ABSTRACT
Cancer cells can escape the antitumor immune response by recruiting immune suppressor cells. However, although innate myeloid-derived suppressor cells (MDSCs) and T regulatory (Treg) cells accumulate normally in tumor-bearing CD81-deficient mice, both populations are impaired in their ability to suppress the antitumor immune response.
Tregs and MDSCs regularly suppress the antitumor immune response and, in most models, depletion of these cells reduces tumor growth and metastasis.Citation1,2 Yet, in tumor-bearing CD81KO mice, these immune suppressor cells accumulate normally, but primary tumor growth and lung metastases were reduced.Citation3 This was due to impaired suppressive function of both Tregs and MDSCs in the absence of CD81. Identifying molecules that modulate the interaction between tumors and the immune system is important, because it can lead to new therapies against cancer.Citation4
Tetraspanins
Tetraspanins are a large evolutionary conserved family of proteins that are widely expressed in most cells of multicellular organisms. They form tetraspanin-enriched microdomains (TEM) in the cell membrane with their partner proteins, the latter differ in the various cell types, thereby allowing individual tetraspanin members to play different functions according to the specific cell type. The association of tetraspanins with integrins and members of the immunoglobulin superfamily influences cell adhesion, migration and invasion.Citation5 It is therefore not surprising that tetraspanins play a role in tumor progression. However, certain members, such as CD151 and Tspan8 are known as promoters of tumor growth, whereas CD82/KAI-1 is known to inhibit tumor growth.Citation6
Role of CD81 in the immune system
The role of CD81 in cell–cell interactions is best illustrated by its location in immune synapses at the central supermolecular activation complex in both B and T cells.Citation7 In B cells CD81 associates with CD19 and facilitates its cell surface expression. This dependence on CD81 for the trafficking of CD19 to the cell surface leads to impaired humoral immune responses in CD81KO mice and in a patient with a mutation in CD81.Citation8,9 Mechanistically, CD81 in TEMs facilitates the connection to the actin cytoskeleton and amplifies B cell receptor signaling.Citation10 In T cells, CD81 is a costimulatory molecule with CD3, of a magnitude similar to that of CD28.Citation10
CD81, a promoter of tumor growth and metastasis
CD81 is widely expressed on most tissues and on the majority of tumor cells. However, its function on malignant cells and in the host microenvironment has not been studied until recently.Citation3 To evaluate the contribution of CD81 to cancer progression, we implanted different tumor cell lines orthotopically into two different strains of WT and CD81KO mice. The growth of primary tumors was reduced in CD81KO compared to WT mice. This observation was consistent among different tumor types in two mouse strains, suggesting an intrinsic defect in these hosts. Moreover, reduced tumor growth was observed regardless of CD81 expression in the implanted tumor cells. Importantly, lung metastases were significantly reduced in CD81KO mice both upon orthotopic and intravenous injection of tumor cells. Thus, expression of CD81 in the host is important for tumor progression.
Tregs and MDSCs accumulate normally, but are functionally impaired in tumor-bearing CD81KO mice
Tumors induce the accumulation of immune suppressor cells; we therefore enumerated these suppressor cell populations in tumor-bearing CD81KO and WT mice. Surprisingly, growing tumors induced an equal increase in Tregs and MDSCs in both WT and CD81KO mice, suggesting that CD81 is not needed for development of these two cell types. However, the suppressive ability of Tregs and MDSCs derived from tumor-bearing CD81KO BALB/c and C57BL/6 mice was significantly impaired by comparison to those cells of their WT counterparts. Importantly, WT Tregs promoted tumor growth and metastasis when adoptively transferred together with tumor cells into CD81KO hosts, establishing a link between reduced tumor growth and metastasis with impaired immune suppression in the absence of CD81.
We explored several inhibitory mechanisms that MDSCs or Tregs use, and found that CD81KO Tregs derived from tumor-bearing mice have reduced IL-10 secretion. Although the mechanisms by which CD81 modulates Treg and MDSCs function still need further investigation, it is clear that the presence of CD81 in the host has a major effect on tumor growth and that this effect is mediated at least partially by the immune system.
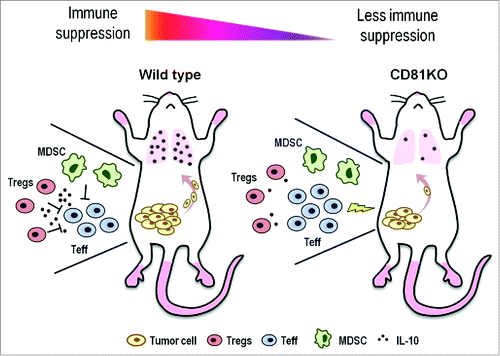
Figure 1. Reduced tumor growth and metastasis as a consequence of impaired immune suppression in CD81KO mice. In wild-type mice, primary tumors grow and recruit immune suppressor T regulatory cells (Tregs), and myeloid-derived suppressor cells (MDSCs). These suppressor cells secrete IL-10 and inhibit the antitumor T effector (Teff) response allowing tumors to grow and metastasize to the lungs (left panel). In contrast, Tregs and MDSCs still accumulate in CD81KO mice, but are impaired at suppressing Teff cells, thereby reducing tumor growth and metastasis (right panel).
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Funding
This work was supported by the Translational Cancer Award from Stanford Cancer Institute and the Breast Cancer Research program from the Department of Defense grant W81XWH-14-1-0397.
References
- Marabelle A, Kohrt H, Levy R. New insights into the mechanism of action of immune checkpoint antibodies. Oncoimmunology 2014; 3:e954869; PMID:25610751
- Gabrilovich DI, Ostrand-Rosenberg S, Bronte V. Coordinated regulation of myeloid cells by tumours. Nat Rev Immunol 2012; 12:253-68; PMID:22437938; http://dx.doi.org/10.1038/nri3175
- Vences-Catalan F, Rajapaksa R, Srivastava MK, Marabelle A, Kuo CC, Levy R, Levy S. Tetraspanin CD81 promotes tumor growth and metastasis by modulating the functions of T regulatory and myeloid-derived suppressor cells. Cancer Res 2015; 75:4517-26; PMID:26329536; http://dx.doi.org/10.1158/0008-5472.CAN-15-1021
- Sharma P, Allison JP. The future of immune checkpoint therapy. Science 2015; 348:56-61; PMID:25838373; http://dx.doi.org/10.1126/science.aaa8172
- Yanez-Mo M, Barreiro O, Gordon-Alonso M, Sala-Valdes M, Sanchez-Madrid F. Tetraspanin-enriched microdomains: a functional unit in cell plasma membranes. Trends Cell Biol 2009; 19:434-46; PMID:19709882; http://dx.doi.org/10.1016/j.tcb.2009.06.004
- Zoller M. Tetraspanins: push and pull in suppressing and promoting metastasis. Nat Rev Cancer 2009; 9:40-55; PMID:19078974; http://dx.doi.org/10.1038/nrc2543
- Mittelbrunn M, Yanez-Mo M, Sancho D, Ursa A, Sanchez-Madrid F. Cutting edge: dynamic redistribution of tetraspanin CD81 at the central zone of the immune synapse in both T lymphocytes and APC. J Immunol 2002; 169:6691-95; PMID:12471100; http://dx.doi.org/10.4049/jimmunol.169.12.6691
- van Zelm MC, Smet J, Adams B, Mascart F, Schandene L, Janssen F, Ferster A, Kuo CC, Levy S, van Dongen JJ, van der Burg M. CD81 gene defect in humans disrupts CD19 complex formation and leads to antibody deficiency. J Clin Invest 2010; 120:1265-74; PMID:20237408; http://dx.doi.org/10.1172/JCI39748
- Maecker HT, Levy S. Normal lymphocyte development but delayed humoral immune response in CD81-null mice. J Exp Med 1997; 185:1505-10; PMID:9126932; http://dx.doi.org/10.1084/jem.185.8.1505
- Levy S. Function of the tetraspanin molecule CD81 in B and T cells. Immunol Res 2014; 58:179-85; PMID:24522698; http://dx.doi.org/10.1007/s12026-014-8490-7
