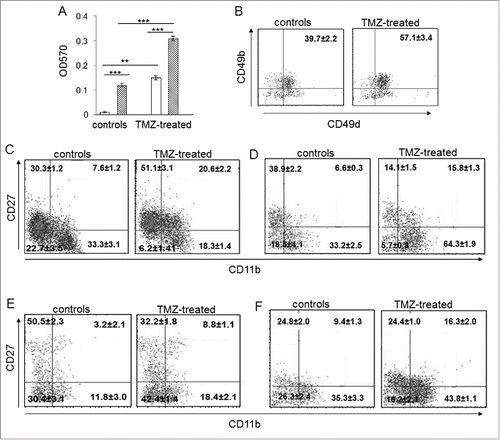
We then analyzed the expression of CD49b and CD49d integrin subunits involved in cellular adhesion and leukocyte tissue infiltration.Citation27 CD49b+CD49d+double positive NK cells increased in blood of TMZ-treated mice compared to controls (57.1 ± 3.4% vs. 39.7 ± 2.2%, respectively, p <0.01), supporting their greater homing ability into tumors ().
We also characterized the effect of TMZ on the maturation status of NK cells by evaluating the surface density of CD11b and CD27, two markers associated with the four-stage developmental program in mice and humans.Citation28,29 In blood, immature CD11blowCD27lowNK cells significantly decreased after 3 d of chemotherapy (p < 0.001 vs. controls). Interestingly, TMZ led to a significant enrichment of the CD11blowCD27high and CD11bhighCD27high NK cell subsets with migratory potential and a simultaneous decrease of the CD11bhighCD27lowcytotoxic subset (p < 0.001 vs. controls; ). The increase of the CD11bhighCD27low cytotoxic subset was observed 5 d after the end of TMZ administration (p = 0.001; ).
On the contrary, in gliomas of TMZ-treated mice there was a strong accumulation of the CD11bhighCD27lowNK effector subset 72 h after the first TMZ administration (p < 0.005, ). This increase persisted at later time points (p < 0.005, ).
These data showed a direct effect of TMZ on the progressive maturation of NK cells, with consequent influence on their migratory and cytotoxic phenotype in blood and gliomas.
Local and systemic NK cell cytotoxicity is triggered by TMZ
We further aimed to verify the cytotoxic ability of NK cells during and after completion of TMZ treatment.
The significant accumulation of the effector subset in blood of TMZ-treated mice suggested an activation of systemic cytotoxicity against tumors. In parallel, IFNγ production in blood-derived NK cells from TMZ-treated mice increased compared to controls (7.5 ± 1.0% vs. 16.9 ± 1.2%; p < 0.005; ).
Figure 5. Cytotoxicity of glioma-infiltrating NK cells is promoted by TMZ treatment. (A) IFNγ production in blood NK cells (n = 4/group); p < 0.005. (B) Cytotoxic specificity of peripheral NK cells from naïve mice, TMZ- and vehicle-treated glioma-bearing mice (n = 10/group) against GL261 cells in vitro. Effector:target ratio (E:T) 10:1, 20:1, 40:1. p < 0.001. (C and D) Relative expression of Prf1, GzmB and IFNγ in TILs at early (C) and later time point (D). *p < 0.01; **p < 0.001; ***p < 0.0001.
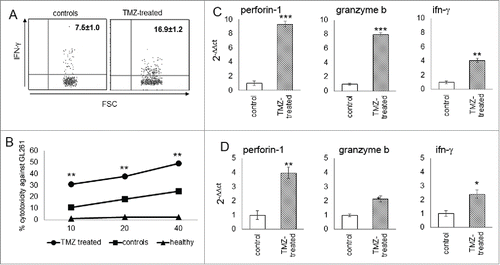
To test the cytotoxic specificity of NK cells, PBLs from naive mice, and TMZ- or vehicle-treated glioma-bearing mice were stimulated with autologous irradiated tumor cells. NK cells from TMZ-treated mice, purified by magnetic sorting, exhibited a greater lytic activity against GL261 cells than NK from vehicle or naïve mice (p < 0.0001, ).
The accumulation of the NK cytotoxic subset in gliomas was associated to increased expression of Perforin 1 (Prf1), Granzyme B (GzmB) and IFNγ (by 9.3 ± 0.04-fold, 8.1 ± 0.02-fold and 4.10 ± 0.03-fold, respectively; p <0.0001) in TMZ-treated mice (). This upregulation persisted on day 19, 5 d after the end of TMZ treatment (Prf1: 3.97 ± 0.07-fold, GzmB: 2.2 ± 0.06-fold and IFNγ: 2.4 ± 0.02-fold, p <0.0001 vs. controls; ).
Thus, TMZ is able to modulate NK cell function increasing their effector activity.
Glioma microenvironment is converted by TMZ into a site permissive for an efficient effector immune response
To investigate whether the glioma microenvironment could be modulated by TMZ, we looked for expression levels of galectin-1, −3 and −9, that suppress NK immune surveillance.Citation30-32 and found that they were expressed at high levels in tumors of vehicle-treated mice and significantly less in those of TMZ-treated mice ().
Figure 6. TMZ modifies glioma microenvironment favoring an NK cell antitumor activity. (A and B) Relative expression of the most immunosuppressive molecules normally expressed in glioma cells: galectin-1, −3, −9 (3.85 ± 0.03-, 2.94 ± 0.02- and 2.22 ± 0.03-fold, respectively vs. controls) and H2-q1 (2.81 ± 0.08-fold vs. controls). (C) Cytokine and chemokine protein array blots with pixel density quantification of lysates from gliomas of control and TMZ-treated mice (n = 5 / group of treatment). Blots are representative of experiments performed on a pool of gliomas from controls or TMZ-treated mice. Cytokines and chemokines which expression changed in both experiments were considered: IFNγ, CCL3, TNF-α, IL-7 and IL-27 were 1.9 ± 0.6-, 1.6 ± 0.4-, 1.7 ± 0.2-, 1.8 ± 0.4-, 1.6 ± 0.2-fold higher, CXCL10 and CCL5 were 0.6 ± 0.7- and 0.7 ± 0.4-fold lower in TMZ-treated compared to controls; *p ≤ 0.01. (D) TGF-β1 and -β2 levels in medium of GL261 cells treated in vitro with DMSO or TMZ: from 23.2 ± 2.5 pg/mL and 478.4 ± 19.3 pg/mL in vehicle to 13.2 ± 2.2 pg/mL and 283.8 ± 25.9 pg/mL in TMZ-treated cells. (E) Relative expression of Nkg2d ligands in GL261 cells treated in vitro with 50 and 150 μM TMZ (striped and black bars, respectively) and DMSO used as vehicle (white bars) at different time points. *p < 0.01; **p < 0.001; ***p < 0.0001.
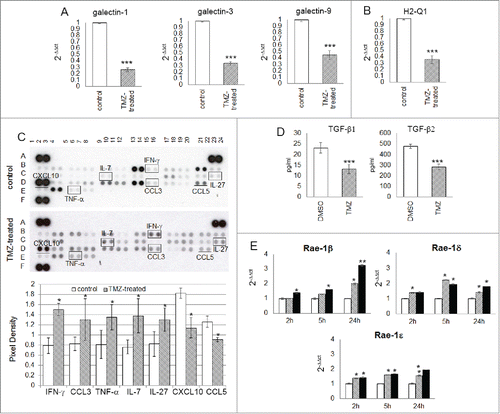
A decrease in the expression of H2-Q1 (hla-e), a non-classical Major Histocompatibility Complex class I (Mhc I) molecule normally implicated in immune escape mechanism and inhibition of NK cell-mediated lysis, was also found in gliomas from TMZ-treated mice (). We also investigated the effects of TMZ administration on intratumor expression of chemokines and cytokines involved in modulating the infiltration of immune cells: CCL3 (whose expression is related to NK cell accumulationCitation10), TNF-α (which is not only involved in antitumor immune response but also responsible for decreased GL261 proliferationCitation33), IL-7 (which plays a role in promoting NK cell survival and inhibiting apoptosisCitation34), and IL-27 (which is an important stimulator of NK cell effector functionCitation35), all increased in gliomas from TMZ-treated mice compared to controls (). In contrast CXCL10, which role in glioma progression is contradictory,Citation36-38 and CCL5, which expression was recently most related with CD8A levels,Citation39 and previously described as immunosuppressor by us and others,Citation10,40 significantly decreased.
Finally, we investigated in vitro the effect of TMZ on glioma immunogenicity. GL261 cells were treated with 50 and 150 μM TMZ or DMSO at different time points. TGF-β1 and TGF-β2 concentrations in the supernatant from TMZ-treated GL261 cells significantly decreased, as evaluated by ELISA (). Nkg2d ligand (Nkg2dl), involved in Nkg2d-mediated NK cell recognition of tumor cells and weakly expressed in GL261 cells, was upregulated in a time- and dose- dependent manner after TMZ treatment (Fig. S2). Similarly, we found a time- and dose-dependent increase of the Rae-1 -β, -ε and –δ, ligands for the Nkg2d receptor (); while B7-h3, a NK cell inhibitory molecule highly expressed in GL261 cells, was significantly decreased (Fig. S3).
These results indicate that TMZ modulates glioma microenvironment into a site favoring NK cell infiltration and antitumor cytotoxicity.
Discussion
Our results show, for the first time, that NK cells in peripheral blood are resistant to chemotherapy due to expression of Abcc3, which was slightly or not expressed by CD8+ T cells. Abcc3 was upregulated and active in NK cells from glioma-bearing mice during TMZ treatment, whereas CD8+ T cells did not exhibit a drug-resistant phenotype. We also confirmed the ability of NK, but not CD8+ T cells, to react to cytotoxic effects of chemotherapy by measuring their apoptosis in vitro, which was low or almost absent in NK cells during TMZ treatment.
ABC transporters promote cell survival independently of cytotoxic drug efflux, as shown in a study where the inhibition of endogenous expression of ABC transporters resulted in reduced expression of Bcl2 protein levels and activation of the apoptotic cascade.Citation41 Based on these observations, ABC transporters can be responsible for the drug-resistance phenotype through direct drug efflux and by other intrinsic pathways, including the phosphorylation of Akt, a key regulatory molecule involved in cell survival, that is activated in response to TMZ contributing to chemo-resistance.Citation42 This is supported by data presented here showing that Abcc3-expressing NK cells from TMZ-treated glioma-bearing mice exhibit significant Akt phosphorylation, a protective mechanism against cell death.Citation43 Notably, Akt activation has been correlated with increased expression and activity of some ABC transporters.Citation44-46 and there is evidence that Akt and PI3K/Akt pathway are responsible for cytotoxic and killing ability of NK cells.Citation47-50 Our data showing that Akt is phosphorylated only in Abcc3+ NK cells support the involvement of Abcc3 in the cytotoxicity of NK cells.
We also found that TMZ led to an enrichment of NK cells with migratory function, as observed by investigating the four-stage model of NK cell maturation.Citation28 While different studies have shown that ABC transporters play a role in the migration of cancer and normal cells, including immune cells,Citation51,52 a direct action of TMZ on NK cell maturation/migration has not been previously reported. The increase of migratory subsets is concomitant with a consistent modulation of the glioma microenvironment of TMZ-treated mice, where we found a significant increase of chemokines that are important for NK cell recruitment.Citation53,54
Intriguingly, the expression of galectin-1 and other galectins, that are potent suppressors of antitumor immune surveillance, decreased in glioma-bearing mice treated with TMZ. Recently, galectin-1 was found responsible for the inhibition of NK cell function and viability: galectin-1 deficient gliomas could be eradicated by infiltrating NK cells with cytotoxic function.Citation30
The evidence that the “cytotoxicity signature” after three chemotherapy treatments resulted down-modulated in the periphery and upregulated in the gliomas of TMZ-treated mice, supports the contention that TMZ can modulate glioma microenvironment facilitating NK cell infiltration and cytotoxic function.
It has long been known that NK cells can control and reinforce antitumor immune responses mediated by DC.Citation57 NK cells and DCs work in synergy, taking advantage of one another.Citation58,59 The reciprocal interaction of NK cells and DCs and the ability of NK cells to recognize and kill tumor cells represent an important rationale for their monitoring during immunotherapeutic approaches with DCs in correlation with clinical outcomes. Our results obtained on a group of recurrent GBM patients treated with DCs loaded with autologous tumor lysate showed a significant increase of NK cells producing IFNγ in patients with prolonged survival.Citation11 We are now evaluating the combination of DC immunotherapy with TMZ in patients with first diagnosis GBM. The NK cell response significantly correlates with survival, whereas the CD8+ T cell response does not appear to influence clinical outcomes. Patients with evidence of an NK cell response showed a significant upregulation of ABCC3 in PBLs in comparison with non-responders (manuscript in preparation).
In agreement with these results, we observed that trafficking and NK cell homing increased in glioma-bearing mice during TMZ treatment. Notably, we also found that murine NK cells can efficiently overcome the drug-mediated toxicity of chemotherapy by expressing multidrug-resistance genes. It has been previously described that NK cells are resistant to chemotherapy.Citation56,60 After chemotherapy treatment, NK cells are the first lymphoid cells to recover and may represent the principal lymphocytes for the initial months after treatment, suggesting a more rapid reaction to cytotoxic effect of drugs than other immune cells.Citation60 Murine and human NK cells were found to express high levels of multidrug transporters, which could confer protection against chemotherapeutic agents.Citation61 TMZ administration, similar to other chemotherapeutics, can cause the development of moderate to severe lymphopenia and myelosuppression,Citation62,63 indicating that immune inhibitory effects take place through selective toxicity on proliferating lymphocytes and inhibition of immune effector differentiation.Citation63,64 However, there is evidence that following a transient chemotherapy-induced lymphopenia, lymphocytes undergo homeostatic proliferation that enhances antitumor, vaccine-induced immune responsesCitation6,65,66 with positive clinical outcome correlation.Citation67 Discrepancies have been reported about the effect of TMZ on NK cells. It has been shown that TMZ significantly decrease the absolute number of CD3− CD56+ effector cells in blood of GBM patients.Citation68 However, other studies have reported that circulating NK cells are relatively resistant to chemotherapy, with their frequency and absolute number, as well as their effector functions, unaffected by TMZ.Citation55,56
In conclusion, our data indicate that chemotherapy is able to modulate tumor microenvironment and reinforce tumor infiltration of NK effector cells and that can contribute to the adjuvant effect of chemotherapy. The different sensitivity of NK and T cells to TMZ, however, may disrupt their interactions, like that of 2B4 (CD244) and CD48,Citation69,70 relevant to generate a T cell memory and possibly amplify antitumor responses. Confirmation of this hypothesis, driven by the results we reported here, may imply a careful re-evaluation of chemotherapy/immunotherapy schedules.
Materials & methods
Cell culture
GL261 cells were cultured as neurospheres in a stem cell growth medium containing DMEM-F12 Glutamax, B-27 (Life Technologies), penicillin/streptomycin, human recombinant epidermal growth factor (EGF; 20 ng/mL) and human recombinant fibroblast growth factor-2 (FGF-2; 20 ng/mL; Peprotech). PBLs were cultured in RPMI 1640 containing 10% fetal bovine serum (FBS), β-mercaptoethanol, sodium pyruvate, nonessential amino acids, L-glutamine, and penicillin/streptomycin (all from EuroClone). Human recombinant interleukin-2 (hIL-2; 10 U/ml; Roche) was added to the medium.
In vivo experiments
C57BL/6N 6-week-old female mice (Charles River Laboratories) received intracranial injections of 105 GL261 cells using specific stereotactic coordinates into the nucleus caudatum (0.7 mm posterior, 3 mm left lateral and 3.5 mm deep, with respect to the bregma). Mice were divided in two groups, treated 9 d after glioma implantation with intraperitoneal injections (i.p.) of 5 mg/kg TMZ or vehicle (DMSO) on days 1–5 and sacrificed at different time points. The animals were monitored every day and euthanized when suffering, in accordance with the current directives of the Campus animal IFOM-IEO house facility and the Minister of Health. Animal experiments were performed in accordance to the Italian Principle of Laboratory Animal Care (D.Lgs. 26/2014) and European Communities Council Directives (86/609/EEC and 2010/63/UE).
Isolation of local and systemic lymphocytes
PBLs were isolated using Lympholyte-M (Cedarlane Labs) according to the manufacturer's instructions. An indirect magnetic labeling system was used to immune-isolate CD8+ T and NK cells (NK and CD8+ T Cell Isolation Kits, Miltenyi Biotec) resulting in a 97 ± 1.5% and 93.2 ± 2.9% pure CD8+T and NK cell population, respectively, as evaluated by flow cytometry. TILs were isolated with the Tumor Dissociation Kit and GentleMACS (Miltenyi Biotec) according to the manufacturer's instruction. A Percoll-density gradient centrifugation (30%–40%–80%–100% isotonic Percoll, 400xg, 15 min at 20°C) was used to separate lymphocytes from the tumor single cell suspension. Immune cells were recovered from the 40–80 gradient interphase.
Flow cytometry
Cells were stained in a cold staining buffer at 4°C in the dark. The following antibodies were used: CD45, CD8+, CD4+, CD3, CD11b, CD27, NK1.1, NKp46, CD49b, CD49d, IFNγ and pAkt (Miltenyi Biotec), Abcc3 (Abcam), B7-H3 (Biolegend) and NKG2D-L (eBioscience). DAPI was added to exclude dead cells. Flow cytometry acquisition was performed on a MACSQuant instrument, and data were analyzed with MACSQuantify Software (Miltenyi Biotec).
Microarray analyses and Real Time-PCR
Total RNA from purified NK and CD8+ T cells was extracted with TRIzol reagent (Life Technologies) using RNeasy Mini Kit and RNase-Free DNase Set (Qiagen). Microarray analyses were performed after three TMZ or DMSO administrations. Mouse Gene 2.0 ST Array GeneChip (Affymetrix), which includes 35,240 mouse transcripts, was used following standard procedures. Differentially expressed genes were identified using a fold-change threshold ≥ 2 for all transcript comparisons. The functional annotation of genes that passed the FC and expression signal cut-offs was performed using the Gene Ontology (GO) Biological Process category. Fast SYBR Green chemistry (Life Technologies) was used for real-time PCR expression analyses. Relative mRNA levels were measured using a ViiA7 Real Time-PCR System (Life Technologies) and calculated using the ΔΔCt method normalizing to the housekeeping Gapdh, Actin and β2M levels. The primer sequences (Primm S.r.l.) are reported in Supplemental Materials.
Migration assay
Migration was assessed in vitro using 8 μm Transwell migration chambers. Purified NK cells (4 ×105/transwell) were placed in the upper chamber and evaluated for their ability to transmigrate toward the lower chamber. Chemoattractant in the lower chamber was represented by medium from GL261 cells previously treated for 24 h with DMSO or 150 μM TMZ. After 12 h, migrating cells were stained with crystal violet solubilized in 10% acetic acid.
Western blot analysis and proteome profiler array
Briefly, cells were washed with cold PBS and lysed in a buffer supplemented with protease and phosphatase inhibitors. Membranes with transferred proteins were incubated with the primary antibody anti-pAKT (Ser473) (1:1000, Cell Signaling) or anti-vinculin (1:10000). The primary antibody incubation was followed by incubation with peroxidase conjugated to the secondary antibody (anti-rat, 1:10000). A chemiluminescence reaction using the ECL Plus kit (GE Healthcare) was detected using G:BOX iChemi system (Syngene). Tumor relative levels of cytokines and chemokines were measured using the Mouse Cytokine Array Panel A kit (R&D Systems) following the manufacturer's instructions. Images of the blots were acquired with G:BOX Chemi system (Syngene) and quantitative analyses were performed using ImageJ software. The 40 cytokines and chemokines of interest were normalized to the corresponding positive controls.
Apoptosis assay
Resistance of NK and CD8+ T cells to the cytotoxic effects of TMZ was evaluated with Annexin V (Biolegend) and propidium (PI). Early and later apoptosis were distinguished with Annexin V positivity and Annexin V-PI double positivity, respectively. The selective MRP inhibitor MK571 at a concentration of 25 μM was used to test the Abcc3 role in chemo-resistance. Briefly, naive lymphocytes were treated with 1 µM TMZ or DMSO for 4 h in vitro. MK571 was added to the medium 30 min before or after the pharmacological treatment and apoptosis was evaluated.
ABC transporter activity
The eFluxx-ID® Green Multidrug-Resistance Assay (Enzo Life Sciences) was used to detect the multidrug-resistant phenotype of NK and CD8+ T cells by monitoring the efflux activity of the three major multidrug-resistance proteins: MDR1, MRP and BCRP. Following the manufactures' instructions, specific inhibitors (Verapamil, MK571 and Novobiocin) were used to define the resistance activity factor (MAF) in PBLs from naïve mice treated with 1 µM TMZ or DMSO for 4 h in vitro. MAF values >25 are indicative of multidrug-resistance positive phenotype.
IFNγ production and LDH assay
PBLs were cultured in a 24-well tissue culture plate in 1 mL of completed RPMI supplemented with 10 U/mL IL-2. Unspecific stimulation was performed with 50 ng/mL phorbol myristate acetate (PMA), 1 µg/mL Ionomycin and 10 µg/mL Brefeldin A for a total of 4 h. Lymphocytes were stained for IFNγ (BD Cytofix/Cytoperm Fixation/Permeabilization Solution Kit, BD Bioscience) according to the manufacturer's instructions. A non-radioactive cytotoxic assay (Cytotoxicity detection kitplus LDH, Roche) was performed to test PBL capacity of TMZ-treated, control and naïve mice to recognize and lyse GL261 cells, according to manufactures' instructions. PBLs were pre-stimulated for 5 d in the presence of 20 Gy-irradiated GL261 cells and tested 5 d later for GL261 cell specific cytotoxicity.
Histology and immunofluorescence
Double immunofluorescence was performed on 5 μm paraffin-embedded sections. Paraffin was removed with xylene and the sections were rehydrated in graded alcohol. Tumor sections were incubated with anti-CD8+ (1:10, BD Bioscience) and anti-NK1.1 (1:10, Miltenyi) antibodies overnight at 4°C. After a counterstained with DAPI, sections were examined using a Nikon confocal microscope and analyses were performed on 3 to 5 independent fields per tumor using the 40X objective. Tumor sections were also stained with hematoxylin and eosin to assess the volume of tumor growth and acquired using the Aperio ScanScope slide scanner (Leica).
Statistical analysis
Statistical comparison was performed using two-tailed Student's t-test. Results were considered significant at p < 0.05.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Supplementary files
Download Zip (464.4 KB)Acknowledgments
We thank the service mouse at IFOM-IEO Campus, and particularly Alberto Gobbi and Manuela Capillo for help and advice with animal experiments.
Additional information
Funding
References
- Stupp R, Mason W, van den Bent MJ, Weller M, Fisher BM, Taphoorn MJB, Belanger K, Brandes AA, Marosi C, Bogdahn U et al. Radiotherapy plus Concomitant\nand Adjuvant Temozolomide for Glioblastoma. N Engl J Med 2005; 352:987-96; PMID:15758009; http://dx.doi.org/10.1056/NEJMoa043330
- Stupp R, Hegi ME, Mason WP, van den Bent MJ, Taphoorn MJB, Janzer RC, Ludwin SK, Allgeier A, Fisher B, Belanger K et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol 2009; 10:459-66; PMID:19269895; http://dx.doi.org/10.1016/S1470-2045(09)70025-7
- Zitvogel L, Apetoh L, Ghiringhelli F, Kroemer G. Immunological aspects of cancer chemotherapy. Nat Rev Immunol 2008; 8:59-73; PMID:18097448; http://dx.doi.org/10.1038/nri2216
- Galluzzi L, Senovilla L, Zitvogel L, Kroemer G. The secret ally: immunostimulation by anticancer drugs. Nat Rev Drug Discov [Internet] 2012 [cited 2012 Oct 31]; 11:215-33. Available from: http://www.ncbi.nlm.nih.gov/pubmed/22301798; PMID:22301798; http://dx.doi.org/10.1038/nrd3626
- Emens LA, Jaffee EM. Leveraging the activity of tumor vaccines with cytotoxic chemotherapy. Cancer Res 2005; 65:8059-64; PMID:16166275; http://dx.doi.org/10.1158/0008-5472.CAN-05-1797
- Sampson JH, Aldape KD, Archer GE, Coan A, Desjardins A, Friedman AH, Friedman HS, Gilbert MR, Herndon JE, McLendon RE et al. Greater chemotherapy-induced lymphopenia enhances tumor-specific immune responses that eliminate EGFRvIII-expressing tumor cells in patients with glioblastoma. Neuro Oncol 2011; 13:324-33; PMID:21149254; http://dx.doi.org/10.1093/neuonc/noq157
- Jordan JT, Sun W, Hussain SF, DeAngulo G, Prabhu SS, Heimberger AB. Preferential migration of regulatory T cells mediated by glioma-secreted chemokines can be blocked with chemotherapy. Cancer Immunol Immunother [Internet] 2008 [cited 2012 Dec 11]; 57:123-31. Available from: http://www.ncbi.nlm.nih.gov/pubmed/17522861; PMID:17522861; http://dx.doi.org/10.1007/s00262-007-0336-x
- Kim T-G, Kim CKC-H, Park J-S, Park S-D, Kim CKC-H, Chung D-S, Hong Y-K. Immunological factors relating to the antitumor effect of temozolomide chemoimmunotherapy in a murine glioma model. Clin Vaccine Immunol [Internet] 2010 [cited 2014 Jan 9]; 17:143-53. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2812079&tool=pmcentrez&rendertype=abstract; PMID:19889936; http://dx.doi.org/10.1128/CVI.00292-09
- Alizadeh D, Zhang L, Brown CE, Farrukh O, Jensen MC, Badie B. Induction of anti-glioma natural killer cell response following multiple low-dose intracerebral CpG therapy. Clin Cancer Res 2010; 16:3399-408; PMID:20570924; http://dx.doi.org/10.1158/1078-0432.CCR-09-3087
- Cantini G, Pisati F, Pessina S, Finocchiaro G, Pellegatta S. Immunotherapy against the radial glia marker GLAST effectively triggers specific antitumor effectors without autoimmunity. Oncoimmunology [Internet] 2012; 1:884-93. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3489744&tool=pmcentrez&rendertype=abstract; PMID:23162756; http://dx.doi.org/10.4161/onci.20637
- Pellegatta S, Eoli M, Frigerio S, Antozzi C, Bruzzone MG, Cantini G, Nava S, Anghileri E, Cuppini L, Cuccarini V et al. The natural killer cell response and tumor debulking are associated with prolonged survival in recurrent glioblastoma patients receiving dendritic cells loaded with autologous tumor lysates. Oncoimmunology [Internet] 2013; 2:e23401. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3661164&tool=pmcentrez&rendertype=abstract; PMID:23802079; http://dx.doi.org/10.4161/onci.23401
- Banissi C, Ghiringhelli F, Chen L, Carpentier AF. Treg depletion with a low-dose metronomic temozolomide regimen in a rat glioma model. Cancer Immunol Immunother 2009; 58:1627-34; PMID:19221744; http://dx.doi.org/10.1007/s00262-009-0671-1
- Oselin K, Mrozikiewicz PM, Pähkla R, Roots I. Quantitative determination of the human MRP1 and MRP2 mRNA expression in FACS-sorted peripheral blood CD4+, CD8+, CD19+, and CD56+ cells. Eur J Haematol 2003; 71:119-23; PMID:12890151; http://dx.doi.org/10.1034/j.1600-0609.2003.00100.x
- Donnenberg VS, Burckart GJ, Zeevi A, Griffith BP, Iacono A, McCurry KR, Wilson JW, Donnenberg AD. P-glycoprotein activity is decreased in CD4+ but not CD8+ lung allograft-infiltrating T cells during acute cellular rejection. Transplantation 2004; 77:1699-706; PMID:15201669; http://dx.doi.org/10.1097/01.TP.0000131163.43015.85
- Van de Ven R, Oerlemans R, van der Heijden JW, Scheffer GL, de Gruijl TD, Jansen G, Scheper RJ. ABC drug transporters and immunity: novel therapeutic targets in autoimmunity and cancer. J Leukoc Biol 2009; 86:1075-87; PMID:19745159; http://dx.doi.org/10.1189/jlb.0309147
- Glavinas H, Krajcsi P, Cserepes J, Sarkadi B. The role of ABC transporters in drug resistance, metabolism and toxicity. Curr Drug Deliv [Internet] 2004 [cited 2015 Feb 9]; 1:27-42. Available from: http://www.ncbi.nlm.nih.gov/pubmed/16305368; PMID:16305368; http://dx.doi.org/10.2174/1567201043480036
- Liston P, Fong WG, Korneluk RG. The inhibitors of apoptosis: there is more to life than Bcl2. Oncogene [Internet] 2003 [cited 2015 Jan 13]; 22:8568-80. Available from: http://www.ncbi.nlm.nih.gov/pubmed/14634619; PMID:14634619; http://dx.doi.org/10.1038/sj.onc.1207101
- Kuwata K, Watanabe H, Jiang S-Y, Yamamoto T, Tomiyama-Miyaji C, Abo T, Miyazaki T, Naito M. AIM inhibits apoptosis of T cells and NKT cells in Corynebacterium-induced granuloma formation in mice. Am J Pathol [Internet] 2003 [cited 2015 Feb 9]; 162:837-47. Available from:http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=1868086&tool=pmcentrez&rendertype=abstract; PMID:12598318; http://dx.doi.org/10.1016/S0002-9440(10)63880-1
- Cai W, Shen F, Li J, Feng Z, Wang Y, Xiao H, Xu B. Activated protease receptor-2 induces GATA6 expression to promote survival in irradiated colon cancer cells. Arch Biochem Biophys [Internet] 2014 [cited 2015 Feb 9]; 555-556:28-32. Available from: http://www.ncbi.nlm.nih.gov/pubmed/24887481; PMID:24887481; http://dx.doi.org/10.1016/j.abb.2014.05.021
- Wan X, Zhang L, Jiang B. Role of endothelin B receptor in oligodendroglioma proliferation and survival: In vitro and in vivo evidence. Mol Med Rep [Internet] 2014 [cited 2015 Feb 9]; 9:229-34. Available from: http://www.ncbi.nlm.nih.gov/pubmed/24145738; PMID:24145738; http://dx.doi.org/10.3892/mmr.2013.1746
- Zamora DO, Babra B, Pan Y, Planck SR, Rosenbaum JT. Human leukocytes express ephrinB2 which activates microvascular endothelial cells. Cell Immunol [Internet] 2006 [cited 2015 Feb 9]; 242:99-109. Available from: http://www.ncbi.nlm.nih.gov/pubmed/17123494; PMID:17123494; http://dx.doi.org/10.1016/j.cellimm.2006.10.001
- Bergström S-E, Bergdahl E, Sundqvist K-G. A cytokine-controlled mechanism for integrated regulation of T-lymphocyte motility, adhesion and activation. Immunology [Internet] 2013 [cited 2015 Feb 9]; 140:441-55. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3839648&tool=pmcentrez&rendertype=abstract; PMID:23866045; http://dx.doi.org/10.1111/imm.12154
- Ewen CL, Kane KP, Bleackley RC. A quarter century of granzymes. Cell Death Differ [Internet] 2012 [cited 2015 Feb 9]; 19:28-35. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3252830&tool=pmcentrez&rendertype=abstract; PMID:22052191; http://dx.doi.org/10.1038/cdd.2011.153
- Ostermann S, Csajka C, Buclin T, Leyvraz S, Lejeune F, Decosterd LA, Stupp R. Plasma and cerebrospinal fluid population pharmacokinetics of temozolomide in malignant glioma patients. Clin Cancer Res 2004; 10:3728-36; PMID:15173079; http://dx.doi.org/10.1158/1078-0432.CCR-03-0807
- Bommhardt U, Chang KC, Swanson PE, Wagner TH, Tinsley KW, Karl IE, Hotchkiss RS. Akt Decreases Lymphocyte Apoptosis and Improves Survival in Sepsis. J Immunol [Internet] 2004; 172:7583-91. Available from: http://www.jimmunol.org/cgi/doi/10.4049/jimmunol.172.12.7583; PMID:15187138; http://dx.doi.org/10.4049/jimmunol.172.12.7583
- Plas DR, Rathmell JC, Thompson CB. Homeostatic control of lymphocyte survival: potential origins and implications. Nat Immunol 2002; 3:515-21; PMID:12032565; http://dx.doi.org/10.1038/ni0602-515
- Gan Y, Liu R, Wu W, Bomprezzi R, Shi FD. Antibody to α4 integrin suppresses natural killer cells infiltration in central nervous system in experimental autoimmune encephalomyelitis. J Neuroimmunol 2012; 247:9-15; PMID:22503411; http://dx.doi.org/10.1016/j.jneuroim.2012.03.011
- Chiossone L, Chaix J, Fuseri N, Roth C, Vivier E, Walzer T. Maturation of mouse NK cells is a 4-stage developmental program. Blood [Internet] 2009 [cited 2013 Apr 4]; 113:5488-96. Available from: http://www.ncbi.nlm.nih.gov/pubmed/19234143; PMID:19234143; http://dx.doi.org/10.1182/blood-2008-10-187179
- Fu B, Wang F, Sun R, Ling B, Tian Z, Wei H. CD11b and CD27 reflect distinct population and functional specialization in human natural killer cells. Immunology [Internet] 2011 [cited 2013 Dec 7]; 133:350-9. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3112344&tool=pmcentrez&rendertype=abstract; PMID:21506999; http://dx.doi.org/10.1111/j.1365-2567.2011.03446.x
- Baker GJ, Chockley P, Yadav VN, Doherty R, Ritt M, Sivaramakrishnan S, Castro MG, Lowenstein PR. Natural killer cells eradicate galectin-1-deficient glioma in the absence of adaptive immunity. Cancer Res [Internet] 2014 [cited 2014 Oct 21]; 74:5079-90. Available from: http://www.ncbi.nlm.nih.gov/pubmed/25038230; PMID:25038230; http://dx.doi.org/10.1158/0008-5472.CAN-14-1203
- Wang W, Guo H, Geng J, Zheng X, Wei H, Sun R, Tian Z. Tumor-released Galectin-3, a soluble inhibitory ligand of human NKp30, plays an important role in tumor escape from NK cell attack. J Biol Chem 2014; 289:33311-9; PMID:25315772; http://dx.doi.org/10.1074/jbc.M114.603464
- Golden-Mason L, McMahan RH, Strong M, Reisdorph R, Mahaffey S, Palmer BE, Cheng L, Kulesza C, Hirashima M, Niki T et al. Galectin-9 functionally impairs natural killer cells in humans and mice. J Virol [Internet] 2013; 87:4835-45. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3624298&tool=pmcentrez&rendertype=abstract; PMID:23408620; http://dx.doi.org/10.1128/JVI.01085-12
- Pellegatta S, Poliani PL, Stucchi E, Corno D, Colombo CA, Orzan F, Ravanini M, Finocchiaro G. Intra-tumoral dendritic cells increase efficacy of peripheral vaccination by modulation of glioma microenvironment. Neuro Oncol 2010; 12:377-88; PMID:20308315; http://dx.doi.org/10.1093/neuonc/nop024
- Michaud A, Dardari R, Charrier E, Cordeiro P, Herblot S, Duval M. IL-7 enhances survival of human CD56bright NK cells. J Immunother 2010; 33:382-90; PMID:20386468; http://dx.doi.org/10.1097/CJI.0b013e3181cd872d
- Ziblat A, Domaica CI, Spallanzani RG, Iraolagoitia XLR, Rossi LE, Avila DE, Torres NI, Fuertes MB, Zwirner NW. IL-27 stimulates human NK-cell effector functions and primes NK cells for IL-18 responsiveness. Eur J Immunol 2015; 45:192-202; PMID:25308526; http://dx.doi.org/10.1002/eji.201444699
- Cai J, Zhang W, Yang P, Wang Y, Li M, Zhang C, Wang Z, Hu H, Liu Y, Li Q et al. Identification of a 6-cytokine prognostic signature in patients with primary glioblastoma harboring M2 microglia/macrophage phenotype relevance. PLoS One 2015; 10:e0126022; PMID:25978454; http://dx.doi.org/10.1371/journal.pone.0126022
- Huang B, Lei Z, Zhao J, Gong W, Liu J, Chen Z, Liu Y, Li D, Yuan Y, Zhang GM et al. CCL2/CCR2 pathway mediates recruitment of myeloid suppressor cells to cancers. Cancer Lett 2007; 252:86-92; PMID:17257744; http://dx.doi.org/10.1016/j.canlet.2006.12.012
- Maru S V, Holloway KA, Flynn G, Lancashire CL, Loughlin AJ, Male DK, Romero IA. Chemokine production and chemokine receptor expression by human glioma cells: role of CXCL10 in tumour cell proliferation. J Neuroimmunol [Internet] 2008 [cited 2015 Aug 28]; 199:35-45. Available from: http://www.ncbi.nlm.nih.gov/pubmed/18538864; PMID:18538864; http://dx.doi.org/10.1016/j.jneuroim.2008.04.029
- Triozzi PL, Schoenfield L, Plesec T, Saunthararajah Y, Tubbs RR, Singh AD. Molecular profiling of primary uveal melanomas with tumor-infiltrating lymphocytes. OncoImmunology 2014; 1-8; http://dx.doi.org/10.4161/21624011.2014.947169
- Conforti R, Ma Y, Morel Y, Paturel C, Terme M, Viaud S, Ryffel B, Ferrantini M, Uppaluri R, Schreiber R et al. Opposing effects of toll-like receptor (TLR3) signaling in tumors can be therapeutically uncoupled to optimize the anticancer efficacy of TLR3 ligands. Cancer Res 2010; 70:490-500; PMID:20068181; http://dx.doi.org/10.1158/0008-5472.CAN-09-1890
- Peaston AE, Gardaneh M, Franco A V, Hocker JE, Murphy KM, Farnsworth ML, Catchpoole DR, Haber M, Norris MD, Lock RB et al. MRP1 gene expression level regulates the death and differentiation response of neuroblastoma cells. Br J Cancer 2001; 85:1564-71; PMID:11720446; http://dx.doi.org/10.1054/bjoc.2001.2144
- Caporali S, Levati L, Graziani G, Muzi A, Atzori MG, Bonmassar E, Palmieri G, Ascierto PA, D’Atri S. NF-κB is activated in response to temozolomide in an AKT-dependent manner and confers protection against the growth suppressive effect of the drug. J Transl Med [Internet] 2012; 10:252. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3551789&tool=pmcentrez&rendertype=abstract; PMID:23259744; http://dx.doi.org/10.1186/1479-5876-10-252
- Bellacosa A, Kumar CC, Cristofano A Di, Testa JR. Activation of AKT kinases in cancer: Implications for therapeutic targeting. Adv. Cancer Res.2005; 94:29-86; PMID:16095999; http://dx.doi.org/10.1016/S0065-230X(05)94002-5
- Chiarini F, Del Sole M, Mongiorgi S, Gaboardi GC, Cappellini A, Mantovani I, Follo MY, McCubrey JA, Martelli AM. The novel Akt inhibitor, perifosine, induces caspase-dependent apoptosis and downregulates P-glycoprotein expression in multidrug-resistant human T-acute leukemia cells by a JNK-dependent mechanism. Leukemia 2008; 22:1106-16; PMID:18385752; http://dx.doi.org/10.1038/leu.2008.79
- Tazzari PL, Cappellini A, Ricci F, Evangelisti C, Papa V, Grafone T, Martinelli G, Conte R, Cocco L, McCubrey JA et al. Multidrug resistance-associated protein 1 expression is under the control of the phosphoinositide 3 kinase/Akt signal transduction network in human acute myelogenous leukemia blasts. Leukemia 2007; 21:427-38; PMID:17215852; http://dx.doi.org/10.1038/sj.leu.2404523
- Bleau AM, Hambardzumyan D, Ozawa T, Fomchenko EI, Huse JT, Brennan CW, Holland EC. PTEN/PI3K/Akt Pathway Regulates the Side Population Phenotype and ABCG2 Activity in Glioma Tumor Stem-like Cells. Cell Stem Cell 2009; 4:226-35; PMID:19265662; http://dx.doi.org/10.1016/j.stem.2009.01.007
- Su YC, Li SC, Hsu CK, Yu CC, Lin TJ, Lee CY, Liao HF. G-CSF downregulates natural killer cell-mediated cytotoxicity in donors for hematopoietic SCT. Bone Marrow Transplant 2012; 47:73-81; PMID:21358682; http://dx.doi.org/10.1038/bmt.2011.22
- Segovis CM, Schoon RA, Dick CJ, Nacusi LP, Leibson PJ, Billadeau DD. PI3K links NKG2D signaling to a CrkL pathway involved in natural killer cell adhesion, polarity, and granule secretion. J Immunol 2009; 182:6933-42; PMID:19454690; http://dx.doi.org/10.4049/jimmunol.0803840
- Kim N, Saudemont A, Webb L, Camps M, Ruckle T, Hirsch E, Turner M, Colucci F. The p110delta catalytic isoform of PI3K is a key player in NK-cell development and cytokine secretion. Blood 2007; 110:3202-8; PMID:17644738; http://dx.doi.org/10.1182/blood-2007-02-075366
- Wai L-E, Fujiki M, Takeda S, Martinez OM, Krams SM. Rapamycin, But Not Cyclosporine or FK506, Alters Natural Killer Cell Function. Transplantation 2008; 85:145-9; PMID:18192925; http://dx.doi.org/10.1097/01.tp.0000296817.28053.7b
- Miletti-González KE, Chen S, Muthukumaran N, Saglimbeni GN, Wu X, Yang J, Apolito K, Shih WJ, Hait WN, Rodriguez-Rodriguez L. The CD44 receptor interacts with P-glycoprotein to promote cell migration and invasion in cancer. Cancer Res 2005; 65:6660-7; PMID:16061646; http://dx.doi.org/10.1158/0008-5472.CAN-04-3478
- Honig SM, Fu S, Mao X, Yopp A, Gunn MD, Randolph GJ, Bromberg JS. FTY720 stimulates multidrug transporter- and cysteinyl leukotriene-dependent T cell chemotaxis to lymph nodes. J Clin Invest 2003; 111:627-37; PMID:12618517; http://dx.doi.org/10.1172/JCI200316200
- Robertson MJ. Role of chemokines in the biology of natural killer cells. J Leukoc Biol [Internet] 2002; 71:173-83. Available from: http://www.ncbi.nlm.nih.gov/pubmed/11818437; PMID:11818437
- Franciszkiewicz K, Boissonnas A, Boutet M, Combadière C, Mami-Chouaib F. Role of chemokines and chemokine receptors in shaping the effector phase of the antitumor immune response. Cancer Res 2012; 72:6325-32; PMID:23222302; http://dx.doi.org/10.1158/0008-5472.CAN-12-2027
- Alvino E, Pepponi R, Pagani E, Lacal PM, Nunziata C, Bonmassar E, D’Atri S. O(6)-benzylguanine enhances the in vitro immunotoxic activity of temozolomide on natural or antigen-dependent immunity. J Pharmacol Exp Ther 1999; 291:1292-300; PMID:10565854
- Ellsworth S, Balmanoukian A, Kos F, Nirschl CJ, Nirschl TR, Grossman S A, Luznik L, Drake CG. Sustained CD4(+) T cell-driven lymphopenia without a compensatory IL-7/IL-15 response among high-grade glioma patients treated with radiation and temozolomide. Oncoimmunology [Internet] 2014; 3:e27357. Available from: http://www.ncbi.nlm.nih.gov/pubmed/24790790; PMID:24790790; http://dx.doi.org/10.4161/onci.27357
- Fernandez NC, Lozier A, Flament C, Ricciardi-Castagnoli P, Bellet D, Suter M, Perricaudet M, Tursz T, Maraskovsky E, Zitvogel L. Dendritic cells directly trigger NK cell functions: cross-talk relevant in innate anti-tumor immune responses in vivo. Nat Med [Internet] 1999; 5:405-11. Available from: http://www.ncbi.nlm.nih.gov/pubmed/10202929; PMID:10202929; http://dx.doi.org/10.1038/7403
- Moretta A. Natural killer cells and dendritic cells: rendezvous in abused tissues. Nat Rev Immunol [Internet] 2002 [cited 2013 Jun 7]; 2:957-64. Available from: http://www.ncbi.nlm.nih.gov/pubmed/12461568; PMID:12461568; http://dx.doi.org/10.1038/nri956
- Van Elssen CHMJ, Oth T, Germeraad WT V, Bos GMJ, Vanderlocht J. Natural killer cells: the secret weapon in dendritic cell vaccination strategies. Clin Cancer Res [Internet] 2014 [cited 2014 Mar 29]; 20:1095-103. Available from: http://www.ncbi.nlm.nih.gov/pubmed/24590885; PMID:24590885; http://dx.doi.org/10.1158/1078-0432.CCR-13-2302
- Lowdell MW. Natural killer cells in haematopoietic stem cell transplantation. Transfus Med [Internet] 2003; 13:399-404. Available from: http://www.ncbi.nlm.nih.gov/pubmed/14651745; PMID:14651745; http://dx.doi.org/10.1111/j.1365-3148.2003.00467.x
- Köck K, Grube M, Jedlitschky G, Oevermann L, Siegmund W, Ritter CA, Kroemer HK. Expression of adenosine triphosphate-binding cassette (ABC) drug transporters in peripheral blood cells: relevance for physiology and pharmacotherapy. Clin Pharmacokinet [Internet] 2007; 46:449-70. Available from: http://www.ncbi.nlm.nih.gov/pubmed/17518506; PMID:17518506; http://dx.doi.org/10.2165/00003088-200746060-00001
- Sengupta S, Marrinan J, Frishman C, Sampath P. Impact of temozolomide on immune response during malignant glioma chemotherapy. Clin Dev Immunol 2012; 2012:831090; PMID:23133490; http://dx.doi.org/10.1155/2012/831090
- Litterman AJ, Zellmer DM, Grinnen KL, Hunt M A, Dudek AZ, Salazar AM, Ohlfest JR. Profound Impairment of Adaptive Immune Responses by Alkylating Chemotherapy. J Immunol [Internet] 2013 [cited 2013 May 28]; PMID:23686484; http://dx.doi.org/10.4049/jimmunol.1203539.
- Roos W, Baumgartner M, Kaina B. Apoptosis triggered by DNA damage O6-methylguanine in human lymphocytes requires DNA replication and is mediated by p53 and Fas/CD95/Apo-1. Oncogene 2004; 23:359-67; PMID:14724564; http://dx.doi.org/10.1038/sj.onc.1207080
- Asavaroengchai W, Kotera Y, Mulé JJ. Tumor lysate-pulsed dendritic cells can elicit an effective antitumor immune response during early lymphoid recovery. Proc Natl Acad Sci U S A 2002; 99:931-6; PMID:11792864; http://dx.doi.org/10.1073/pnas.022634999
- Sanchez-Perez LA, Choi BD, Archer GE, Cui X, Flores C, Johnson LA, Schmittling RJ, Snyder D, Herndon JE, Bigner DD et al. Myeloablative temozolomide enhances CD8+ T-cell responses to vaccine and is required for efficacy against brain tumors in mice. PLoS One [Internet] 2013 [cited 2014 Mar 16]; 8:e59082. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3601076&tool=pmcentrez&rendertype=abstract; PMID:23527092; http://dx.doi.org/10.1371/journal.pone.0059082
- Iversen TZ, Brimnes MK, Nikolajsen K, Andersen RS, Hadrup SR, Andersen MH, Bastholt L, Svane IM. Depletion of T lymphocytes is correlated with response to temozolomide in melanoma patients. Oncoimmunology [Internet] 2013; 2:e23288. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3601183&tool=pmcentrez&rendertype=abstract; PMID:23525955; http://dx.doi.org/10.4161/onci.23288
- Fadul CE, Fisher JL, Gui J, Hampton TH, Côté AL, Ernstoff MS. Immune modulation effects of concomitant temozolomide and radiation therapy on peripheral blood mononuclear cells in patients with glioblastoma multiforme. Neuro Oncol 2011; 13:393-400; PMID:21339188; http://dx.doi.org/10.1093/neuonc/noq204
- Lee KM, Bhawan S, Majima T, Wei H, Nishimura MI, Yagita H, Kumar V. Cutting edge: the NK cell receptor 2B4 augments antigen-specific T cell cytotoxicity through CD48 ligation on neighboring T cells. J Immunol 2003; 170:4881-5; PMID:12734329; http://dx.doi.org/10.4049/jimmunol.170.10.4881
- Assarsson E, Kambayashi T, Schatzle JD, Cramer SO, von Bonin A, Jensen PE, Ljunggren HG, Chambers BJ. NK Cells Stimulate Proliferation of T and NK Cells through 2B4/CD48 Interactions. J Immunol 2004; 173:174-80; PMID:15210772; http://dx.doi.org/10.4049/jimmunol.173.1.174
