ABSTRACT
Immune suppression is recognized as a hallmark of cancer and this notion is largely based on studies on cellular immunity. Our recent studies have demonstrated a potential new mechanism of cancer suppression of immunity by impairment of antibody effector function mediated by proteolytic enzymes in the tumor microenvironment.
We recently reported the presence of immunoglobulin G (IgG) hinge cleavage in tumor tissues from a cohort of breast cancer patients and have demonstrated a potentially important, yet overlooked proteolytic mechanism of cancer evasion of host humoral immunity.Citation1 The positive correlations between single hinge cleaved antibodies (scIgGs), expression of matrix metallo-proteinases (MMPs), tumor-associated macrophages, and tumor progression suggest a role for proteolysis within the complex immune interrelationships within the tumor microenvironment.Citation1This clinical study on the impairment of IgG antibodies in breast tumor tissues was built on several earlier in vitro and in vivo animal model investigations from our group and others.Citation2-9
Earlier findings had shown that proteolytic enzymes such as cancer-associated MMPs, as well as several bacterial proteases, catalyze limited cleavages in one chain of the IgG1 lower hinge to fully disable key antibody cell-killing effector functions ().Citation8,9 It is remarkable that a single peptide bond scission in the lower hinge region of the IgG1 antibodies resulted in the loss of ADCC (antibody dependent cellular cytotoxicity) and CDC (complement dependent cytotoxicity) in in vitro assays.Citation7,8 It is well established that some of the IgG1 monoclonal antibody cancer therapies, such as the HER2 targeting trastuzumab depend, in part, on the antibody triggered immune effector functions including ADCC for their tumor inhibition efficacy.Citation10 Therefore, the impairment of antibody-mediated immune effector functions can be expected to compromise the efficacy of these cancer antibody immune therapies. The introduction of single-cleaved IgGs in in vivo models resulted in a similar inability to eradicate cancer cells.Citation3,7,Citation8 The loss of function was traced to an inability of the antibody Fc domain to effectively engage Fc receptors on immune cells or components of the complement system.Citation3,7,Citation8
Figure 1. Proteolytic single hinge cleavage of IgG antibodies and its role in cancer evasion of host antibody (humoral) immunity. (A) A diagram illustrating the proteolytic antibody single strand-hinge cleavage process. scHC, single strand-hinge cleaved heavy chain; Fc(m), Fc monomer (released from IgG1 only under denaturing conditions); HC, heavy chain; and LC, light chain. The hinge region is not drawn to scale to illustrate its proteolytic cleavage. Adapted from Zhang et al, 2015. Clin Cancer Res.Citation1 (B) Working hypothesis that single hinge cleavage of IgG by MMPs leads to resistance to antibody therapies by reducing Fc mediated immune effector functions. In this model, we hypothesize that increased expression of MMP activities in the tumor microenvironment can result in the single hinge cleavage of anticancer antibodies in patients. The single hinge cleaved antibody lacks Fc-mediated effector functions such as ADCC and CDC, which leads to loss of efficacy and development of antibody resistance. ADCC, antibody dependent cellular cytotoxicity; CDC, complement dependent cytotoxicity.
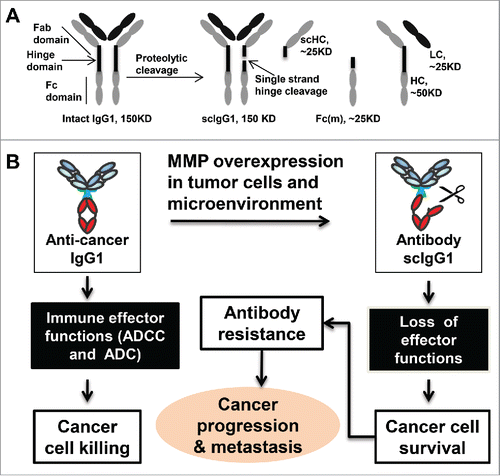
The overall structural change in IgG caused by proteolytic cleavage is subtle and is virtually undetectable in biological samples without the development of specific tools to distinguish “damaged” IgGs from the high, normal levels of intact IgGs. Thus, a localized impairment of IgGs that could otherwise participate in the eradication of pathogenic cells such as cancer cells has been a largely unacknowledged mechanism of tumor escape from host humoral immunity. By developing specific anti-hinge antibody reagents against distinct neo-epitopes on the IgG1 lower hinge caused by protease cleavage, we acquired the means to specifically detect the presence of IgG breakdown in the complex tissue environments.
In the recent Clinical Cancer Research paper,Citation1 we investigated the impairment of IgGs in breast tumor tissues from a cohort of 60 cancer patients in comparison with that of 20 healthy donors.Citation1 Immunohistochemical analysis was used to visualize the presence of IgGs with hinge cleavage in tumor tissues. A cocktail of antibodies specific for individual cleavage points with exposed C-termini corresponding to likely protease sites in the IgG hinge provided the necessary specificity without appreciable binding to intact IgG.Citation1 The results showed that levels of single-cleaved IgGs were substantially higher in tumors compared to normal tissue samples. The intratumoral nature of protease action was also supported by low concentrations of cleaved IgG detected in the blood from the same patients. The study of proteolytic hinge cleavage in cancer tumor tissues indicated a significant trend toward a higher incidence of IgG breakdown in tumor samples and potential impairment of antibody immunity in cancer patients.
This study demonstrated that single-cleaved host IgGs are abundant in freshly obtained tumor samples. The observation was further associated with other immune phenomena of importance in cancer. Specifically, an incursion of tumor-associated macrophages (linked to poor clinical outcomes in cancer patients) was strikingly increased in tumors with higher levels of single-cleaved IgGs. Not surprisingly, increased MMP levels (especially MMP-9) were positively correlated with the levels of detectable IgG cleavage. An additional association emerged when it was noted that scIgG levels were stratified among cancer subtypes with the triple-negative group of breast cancer patients exhibiting a higher mean single-cleaved IgG signal. These results further confirmed the complex concerted interplay among various factors in the tumor environment with particular emphasis on the roles of IgG cleavage.
In the Clinical Cancer Research report,Citation1 we focused on the potentially harmful effects of cancer proteases on host immunity and endogenous IgGs. It is an obvious step to envision that a similar disablement could occur in the context of immunotherapies and that this might help to clarify the well-known ability of some tumors to resist even highly specific, antitumor monoclonal antibody therapies (). In related work, we had employed an anti-hinge monoclonal antibody with resistance to proteolytic cleavage to provide a surrogate Fc domain for restoring cell-killing functions.Citation3 Those findings, both in vitro and in vivo, highlighted potential therapeutic opportunities that exist to counter and potentially reverse the disablement of IgGs in tumors. The present study provides convincing set of data from a cohort of cancer patients to indicate that specific cleavage of endogenous host IgGs occurs in this important clinical setting. The present findings point to a new direction for investigating the mechanism behind the phenomenon of antibody hinge cleavage and impairment of Fc effector function and its role in evasion of host humoral immunity in cancer.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Funding
This study was partially funded by grants from Janssen R&D, LLC, the Texas Emerging Technology Fund, CPRIT (RP150230), and the Welch Foundation grant no. AU0042.
References
- Zhang N, Deng H, Fan X, Gonzalez A, Zhang S, Brezski RJ, Choi BK, Rycyzyn M, Strohl W, Jordan R et al. Dysfunctional Antibodies in the Tumor Microenvironment Associate with Impaired Anticancer Immunity. Clin Cancer Res 2015; 21(23):5380-90; PMID:26224871; http://dx.doi.org/10.1158/078-0432.CCR-15-1057
- Kinder M, Greenplate AR, Strohl WR, Jordan RE, Brezski RJ. An Fc engineering approach that modulates antibody-dependent cytokine release without altering cell-killing functions. MAbs 2015; 7:494-504; PMID:25933349;http://dx.doi.org/10.1080/19420862.2015.1022692
- Fan X, Brezski RJ, Deng H, Dhupkar PM, Shi Y, Gonzalez A, Zhang S, Rycyzyn M, Strohl WR, Jordan RE et al. A Novel Therapeutic Strategy to Rescue the Immune Effector Function of Proteolytically Inactivated Cancer Therapeutic Antibodies. Mol Cancer Ther 2015; 14:681-91; PMID:25552368.
- Biancheri P, Brezski RJ, Di Sabatino A, Greenplate AR, Soring KL, Corazza GR, Kok KB, Rovedatti L, Vossenkämper A, Ahmad N et al. Proteolytic Cleavage and Loss of Function of Biologic Agents that Neutralize Tumor Necrosis Factor in the Mucosa of Patients with Inflammatory Bowel Disease. Gastroenterology 2015; 149:1564-74; PMID:26170138; http://dx.doi.org/10.1053/j.gastro.2015.07.002
- Malia TJ, Teplyakov A, Brezski RJ, Luo J, Kinder M, Sweet RW, Almagro JC, Jordan RE, Gilliland GL. Structure and specificity of an antibody targeting a proteolytically-cleaved IgG hinge. Proteins 2014; 82:1656-67; PMID:24638881; http://dx.doi.org/10.1002/prot.24545
- Kinder M, Greenplate AR, Grugan KD, Soring KL, Heeringa KA, McCarthy SG, Bannish G, Perpetua M, Lynch F, Jordan RE et al. Engineered Protease-resistant Antibodies with Selectable Cell-killing Functions. J Biol Chem 2013; 288:30843-54; PMID:23986451; http://dx.doi.org/10.1074/jbc.M113.486142
- Fan X, Brezski RJ, Fa M, Deng H, Oberholtzer A, Gonzalez A, Dubinsky WP, Strohl WR, Jordan RE, Zhang N et al. A single proteolytic cleavage within the lower hinge of trastuzumab reduces immune effector function and in vivo efficacy. Breast Cancer Res 2012; 14:R116; PMID:22873525; http://dx.doi.org/10.1186/bcr3240
- Brezski RJ, Vafa O, Petrone D, Tam SH, Powers G, Ryan MH, Luongo JL, Oberholtzer A, Knight DM, Jordan RE. Tumor-associated and microbial proteases compromise host IgG effector functions by a single cleavage proximal to the hinge. Proc Natl Acad Sci U S A 2009; 106:17864-9; PMID:19815504; http://dx.doi.org/10.1073/pnas.0904174106
- Ryan MH, Petrone D, Nemeth JF, Barnathan E, Bjorck L, Jordan RE. Proteolysis of purified IgGs by human and bacterial enzymes in vitro and the detection of specific proteolytic fragments of endogenous IgG in rheumatoid synovial fluid. Mol Immunol 2008; 45:1837-46; PMID:18157932; http://dx.doi.org/10.1016/j.molimm.2007.10.043
- Shi Y, Fan X, Deng H, Brezski RJ, Rycyzyn M, Jordan RE, Strohl WR, Zou Q, Zhang N, An Z. Trastuzumab Triggers Phagocytic Killing of High HER2 Cancer Cells In Vitro and In Vivo by Interaction with Fcgamma Receptors on Macrophages. J Immunol 2015; 194:4379-86; PMID:25795760
