ABSTRACT
CD245 is a human surface antigen expressed on peripheral blood lymphocytes, initially delineated by two monoclonal antibodies DY12 and DY35. Until now, CD245 molecular and functional characteristics remained largely unknown. We combined immunological and proteomic approaches and identified CD245 as the unconventional myosin 18A, a highly conserved motor enzyme reported as a receptor for the surfactant protein A (SP-A), that plays a critical role in cytoskeleton organization and Golgi budding. We report that the recruitment of CD245 strongly enhanced NK cell cytotoxicity. Further, we show that the enhancement of the NK lymphocytes killing ability toward CD137-ligand expressing target cells could result from the induction of CD137 expression following CD245 engagement. The SP-A receptor could therefore represent a novel and promising target in cancer immunotherapy.
Abbreviations
| ADCC | = | antibody-dependent cell cytotoxicity |
| EBV | = | Epstein-Barr Virus |
| ECL | = | enhanced chemiluminescence |
| E/T | = | effector/target |
| FCS | = | fetal calf serum |
| LAK | = | lymphokine-activated killer |
| MHC | = | major histocompatibility complex |
| mAb | = | monoclonal antibody |
| MS | = | Mass spectrometry |
| SP-R | = | surfactant protein A-receptor |
| NK | = | natural killer |
| NKR | = | NK receptors |
| NCR | = | natural cytotoxicity receptor |
| NKG2D | = | NKG2 C-type lectin receptors family D |
| PBMC | = | Peripheral blood mononuclear cells |
| PB-NK | = | peripheral blood NK cells |
| PBS | = | phosphate buffer saline |
| RPMI | = | Roswell Park Memorial Institute |
| SP-A | = | surfactant protein A |
| SLAM | = | signaling lymphocytic activation molecule. |
Introduction
Natural killer (NK) cells were identified over 40 y ago as a subset of lymphocytes able to spontaneously kill tumor cells in the absence of pre-stimulation.Citation1 Present in most mammalian and avian species, NK cells play a critical role in the antitumorCitation2 and anti-infectious immunity and in reproduction.Citation3 NK cell cytotoxicity is tightly controlled by a balance between signals delivered through the engagement of activating and inhibitory receptors.Citation4 Activating NK receptors (NKR) include several members belonging to the natural cytotoxicity receptors (NCR) family (such as NKp46, NKp44 and NKp30), the NKG2 C-type lectin receptors family (NKG2D), the major histocompatibility complex (MHC) class I-specific killer cell Ig-like receptors (KIRs) family, the Ig-like signaling lymphocytic activation molecule (SLAM) family (2B4), as well as unclassified receptors such as CD160.Citation5,6 Beside these receptors, costimulatory molecules co-exist at the surface of activated NK cells to potentiate NK cells functions. 4-1BB (CD137) is a costimulatory receptor expressed on T, B and NK cellsCitation7 whose expression is triggered by engagement of the Fc receptors on the NK cell surface, as during antibody-dependent cell cytotoxicity (ADCC).Citation8 Consequently, the engagement of CD137 increases cetuximab-, rituximab-, and trastuzumab-dependent NK cell cytotoxic function in different cancer models.Citation9-11 NK cell activation is dominantly suppressed if the inhibitory NKR bind to MHC class I molecules on target cells.Citation2 In humans, these receptors mainly belong to C-type lectin receptors, as the NKG2A heterodimer, or to the KIRs family. We previously described CD245 as an unidentified structure on the surface of human peripheral blood lymphocytes that was recognized by the monoclonal antibodies DY12 and DY35. We also reported that CD245 on NK lymphocytes was associated with tyrosine phosphatase activities.Citation12 To further understand the NK lymphocyte biology, we characterize the molecular and functional properties of CD245 and identify a novel co-activation pathway that potentiates the NK cell cytotoxicity toward virally infected and tumor cells. Our data suggest that CD245 might be a promising target in the field of human cancer immunotherapy.
Methods
Cells
Peripheral blood mononuclear cells (PBMC) were isolated from heparinized venous blood obtained from healthy donors by density gradient centrifugation over lymphocytes separation medium (PAA Laboratories/GE Healthcare Europe, Vélizy-Villacoublay, France). Fresh peripheral blood NK cells (PB-NK) were isolated by magnetic cell sorting using an NK cell isolation kit according to the manufacturers' recommendations (MiltenyiBiotec, BergischGladbach, Germany). PB-NK cell purity was shown to be >95% as assessed by flow cytometry. The YT2C2 NK cell line, the murine mastocytoma cell line P815, and the Burkitt-lymphoma B-cell line Raji (all purchased from the ATCC, Manassas, USA), the Epstein-Barr Virus (EBV)-infected B cell line (locally produced Citation13) were cultured in Roswell Park Memorial Institute (RPMI) 1640 medium supplemented with 1% penicillin/streptomycin, 2 mML-glutamine, and 10% heat-inactivated fetal calf serum (FCS) (Perbio Science, Villebon-sur-Yvette, France).
Immunohistochemistry
Formalin-fixed and paraffin-embedded PB-NK cells were analyzed for CD245 expression using a standard peroxidase method. Mouse anti-human CD245 (DY12), or granzyme B (clone GrB-7, DAKO) monoclonal antibody (mAb) was used as primary antibody, followed by biotin-conjugated anti-mouse Ig antibody and revealed with the streptavidin-peroxidase (LSAB kit, Dako, Les Ulis, France). The peroxidase reaction was then developed using 3-amino-9-ethyl carbazole substrate for 5 to 8 min.
Cell surface biotinylation
Cells were biotinylated by a sulfosuccinimidobiotin (Sulfo-NHS-biotin, Pierce, Rockford, USA) procedure. Briefly, after three washes in PBS, cells were resuspended at a density of 10 × 106/mL in PBS containing 1 mg/mL of Sulfo-NHS-biotin. After a 30-min incubation at 4°C, cells were washed three times with culture medium and processed for immunoprecipitation (see below).
Immunoprecipitation and western blot
Immunoprecipitations were performed on YT2C2 cell or human lung extracts as previously described.Citation13 Briefly, 2 to 10 × 106cells or 100 mg of tissue were lysed in 1% Nonidet-P40 or Brij58 lysis buffer (Sigma) for 1 h at 4°C. After centrifugation at 10,000 rpm and 4°C, post-nuclear lysates were subjected to immunoprecipitation with either DY12 or control IgG mAb and protein G-Sepharose beads. The precipitated proteins were separated by SDS-8% PAGE and transferred onto a nitrocellulose membrane (Millipore, Bedford, USA). Immunoblot analyses were performed using rabbit anti-myosin 18A antibodies (Protein Tech Group, Manchester, UK) or anti-SHP-1, anti-SHP-2, anti-SHIP and anti-PAK2 polyclonal Abs (Cell Signaling Technologies, Beverly, USA), followed by horseradish peroxidase (HRP)-conjugated goat anti-rabbit Igs secondary antibodies (Jackson ImmunoResearch Laboratories, West Grove, USA) and revealed with enhanced chemiluminescence (ECL) reagents (Amersham Biosciences, GE Healthcare Europe). For the biotinylation experiments, HRP-coupled-streptavidin was used.
Mass spectrometry (MS)
After immunoprecipitation with DY12 mAb or control IgG, the area of interest was cut out with a scalpel from the nitrocellulose and processed for MS analysis without chemical treatment as previously described.Citation14,15 Proteins were digested with trypsin and MS analysis was carried out using a MALDI TOF/TOF ABI 4800 apparatus (Applied Biosystems, Foster City, USA). The masses obtained by MS-MALDI were analyzed using the Expasy database and software (http://www.expasy.org) and a local Visual Basic for Applications (VBA) software (Microsoft Excel, Microsoft, Redmond, USA).
Cytotoxicity assays
Cytotoxicity assays were performed according to a standard 51Cr-release method. Target cells were labeled with 100 μCi of Na51CrO4 for 90 min at 37°C, washed three times in culture medium and plated in 96-well V-bottom microtiter plates (Greiner BioOne, Courtaboeuf, France).
In redirected cytotoxicity assays on P815 cells, PB-NK cells were left untreated or pre-activated with recombinant human IL-2 (100 IU/mL, Peprotech) for 24 h. 51Cr-labeled P815 cells were incubated with DY12 or isotype control mAb, alone or in combination with anti-CD335 (NKp46) or anti-CD337 (NKp30) mAb (10 μg/mL each).
In lymphokine-activated killer (LAK) assays against an EBV-infected B cell line, PB-NK lymphocytes were left untreated or preactivated with recombinant human IL-2 for 24 h and then treated with F(ab')2 fragments of DY12 or isotype control mAb (10 μg/mL) or recombinant surfactant protein A (SP-A, 100 ng/mL) (USCNK, Houston, USA) for 1 h at room temperature.
For all assays, a fixed number of 103 target cells/well was used and NK cells were added at various E/T cell ratios, as indicated. All conditions were done in triplicate.
After 4 h of culture at 37°C, the plates were spun down and 100 μL of the cell supernatant were collected from each well. The 51Cr release was quantified using a gamma-counter (Packard Instrument Company, Meriden, USA). The percentage of specific lysis was calculated as follows: % Specific lysis = [(Sample cpm − Spontaneous Lysis Control cpm)/(Maximum Lysis Control cpm − Spontaneous Lysis Control cpm)] × 100. The specific lysis was considered significant if >10%.
Lymphocytes activation
For the study of NK cell activating receptors expression, freshly isolated PB-NK cells were first in vitro stimulated for 1 h at 37°C with DY12 or control IgG1 mAb (10 μg/mL), washed and incubated with rabbit anti-mouse IgG antibodies to allow mAb cross-linking (Jackson ImmunoResearch Laboratories) (10 μg/mL). To activate T lymphocytes, freshly isolated PBMC were cultured in flat bottom 96-wells plate coated with 0.3 µg/well of anti-CD3 mAb. For B lymphocytes activation, PBMC were cultured in round bottom 96-wells plate in complete RPMI medium with 10 µg/mL of polyclonal goat anti-human anti-IgM Ab. After 72 h of culture in complete RPMI medium, cells were harvested and washed with PBS before straining.
NK cell degranulation assay and blocking of the CD137/CD137 ligand (CD137L) interaction
Freshly isolated PB-NK cells were activated as described above. Raji target cells were then added to a final volume of 150 μL/well at various E/T ratios. After 4 h of culture at 37°C in the presence of PE-Cy7-conjugated anti-CD107a (Becton Dickinson), cells were washed and prepared for flow cytometry analysis. In some experiments, human 4-1BB-Ligand/TNFSF9 affinity purified polyclonal Ab (R&D systems, Minneapolis, USA) was added to the culture at a final concentration of 10 μg/mL to block the CD137/CD137L interaction.
Flow cytometry analysis
The mAbs used were the following: anti-CD3, anti-CD4, anti-CD8, anti CD19, anti-CD20, anti-CD56, anti-CD197 (C-C chemokine receptor type 7 (CCR7)), anti-γδ T-cell receptor mAb (MiltenyiBiotec), and anti-CD245 mAb (DY12, mouse IgG1, locally produced). Irrelevant isotype-matched mAbs were used as negative controls. Fluorescein isothiocyanate (FITC), allophycocyanin (APC)- or R-phycoerythrin (RPE)-conjugated goat anti-mouse IgG or IgM antibodies (Beckman Coulter, Brea, USA) were used as secondary reagents. Briefly, cells were incubated with the specific mAb for 30 min at 4°C, washed twice in phosphate buffer saline (PBS) (Life Technologies, Carlsbad, USA), and further incubated with the appropriate secondary Abs. Cells were washed and analyzed by flow cytometry on a FC500 analyzer (Beckman Coulter). In some experiments, PBMC were activated with anti-CD3 or anti-IgM antibodies for 72 h before labeling.
To characterize the expression of NK cell activating receptors after CD245 engagement, NK cells were activated as described in the “Activation of NK cells” section, washed and labeled with Fixable Viability Stain 450 (Becton Dickinson, Franklin Lakes, USA) and the following antibodies to human cell surface antigens: APC-conjugated anti-CD137, PE-conjugated anti-NKG2D, FITC-conjugated anti-DNAX Accessory Molecule-1 (DNAM-1, CD226), PE-conjugated anti-CD160 (Becton Dickinson), PE-conjugated anti-NKp30 (CD337), anti-NKp44 (CD336), and anti-NKp46 (CD335) (Beckman-Coulter).
To study CD137L expression on Raji cells, Raji cell lines were cultured and treated as described above, washed and stained with Fixable Viability Stain 450 (Becton Dickinson) and PE-conjugated anti-CD137L (Becton Dickinson) for flow cytometry analysis. Cells were washed and analyzed on a Canto II Flow-Cytometer (Becton Dickinson).
Analysis
Flow cytometry analysis was carried out using the FlowJo software version X. All values are expressed as means of fluorescence intensity (MFI). Values are plotted with their mean and standard deviation and compared between groups with Prism software (Graph Pad version 6) by two-tailed Mann–Whitney U test or ANOVA (for cytotoxicity tests) to compare continuous variables. p ≤ 0.05 was considered as statistically significant.
Results
Human NK cells express the long (α) and short (β) isoforms of myosin 18A (CD245)
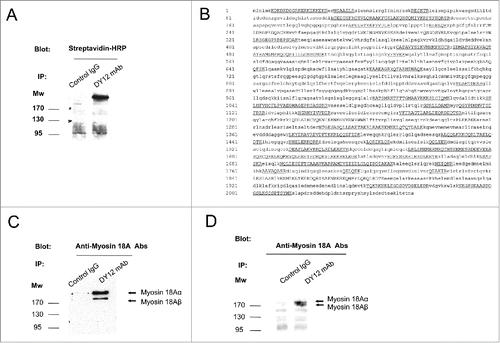
By using the two mAbs DY12 and DY35, we previously described CD245 as a surface protein with an apparent molecular weight of approximately 220 kDa expressed by a large panel of normal and malignant human hematopoietic cells.Citation12 In order to identify CD245 protein sequence, YT2C2 cells (the leukemic NK cell line used in the original immunization program leading to the selection of the anti-CD245 mAbs) were biotinylated and cell lysates were subjected to immunoprecipitation with DY12 or a control IgG1 mAb. As shown in , after migration of the immunoprecipitates on SDS-PAGE and immunoblot analysis with HRP-conjugated streptavidin, we confirmed the detection of CD245 molecules in the 220–240 kDa area. This area was cut out from the nitrocellulose, subjected to trypsin digestion and then processed for mass spectrometry (MS) analysis. In the list of the 239 masses of tryptic peptides obtained, 59 corresponded to those of myosin 18A, with a difference lower than 36 ppm from the corresponding theoretical mass (). To further confirm that the CD245 molecule expressed by the YT2C2 cell line was indeed the unconventional myosin 18A, YT2C2 cell lysates were immunoprecipitated using DY12 mAb or an IgG1 control isotype and the immunoprecipitates were subjected to immunoblotting using polyclonal anti-myosin 18A antibodies. This led to the specific detection of the α (230 kDa) and β (190 kDa) isoforms of myosin 18A in DY12 immunoprecipitate (). Thus, CD245 expressed at the cell surface of human YT2C2 NK cell line is the bona fide myosin 18A. Of note, both α and β isoforms were expressed in YT2C2 cells, whereas only the α isoform was found in biotinylated YT2C2 immunoprecipitate, suggesting that only the α isoform is expressed at the cell surface of YT2C2 cells. These data are consistent with previous studies in mice that showed that myosin 18Aα (p230) and β (p190) had different subcellular localizations, the former colocalizing with the endoplasmic reticulum and Golgi structures.Citation16Myosin 18A was reported as a receptor for the surfactant protein A (SP-A),Citation17 a collectin notably present in human lung.Citation18 Thus, we performed immunoprecipitation with DY12 mAb using fresh human lung extracts. The results show the recognition of both myosin 18A isoforms by DY12 mAb in these cellular extracts ().
Figure 1. NK cells express the long (α) and short (β) isoforms of myosin 18A. (A) Biotinylated YT2C2 leukemic cell lines were lysed and immunoprecipitated with DY12 mAb or control IgG. After migration on SDS-PAGE and transfer to nitrocellulose, the immunoprecipitated proteins were revealed with HRP-conjugated streptavidin and an ECL detection system. (B) Amino acid sequence of the 220–240 kDa protein immunoprecipitated by DY12 mAb as determined by mass spectrometry (MS) analysis. Underlined are the sequences common to that of myosin 18A. (C) YT2C2 cell lysates were immunoprecipitated using DY12 mAb or IgG control isotype. Immunoprecipitates were further subjected to immunoblotting using polyclonal anti-myosin 18A antibodies. Positions of the α (230 kDa) and β (190 kDa) isoforms of myosin 18A are indicated. (D) Fresh and healthy lung tissue from a human subject was lysed and extracts were immunoprecipitated with DY12 or control IgG mAb. Immunoprecipitates were immunoblotted using polyclonal anti-myosin 18A antibodies followed by HRP-conjugated goat anti-rabbit antibodies. The proteins immunoprecipitated by DY12 mAb corresponded to the α (230 kDa) and β (190 kDa) isoforms of myosin 18A.
CD245 expression at the cell surface of peripheral blood lymphocytes is constitutive and increased by activation
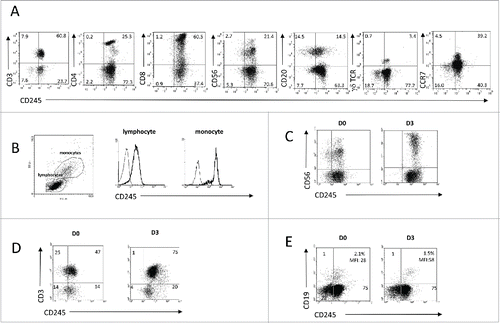
To investigate the expression of CD245 on distinct subsets of PBMCs, freshly isolated PBMCs from healthy subjects were analyzed by flow cytometry. As shown in , all investigated peripheral blood lymphocyte subsets expressed significantly CD245 although at various levels. Most CD3+ T cells including γδ lymphocytes, CD56bright+ as well as CD56dim+ NK cells, and half of the CD20+ B cells expressed CD245. This expression was also associated with the one of CCR7, a chemokine receptor expressed in T, B and CD56bright+ NK cells, involved in lymph node homing. In addition, the expression of CD245 is higher on monocytes as compared to lymphocytes () and was found increased after 3 d of activation of NK cells with IL-2 (), and of T and B cells with anti-CD3 or anti-IgM antibodies, respectively ().
Figure 2. Myosin 18A/CD245 expression at the cell surface of peripheral blood mononuclear cells is constitutive and increased by activation. (A) PBMC from healthy donors were isolated and subjected to flow cytometry analysis using anti-CD245 DY12 mAb plus PE-coupled goat anti-mouse IgG antibodies and fluorochrome-conjugated, anti-CD3, -CD4, -CD8, -CD20, -CD56, -γδ TCR, or -CCR7 mAbs. (B) Flow cytometry analysis of CD245 expression on the lymphocytes and monocytes gates was assessed using DY12 mAb as in (A) Representative results from one experiment out of two are shown. (C–E) PBMC were stimulated for 72 h with IL-2 (100 IU/mL) (C), immobilized anti-CD3 mAb (D) or soluble anti-IgM antibodies (E) Expression of CD245/myosin 18A on NK, T and B cells was assessed using DY12 mAb as described in (A) and anti-CD56 (C), -CD3 (D) or CD19 (E) mAb, respectively. For each type of activation, representative results from one experiment out of two are shown.
Recruitment of CD245 on peripheral blood NK cells enhances the CD335/NKp46 and CD337/NKp30-mediated NK cell redirected cytotoxicity toward the mastocytoma cell line P815
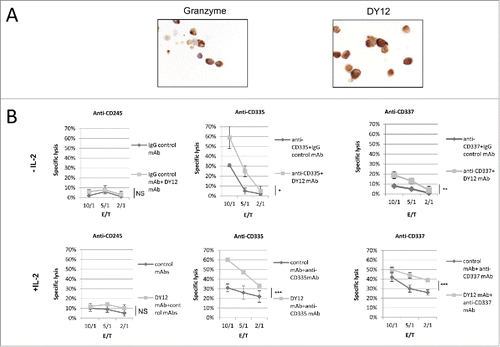
SP-A, a ligand for myosin 18A/CD245, has previously been shown to stimulate the anti-tumor immunity in vivo in a xenograft mouse model.Citation19 This antitumor effect of SP-A was dependent on NK cells in vivoCitation19 although the exact mechanisms remained unknown. To address this issue, we investigated whether the engagement of CD245 was able to modulate the NK cell redirected cytotoxicity against the murine mastocytoma cell line P815. Prior to functional investigations of CD245 role on human PB-NK cells, its expression was confirmed by immunohistochemistry using the DY12 mAb. Anti-granzyme B antibodies were used as positive control. The resulting data clearly show the presence of myosin 18A at the cell membrane and in the cytoplasm of PB-NK lymphocytes (). As shown in (upper panels), NK cells stimulated with DY12 mAb alone exhibited a poor cytotoxic activity against P815 cell line (specific lysis of 6% at an E/T ratio of 10/1). In contrast, engagement of CD335 and CD337 induced the cellular cytotoxicity of PB-NK cells that was significantly enhanced by the coengagement of CD245. Similar results were obtained when using IL-2-activated NK cells (, lower panel). These data identify CD245 as a co-activator of NK cell cytotoxic activity triggered by the NCR CD335 and CD337, and support the hypothesis that the antitumor effect of SP-A in vivo might be mediated by its interaction with CD245 on NK cells.
Figure 3. The engagement of myosin 18A/CD245 with DY12 mAb increases NK cell cytotoxicity. (A) Freshly isolated PB-NK lymphocytes from healthy donors were assessed for CD245 expression by immunohistochemistry using the DY12 mAb. Anti-granzyme B antibodies were used as positive control. (B) CD245-induced reverse cytotoxicity toward the P815 mastocytoma cell line. Cytotoxicity assays were performed according to a standard 51Cr-release method. Effector cells were left untreated (upper panels) or IL-2-activated (lower panels) prior to contact with the target cells. The target cells P815 were pre-incubated with DY12 or control mAb alone or together with an anti-CD335 (NKp46) or -CD337 (NKp30) mAb at 10 μg/mL. Assays were performed in triplicate at various E/T cell ratios, as indicated. Results are shown as mean percentages ± SD. Statistics were calculated using the ANOVA test, *p < 0.05, **p < 0.01, ***p < 0.001.
CD245 engagement by DY12 mAb or by its physiological ligand SP-A increases the IL-2 activated NK cell cytotoxicity toward EBV-infected B cells
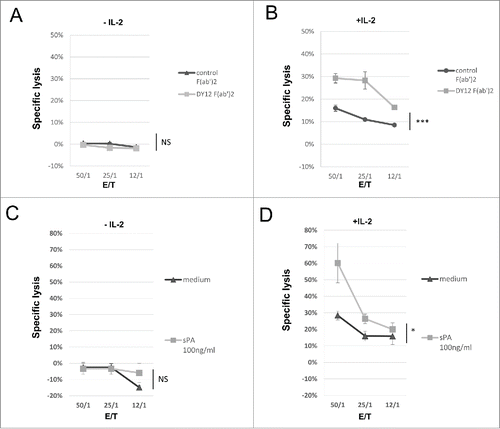
Because (i) NK cells play a critical and first-line role in the antiviral immune defense,Citation20 (ii) human NK cells express high levels of CD245, (iii) CD245 cell surface expression is increased in the presence of IL-2 in NK cells and (iv) CD245 corresponds to myosin 18A that has been shown to be a receptor for SP-A,Citation17 a protein involved in viral clearance in the human lung,Citation21 we asked whether engagement of CD245 on the NK cell surface was able to regulate their IL-2-activated killer activity against virally infected cells. As expected, freshly isolated PB-NK lymphocytes failed to kill an EBV-infected B cell line and engagement of CD245 with DY12 mAb was unable to overcome the resistance to NK cell cytotoxicity of these highly MHC class I molecules expressing target cells (). In contrast, we observed that short-term IL-2-activated PB-NK lymphocytes exhibited a weak but significant cytotoxicity (specific lysis of 16% at the 50/1 E/T ratio) against the EBV-infected B cell line and that further engagement of CD245 with F(ab')2 fragments of DY12 mAb significantly increased their cytotoxic activity (29% vs. 16% at the 50/1 E/T ratio, p <0.001, ). The targeting of CD245 did not significantly increase the formation of conjugates between the EBV-infected B cells and the activated NK lymphocytes (32% of EBV-B cells with control isotype vs. 33% with DY12 mAb, p = NS, data not shown). As myosin 18A was reported to be a receptor for SP-A,Citation17 we tested whether recombinant SP-A could have the same effect as DY12 mAb. The results show that resting PB-NK lymphocytes were unable to kill the EBV-infected B cell line and that addition of SP-A induced no detectable killing activity even at the highest E/T ratio (p = NS, ). However, IL-2-activated NK lymphocytes exhibited lymphokine-activated killer (LAK) activity toward the EBV-infected B cells (specific lysis of 28% at the 50/1 E/T ratio) that was significantly increased upon addition of SP-A (60% vs. 28%, p = 0.03, ). These results clearly indicate that DY12 mAb as well as the physiological ligand SP-A trigger the enhancement of the NK lymphocyte cytolytic function through CD245 targeting.
Figure 4. Engagement of CD245 with DY12 mAb or its physiological ligand SP-A increases the NK lymphocytes lymphokine-activated killer activity toward EBV-infected B cells. Cytotoxicity assays were performed according to a standard 51Cr-release method. Effector cells were untreated (A and C) or IL-2 activated (B and D) PB-NK lymphocytes from healthy donors and target cell was an Epstein-Barr Virus (EBV)-infected B cell line. (A and B) Target cells were preincubated with DY12 control F(ab')2 or control F(ab')2 mAb fragment at 10 μg/mL prior to contact with the NK cells. (C and D) The assay was performed in medium alone or the presence of recombinant SP-A at 100 ng/mL as indicated. Assays were performed in triplicate at various E/T cell ratios, as indicated Results are shown as mean percentages of specific lysis ± SD. Statistics were calculated using the ANOVA test, *p < 0.05.
The increased NK cell degranulation mediated through the engagement of CD245 could be 4-1BB (CD137) dependent
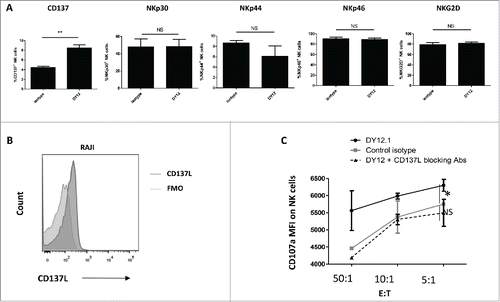
To further understand the mechanism by which recruitment of CD245 increases the NK cell cytotoxicity, we studied the expression of activating NK cell receptors after engagement of CD245 by DY12. As shown in , CD245 targeting did not induce any significant change in the expression of NKp30, NKp44, NKp46 or NKG2D. It also showed no significant impact on the expression of DNAM1 or CD160 (data not shown). In contrast, CD245 engagement increased the membrane expression of CD137 (4-1BB) (). Next, we asked whether NK cell cytotoxicity toward CD137L-expressing cells could depend on the CD137/CD137L interaction. CD137L has been shown to be expressed by human B cells and dendritic cells.Citation22 As Raji is a well-characterized Burkitt B-cell lymphoma cell line, and because Burkitt lymphoma has been shown to express CD137L,Citation23 we wondered whether the NK cell degranulation is increased following incubation with DY12 mAb and in the presence of Raji cells. Prior to using the Raji cell line as target, we confirmed its CD137L expression (). Consequently, in cytotoxic assays, the engagement of CD245 with DY12 mAb increased the NK cell degranulation in the presence of Raji cells, as assessed by the enhanced level of CD107a (). Blocking the CD137-CD137L interaction with an anti-human CD137L Ab completely abrogated the CD245-induced NK cell degranulation in the presence of Raji cells. Note that peripheral blood lymphocytes from some healthy individuals could degranulate in the presence of the so-called NK-cell-resistant target cell line Raji without being activated with IL-2. Altogether, these data suggest that the NK cell cytotoxicity induced by the recruitment of myosin 18A in the presence of CD137L-expressing target cells is CD137 dependent.
Figure 5. CD245-enhanced NK cell degranulation in the presence of 4-1BBL/CD137L-expressing target cells is 4-1BB/CD137-dependent. (A) The expression of the activating NK cell receptors NKp30, NKp44, NKp46 and NKG2D and of CD137 was monitored by flow cytometry on human NK cells triggered with an isotype control or DY12 mAb. FMO was used to determine positivity thresholds. The NK cells from five healthy donors were tested. Shown are the mean percentages ± SD of positive cells for each marker. Statistics were calculated with the Mann–Whitney U-test, **p < 0.01. (B) Raji cells were assessed for CD137L expression by flow cytometry. Unstained control was used to determine the positivity threshold. (C) PB-NK lymphocytes from healthy donors (n = 2) were incubated with DY12 or control IgG mAb (10 μg/mL), followed by cross-linking with rabbit anti-mouse IgG antibodies. The target cells were then added with or without anti-CD137L antibodies (10 μg/mL). CD107a expression was measured on CD3−CD56+ NK cells by flow cytometry. Results shown are mean percentages ± SD of CD107a MFI. Statistics were calculated using the ANOVA test, *p < 0.05.
Myosin 18A interacts with PAK-2 and SHP-2, two key signal transducers of the NK cell activation and degranulation processes
As shown previously, recruitment of myosin 18A enhanced NK cell cytotoxicity against tumor cells or virally infected cell lines (–). NK cell cytotoxicity is dependent on cytoskeleton reorganization, to create the NK immune synapse firstCitation24 and further allow the polarization and exocytosis of the cytolytic granules.Citation25,26 Myosin 18A has been shown to play a role in the cytoskeleton organization and to interact with PAK-2 in epithelial cell lines.Citation27 To further elucidate the mechanisms by which CD245 stimulation increased NK cell degranulation and lysis of target cells that failed to express CD137L, immunoprecipitates were prepared on YT2C2 NK cell line lysates with DY12 or control IgG1 isotype and subjected to electrophoresis and immunoblotting using anti-PAK2 antibody. Whole YT2C2 cell lysate was used as positive control. As shown in , a weak but significant amount of PAK2 was specifically recovered in DY12 immunoprecipitate. This data is in favor of an interaction between myosin 18A and PAK2 in NK cells, therefore identifying PAK2 as a potential signal transducer of the myosin 18A mediated NK cell cytotoxicity.
Figure 6. Myosin 18A co-precipitates with PAK-2 and SHP-2, two key signal transducers in NK cell activation and degranulation processes. Immunoprecipitates were performed on YT2C2 cell lysates with DY12 or control IgG1 mAb and subjected to electrophoresis and immunoblotting using anti-PAK2 (A), SHIP, SHP-1 or SHP-2 (B) antibodies. Total YT2C2 cell lysates were used as positive controls.
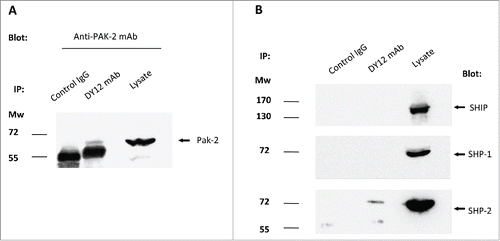
As previously demonstrated, CD245 was found associated with a phosphatase activity in the YT2C2 NK cell line.Citation36 Among the main phosphatases involved in NKRs-mediated signaling pathways are the Src-homology domain-containing phosphatases (SHP) such as the SH2 domain-containing inositol 5′-phosphatase (SHIP), SHP-1 and SHP-2. These phosphatases interact with phosphorylated tyrosine residues on other proteins through their SH2 domains.Citation28-31 We thus investigated whether myosin 18A was able to recruit SHP-1, SHP-2 or SHIP. As demonstrated in , immunoprecipitation of YT2C2 cell lysates with DY12 mAb and further immunoblotting of the resulting immunoprecipitates using anti-SHP-1, -SHP-2 or -SHIP antibodies revealed the presence of the phosphatase SHP-2, but not of SHP-1 or SHIP within myosin 18A immunoprecipitates. Thus, SHP-2 may be involved in the regulation of myosin 18A dependent activating signals.
Discussion
In the present work, we identified CD245, a human cell surface antigen expressed on peripheral blood lymphocytes, as the unconventional myosin 18A, a highly conserved motor enzyme involved in cytoskeleton organization and Golgi budding.Citation32 It is worthy to mention that by using a polyclonal antibody against surfactant protein A-receptor (SP-R)-210 that was previously shown to detect myosin 18A,Citation17 Samten et al. found a reactivity only with a very small fraction of peripheral blood CD3+ lymphocytes, and that this staining increased 5- to 10-fold and was extended to the whole lymphocyte population after stimulation with Mycobacterium tuberculosis.Citation33 Using the DY12 mAb, we found that CD245/myosin 18A was constitutively expressed by most lymphocytes including CD3+ lymphocytes, and that its membrane expression was enhanced on NK, T and B lymphocytes following activation. In addition, monocytes were found to also express CD245/myosin18A, although at higher level than lymphocytes. This discrepancy with the previously published data could be explained by a better specificity or affinity of the DY12 mAb for myosin 18A than the polyclonal antibody against the SP-R-210. Last, Myo18A was found to co-precipitate with SHP-2, a phosphatase with a key role in NK cell receptors-mediated signaling pathways,Citation31 and with PAK-2, a serine/threonine kinase that controls the cytoskeletal organization.Citation27 Of note, SHP-2 has previously been shown to negatively regulate NK cell function,Citation31 although in other settings, it mediated a positive proliferation signalCitation34 and activated the IL-1/Erk-mediated cytoskeleton reorganization.Citation35
Our novel molecular and functional data on a newly described NK cell co-activating receptor have broad potential applications. The unconventional myosin 18A is a member of the myosin superfamily of motor enzymes. Myosins generally contain a conserved catalytic head that catalyzes ATP hydrolysis and binds F-actin, thus promoting motility. The first myosin, M2, was discovered in muscle extracts and is referred to as conventional myosin, whereas other classes, including class 18, are called unconventional myosins. Myosin 2A is required for cytolytic granule exocytosis in human NK cells.Citation36 Class 18 myosins have been involved in fundamental tissular processes in mammalians, including epithelial cell migration,Citation37 stromal cell differentiationCitation38 and tumor suppression.Citation39,40 In humans and mice, myosin 18A is expressed in hematopoietic cells as two splice variants, referred to as α (230 kDa) and β (190 kDa). At the cell level, myosin 18A participates in cytoskeleton organization,Citation41 Golgi buddingCitation32 and DNA-damaged-induced Golgi dispersion by its association with F-actin and Golgi Phosphoprotein 3 (GOLPH3) in epithelial cellsCitation42 but its specific role in NK cells was not shown yet . Myosin 18A was also reported as a receptor for the surfactant protein A (SP-A),Citation17 a collectin present in human lung,Citation18 blood,Citation43 intestinal tractCitation44 and skinCitation45 that participates in the elimination of pathogens.Citation21 SP-A has also been shown to strongly stimulate the anti-tumor immunity in a xenograft mouse model.Citation19 Tumor cells transduced with SP-A grew slower than those transduced with the vector alone. This antitumor effect of SP-A was entirely dependent on NK cells in vivoCitation19 although the exact mechanism remained unknown. We showed that myosin 18A/CD245 is a potent human NK cell co-activating receptor, whose cell surface expression is increased by IL-2. CD245 stimulation by DY12 mAb or its physiological ligand SP-A was able to increase NK cell degranulation and lymphokine-activated killer activity toward tumor B cells and EBV-infected B cells, respectively. Our data support the hypothesis that this major antitumor effect of SP-A in vivo is mediated by its interaction with myosin 18A on NK cells.
We also found that myosin 18A stimulation was able to induce CD137 expression at the NK cell surface and that the myosin 18A induced NK cell cytotoxicity toward CD137L-expressing tumor cells was dependent on the CD137/CD137L interaction. The use of monoclonal antibodies modulating the NK cell antitumor function is a fast growing field of research . On one side, monoclonal antibodies able to induce ADCC by targeting both the cancer cell and the FcγRIIIA/CD16-activating receptor present on NK cells have revolutionized the management of lymphoma and human cancer.Citation46 On the other side, not all malignancies have an identified target and some malignancies with identified targets escape to therapeutic antibodies. In such immunotherapeutic field, strategies that aim at increasing the efficacy of monoclonal antibodies are promising. Stimulation of the CD137 (4-1BB) receptor present on NK cells has been shown to increase the efficacy of cetuximab, trastuzumab and rituximab in both in vitro and in vivo models of human cancer.Citation9-11 The use of monoclonal antibodies that modulate the expression of CD137 at the NK cell surface, such as DY12 mAb (), could be interesting in such application. In conclusion, the NK cell activating receptor CD245 appears as a very promising target in the field of the immunotherapy of human cancer and hematological malignancies. Further in vivo studies are needed to determine the optimal conditions for the use of agonistic antibodies to myosin 18A/CD245 as immunotherapeutic agents in this context.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Author contributions
AdM and JG performed the experiments and analysis. AMC, ND and AB designed the research. AdM, JG, AMC, ND and AB wrote the manuscript. ND, JDB, DG, PV, AT, CG, MB, YM and AB critically reviewed the manuscript.
Acknowledgments
The authors thank Drs Reem Al Daccak, Rachid Hocine and Laura Lauden for their valuable contribution of reactives and protocols.
Funding
AdM was supported by a grant from Institut National du Cancer/Institut Thématique MultiOrganismes Cancer.
References
- Rosenberg EB, Herberman RB, Levine PH, Halterman RH, McCoy JL, Wunderlich JR. Lymphocyte cytotoxicity reactions to leukemia-associated antigens in identical twins. Int J Cancer 1972; 9(3):648-58; PMID:4513057; http://dx.doi.org/10.1002/ijc.2910090323
- Benson DM, Caligiuri MA. Killer immunoglobulin-like receptors and tumor immunity. Cancer Immunol Res 2014; 2(2):99-104; PMID:24592397; http://dx.doi.org/10.1158/2326-6066.CIR-13-0219
- Moffett-King A. Natural killer cells and pregnancy. Nat Rev Immunol 2002; 2(9):656-63; PMID:12209134; http://dx.doi.org/10.1038/nri886
- Lanier LL. Up on the tightrope: natural killer cell activation and inhibition. Nat Immunol 2008; 9(5):495-502; PMID:18425106; http://dx.doi.org/10.1038/ni1581
- Le Bouteiller P, Barakonyi A, Giustiniani J, Lenfant F, Marie-Cardine A, Aguerre-Girr M, Rabot M, Hilgert I, Mami-Chouaib F, Tabiasco J et al. Engagement of CD160 receptor by HLA-C is a triggering mechanism used by circulating natural killer (NK) cells to mediate cytotoxicity. Proc Natl Acad Sci U S A 2002; 99(26):16963-8; PMID:12486241; http://dx.doi.org/10.1073/pnas.012681099
- Bensussan A, Gluckman E, Marsafy S el, Schiavon V, Mansur IG, Dausset J, Boumsell L, Carosella E. BY55 monoclonal antibody delineates within human cord blood and bone marrow lymphocytes distinct cell subsets mediating cytotoxic activity. Proc Natl Acad Sci U S A 1994; 91(19):9136-40; PMID:8090781; http://dx.doi.org/10.1073/pnas.91.19.9136
- Melero I, Johnston JV, Shufford WW, Mittler RS, Chen L. NK1.1 cells express 4-1BB (CDw137) costimulatory molecule and are required for tumor immunity elicited by anti-4-1BB monoclonal antibodies. Cell Immunol 1998; 190(2):167-72; PMID:9878117; http://dx.doi.org/10.1006/cimm.1998.1396
- Lin W, Voskens CJ, Zhang X, Schindler DG, Wood A, Burch E, Wei Y, Chen L, Tian G, Tamada K et al. Fc-dependent expression of CD137 on human NK cells: insights into «agonistic» effects of anti-CD137 monoclonal antibodies. Blood 2008; 112(3):699-707; PMID:18519814; http://dx.doi.org/10.1182/blood-2007-11-122465
- Kohrt HE, Colevas AD, Houot R, Weiskopf K, Goldstein MJ, Lund P, Mueller A, Sagiv-Barfi I, Marabelle A, Lira R et al. Targeting CD137 enhances the efficacy of cetuximab. J Clin Invest 2014; 124(6):2668-82; PMID:24837434; http://dx.doi.org/10.1172/JCI73014
- Kohrt HE, Houot R, Weiskopf K, Goldstein MJ, Scheeren F, Czerwinski D, Colevas AD, Weng WK, Clarke MF, Carlson RW et al. Stimulation of natural killer cells with a CD137-specific antibody enhances trastuzumab efficacy in xenotransplant models of breast cancer. J Clin Invest 2012; 122(3):1066-75; PMID:22326955; http://dx.doi.org/10.1172/JCI61226
- Kohrt HE, Houot R, Goldstein MJ, Weiskopf K, Alizadeh AA, Brody J, Müller A, Pachynski R, Czerwinski D, Coutre S et al. CD137 stimulation enhances the antilymphoma activity of anti-CD20 antibodies. Blood 2011; 117(8):2423-32; PMID:21193697; http://dx.doi.org/10.1182/blood-2010-08-301945
- Marie-Cardine A, Boumsell L, Bensussan A. The T-cell panel DY12 and DY35 mAbs define a novel receptor associated with protein tyrosine phosphatase activity in the leukeamic cell line YT indi. In: Leucocyte Typing VII. Oxford University Press. D Mason; 2002, Oxford University press, New York, USA. Edition D Mason. p. 688.
- Giustiniani J, Marie-Cardine A, Bensussan A. A soluble form of the MHC class I-specific CD160 receptor is released from human activated NK lymphocytes and inhibits cell-mediated cytotoxicity. J Immunol 2007; 178(3):1293-300; PMID:17237375; http://dx.doi.org/10.4049/jimmunol.178.3.1293
- Dufresne-Martin G, Lemay J-F, Lavigne P, Klarskov K. Peptide mass fingerprinting by matrix-assisted laser desorption ionization mass spectrometry of proteins detected by immunostaining on nitrocellulose. Proteomics 2005; 5(1):55-66; PMID:15602772; http://dx.doi.org/10.1002/pmic.200400902
- Mansur I-G, Schiavon V, Giustiniani J, Bagot M, Bensussan A, Marie-Cardine A. Engagement of IL-1 receptor accessory protein (IL-1RAcP) with the monoclonal antibody AY19 provides co-activating signals and prolongs the CD2-induced proliferation of peripheral blood lymphocytes. Immunol Lett 2011; 139(1–2):52-7; PMID:21600927; http://dx.doi.org/10.1016/j.imlet.2011.04.015
- Mori K, Furusawa T, Okubo T, Inoue T, Ikawa S, Yanai N, Mori KJ, Obinata M. Genome structure and differential expression of two isoforms of a novel PDZ-containing myosin (MysPDZ) (Myo18A). J Biochem (Tokyo) 2003; 133(4):405-13; PMID:12761286; http://dx.doi.org/10.1093/jb/mvg053
- Yang C-H, Szeliga J, Jordan J, Faske S, Sever-Chroneos Z, Dorsett B, Christian RE, Settlage RE, Shabanowitz J, Hunt DF et al. Identification of the surfactant protein A receptor 210 as the unconventional myosin 18A. J Biol Chem 2005; 280(41):34447-57; PMID:16087679; http://dx.doi.org/10.1074/jbc.M505229200
- Haagsman HP, Hawgood S, Sargeant T, Buckley D, White RT, Drickamer K, Benson BJ. The major lung surfactant protein, SP 28-36, is a calcium-dependent, carbohydrate-binding protein. J Biol Chem 1987; 262(29):13877-80; PMID:2820982
- Mitsuhashi A, Goto H, Kuramoto T, Tabata S, Yukishige S, Abe S, Hanibuchi M, Kakiuchi S, Saijo A, Aono Y et al. Surfactant protein A suppresses lung cancer progression by regulating the polarization of tumor-associated macrophages. Am J Pathol 2013; 182(5):1843-53; PMID:23499372; http://dx.doi.org/10.1016/j.ajpath.2013.01.030
- Cerwenka A, Lanier LL. Natural killer cells, viruses and cancer. Nat Rev Immunol 2001; 1(1):41-9; PMID:11905813; http://dx.doi.org/10.1038/35095564
- McNeely TB, Coonrod JD. Aggregation and opsonization of type A but not type B Hemophilus influenzae by surfactant protein A. Am J Respir Cell Mol Biol 1994; 11(1):114-22; PMID:8018334; http://dx.doi.org/10.1165/ajrcmb.11.1.8018334
- Pollok KE, Kim YJ, Hurtado J, Zhou Z, Kim KK, Kwon BS. 4-1BB T-cell antigen binds to mature B cells and macrophages, and costimulates anti-mu-primed splenic B cells. Eur J Immunol 1994; 24(2):367-74; PMID:8299685; http://dx.doi.org/10.1002/eji.1830240215
- Zhao S, Zhang H, Xing Y, Natkunam Y. CD137 ligand is expressed in primary and secondary lymphoid follicles and in B-cell lymphomas: diagnostic and therapeutic implications. Am J Surg Pathol 2013; 37(2):250-8; PMID:23095505; http://dx.doi.org/10.1097/PAS.0b013e318268c6ea
- Davis DM, Chiu I, Fassett M, Cohen GB, Mandelboim O, Strominger JL. The human natural killer cell immune synapse. Proc Natl Acad Sci U S A 1999; 96(26):15062-7; PMID:10611338; http://dx.doi.org/10.1073/pnas.96.26.15062
- Rak GD, Mace EM, Banerjee PP, Svitkina T, Orange JS. Natural killer cell lytic granule secretion occurs through a pervasive actin network at the immune synapse. PLoS Biol 2011; 9(9):e1001151; PMID:21931536; http://dx.doi.org/10.1371/journal.pbio.1001151
- Brown ACN, Oddos S, Dobbie IM, Alakoskela J-M, Parton RM, Eissmann P, Neil MA, Dunsby C, French PM, Davis I et al. Remodelling of cortical actin where lytic granules dock at natural killer cell immune synapses revealed by super-resolution microscopy. PLoS Biol 2011; 9(9):e1001152; PMID:21931537; http://dx.doi.org/10.1371/journal.pbio.1001152
- Hsu R-M, Tsai M-H, Hsieh Y-J, Lyu P-C, Yu J-S. Identification of MYO18A as a novel interacting partner of the PAK2/betaPIX/GIT1 complex and its potential function in modulating epithelial cell migration. Mol Biol Cell 2010; 21(2):287-301; PMID:19923322; http://dx.doi.org/10.1091/mbc.E09-03-0232
- Burshtyn DN, Scharenberg AM, Wagtmann N, Rajagopalan S, Berrada K, Yi T, Kinet JP, Long EO. Recruitment of tyrosine phosphatase HCP by the killer cell inhibitor receptor. Immunity 1996; 4(1):77-85; PMID:8574854; http://dx.doi.org/10.1016/S1074-7613(00)80300-3
- Stebbins CC, Watzl C, Billadeau DD, Leibson PJ, Burshtyn DN, Long EO. Vav1 dephosphorylation by the tyrosine phosphatase SHP-1 as a mechanism for inhibition of cellular cytotoxicity. Mol Cell Biol 2003; 23(17):6291-9; PMID:12917349; http://dx.doi.org/10.1128/MCB.23.17.6291-6299.2003
- Mahmood S, Kanwar N, Tran J, Zhang M-L, Kung SKP. SHP-1 phosphatase is a critical regulator in preventing natural killer cell self-killing. PloS One 2012; 7(8):e44244; PMID:22952938; http://dx.doi.org/10.1371/journal.pone.0044244
- Purdy AK, Campbell KS. SHP-2 expression negatively regulates NK cell function. J Immunol 2009; 183(11):7234-43; PMID:19915046; http://dx.doi.org/10.4049/jimmunol.0900088
- Dippold HC, Ng MM, Farber-Katz SE, Lee S-K, Kerr ML, Peterman MC, Sim R, Wiharto PA, Galbraith KA, Madhavarapu S et al. GOLPH3 bridges phosphatidylinositol-4- phosphate and actomyosin to stretch and shape the Golgi to promote budding. Cell 2009; 139(2):337-51; PMID:19837035; http://dx.doi.org/10.1016/j.cell.2009.07.052
- Samten B, Townsend JC, Sever-Chroneos Z, Pasquinelli V, Barnes PF, Chroneos ZC. An antibody against the surfactant protein A (SP-A)-binding domain of the SP-A receptor inhibits T cell-mediated immune responses to Mycobacterium tuberculosis. J Leukoc Biol 2008; 84(1):115-23; PMID:18443188; http://dx.doi.org/10.1189/jlb.1207835
- Mitra S, Beach C, Feng G-S, Plattner R. SHP-2 is a novel target of Abl kinases during cell proliferation. J Cell Sci 2008; 121(20):3335-46; PMID:18827006; http://dx.doi.org/10.1242/jcs.035691
- MacGillivray M, Herrera-Abreu MT, Chow C-W, Shek C, Wang Q, Vachon E, Feng GS, Siminovitch KA, McCulloch CA, Downey GP. The protein tyrosine phosphatase SHP-2 regulates interleukin-1-induced ERK activation in fibroblasts. J Biol Chem 2003; 278(29):27190-8; PMID:12721296; http://dx.doi.org/10.1074/jbc.M213083200
- Andzelm MM, Chen X, Krzewski K, Orange JS, Strominger JL. Myosin IIA is required for cytolytic granule exocytosis in human NK cells. J Exp Med 2007; 204(10):2285-91; PMID:17875677; http://dx.doi.org/10.1084/jem.20071143
- Hsu R-M, Hsieh Y-J, Yang T-H, Chiang Y-C, Kan C-Y, Lin Y-T, Chen JT, Yu JS. Binding of the extreme carboxyl-terminus of PAK-interacting exchange factor β (βPIX) to myosin 18A (MYO18A) is required for epithelial cell migration. Biochim Biophys Acta 2014; 1843(11):2513-27; PMID:25014165; http://dx.doi.org/10.1016/j.bbamcr.2014.06.023
- Furusawa T, Ikawa S, Yanai N, Obinata M. Isolation of a novel PDZ-containing myosin from hematopoietic supportive bone marrow stromal cell lines. Biochem Biophys Res Commun 2000; 270(1):67-75; PMID:10733906; http://dx.doi.org/10.1006/bbrc.2000.2377
- Nakano T, Tani M, Nishioka M, Kohno T, Otsuka A, Ohwada S, Yokota J. Genetic and epigenetic alterations of the candidate tumor-suppressor gene MYO18B, on chromosome arm 22q, in colorectal cancer. Genes Chromosomes Cancer 2005; 43(2):162-71; PMID:15751041; http://dx.doi.org/10.1002/gcc.20180
- Nishioka M, Kohno T, Tani M, Yanaihara N, Tomizawa Y, Otsuka A, Sasaki S, Kobayashi K, Niki T, Maeshima A et al. MYO18B, a candidate tumor suppressor gene at chromosome 22q12.1, deleted, mutated, and methylated in human lung cancer. Proc Natl Acad Sci U S A 2002; 99(19):12269-74; PMID:12209013; http://dx.doi.org/10.1073/pnas.192445899
- Taft MH, Behrmann E, Munske-Weidemann L-C, Thiel C, Raunser S, Manstein DJ. Functional characterization of human myosin-18A and its interaction with F-actin and GOLPH3. J Biol Chem 2013; 288(42):30029-41; PMID:23990465; http://dx.doi.org/10.1074/jbc.M113.497180
- Farber-Katz SE, Dippold HC, Buschman MD, Peterman MC, Xing M, Noakes CJ, Tat J, Ng MM, Rahajeng J, Cowan DM et al. DNA damage triggers Golgi dispersal via DNA-PK and GOLPH3. Cell 2014; 156(3):413-27; PMID:24485452; http://dx.doi.org/10.1016/j.cell.2013.12.023
- Kuroki Y, Tsutahara S, Shijubo N, Takahashi H, Shiratori M, Hattori A, Honda Y, Abe S, Akino T. Elevated levels of lung surfactant protein A in sera from patients with idiopathic pulmonary fibrosis and pulmonary alveolar proteinosis. Am Rev Respir Dis 1993; 147(3):723-9; PMID:8442609; http://dx.doi.org/10.1164/ajrccm/147.3.723
- Rubio S, Lacaze-Masmonteil T, Chailley-Heu B, Kahn A, Bourbon JR, Ducroc R. Pulmonary surfactant protein A (SP-A) is expressed by epithelial cells of small and large intestine. J Biol Chem 1995; 270(20):12162-9; PMID:7744866; http://dx.doi.org/10.1074/jbc.270.20.12162
- Aiad HAS, El-Farargy SM, Soliman MM, El-Wahed Gaber MA, El-Aziz Othman SA. Immunohistochemical staining of surfactant proteins A and B in skin of psoriatic patients before and after narrow-band UVB phototherapy. Am J Clin Dermatol 2012; 13(5):341-8; PMID:22621659; http://dx.doi.org/10.2165/11630720-000000000-00000
- Cunningham D, Humblet Y, Siena S, Khayat D, Bleiberg H, Santoro A, Bets D, Mueser M, Harstrick A, Verslype C et al. Cetuximab monotherapy and cetuximab plus irinotecan in irinotecan-refractory metastatic colorectal cancer. N Engl J Med 2004; 351(4):337-45; PMID:15269313; http://dx.doi.org/10.1056/NEJMoa033025
